Abstract
Objective
SHAPE (Simple Hysterectomy And PElvic node assessment) was an international phase III trial demonstrating that simple hysterectomy was non-inferior to radical hysterectomy for pelvic recurrence risk, but superior for quality of life and sexual health. The objective was to conduct a cost-effectiveness analysis comparing simple vs. radical hysterectomy for low-risk early-stage cervical cancer.
Methods
Markov model compared the costs and benefits of simple vs. radical hysterectomy for early cervical cancer over a 5-year time horizon. Quality-adjusted life years (QALYs) were estimated from health utilities derived from EQ-5D-3L surveys. Sensitivity analyses accounted for uncertainty around key parameters. Monte Carlo simulation estimated complication numbers according to surgical procedure.
Results
Simple hysterectomy was more effective and less costly than radical hysterectomy. Average overall costs were $11,022 and $12,533, and average gains were 3.56 and 3.54 QALYs for simple and radical hysterectomy, respectively. Baseline health utility scores were 0.81 and 0.83 for simple and radical hysterectomy, respectively. By year 3, these scores improved for simple hysterectomy (0.82) but not for radical hysterectomy (0.82). Assuming 800 early cervical cancer patients annually in Canada, the model estimated 3 vs. 82 patients with urinary retention, and 49 vs. 86 patients with urinary incontinence persisting 4 weeks after simple vs. radical hysterectomy, respectively. Results were most sensitive to variability in health utilities after surgery, but stable through wide ranges of costs and recurrence estimates.
Conclusion
Simple hysterectomy is less costly and more effective in terms of quality-adjusted life expectancy compared to radical hysterectomy for early cervical cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT01658930
Keywords: Low Risk, Early Stage Cervical Cancer, Simple Hysterectomy, Radical Hysterectomy, Quality-Adjusted Life Expectancy, Health Utility, Cost-Effectiveness
Synopsis
SHAPE was an international phase III randomized trial demonstrating that simple hysterectomy is non-inferior to radical hysterectomy in terms of pelvic recurrence rate in low-risk early-stage cervical cancer. This cost-effectiveness analysis demonstrated that simple hysterectomy was more effective in terms of quality-adjusted life expectancy, and less costly than radical hysterectomy.
INTRODUCTION
SHAPE (Simple Hysterectomy And PElvic node assessment) was an international phase III randomized trial comparing radical hysterectomy to simple hysterectomy for early-stage cervical cancer [1]. The rationale for the trial was based on retrospective evidence that conservative surgery (simple hysterectomy) appeared to be comparable to radical hysterectomy in terms of oncologic outcomes such as recurrence and mortality [2,3,4,5,6], but with much less perioperative morbidity such as intraoperative injuries and postoperative bowel, bladder, sexual dysfunction, and lower extremity lymphedema [7,8,9,10,11,12]. The morbidity associated with radical hysterectomy is attributed to removing the parametrium, or connective tissue surrounding the cervix, which was historically required to achieve a wide margin around the primary tumor in the cervix [13]. Removing the parametrium during radical hysterectomy causes disruption of the autonomic fibers innervating the bladder, which can cause bladder dysfunction [14], but the parametria are spared during simple hysterectomy. The primary outcome of the SHAPE trial was pelvic recurrence rate at 3 years. The hypothesis was that less radical surgery (simple hysterectomy) would yield a comparable pelvic recurrence rate despite sparing the parametrium, but it would be superior in terms of adverse events and quality of life compared to radical hysterectomy. The trial confirmed that simple hysterectomy was non-inferior to radical hysterectomy in terms of 3-year pelvic recurrence rate, but the postoperative complication rate was higher in those having radical hysterectomy, notably bladder dysfunction immediately after and persisting beyond 4 weeks after surgery [15]. Quality of life and sexual health scores were significantly lower among those having radical hysterectomy [16]. All of these non-oncologic outcomes are associated with a cost, and they can influence an individual’s quality-adjusted life expectancy. The objective of this study was to conduct a model-based cost-effectiveness analysis based on prospectively-collected data from the SHAPE trial comparing the 2 surgical treatment strategies for early-stage cervical cancer.
MATERIALS AND METHODS
A Markov model was constructed using TreeAge Pro 2023 (Williamstown, MA, USA) to compare costs in 2023 Canadian dollars and benefits in terms of quality-adjusted life expectancy gains, for early stage cervical cancer as per the SHAPE trial (squamous cell, adenocarcinoma, or adenosquamous carcinoma, maximum tumor diameter 2 cm, maximum depth of invasion 10 mm or less than 50% cervical stromal invasion on magnetic resonance imaging, no evidence of metastatic disease on preoperative imaging) [1]. The 2 surgical treatment strategies were: 1) radical hysterectomy, and 2) simple hysterectomy, and both surgical procedures included pelvic lymph node assessment (sentinel node biopsy, and/or complete pelvic lymphadenectomy). This study was exempt from Research Ethics Board review as there were no individual level data required.
The Markov model consists of 3 mutually exclusive health states: 1) alive and at risk for cervical cancer recurrence; 2) cervical cancer recurrence; 3) dead of cervical cancer or other cause. All patients begin in the “alive and at risk for cervical cancer recurrence” state. They transition from one state to another based on annual transition probabilities for recurrences and deaths from the SHAPE trial. They entered the model at primary diagnosis of cervical cancer at an average age of 44. They had all of the prerequisite imaging and pathology for consideration of simple hysterectomy [15]. They had not received any other treatment for their cancer apart from LEEP/cone biopsy. We did not model treatment of recurrences. The median follow-up in the trial was 4.5 years, and therefore we modeled a time horizon of 5 years.
Quality adjusted life expectancy was calculated based on health utilities derived from the study. Health utilities represent patient preferences for health states, where 1 represents perfect health and 0 implies death, and these scores were multiplied by life years gained to generate quality-adjusted life years (QALYs). These utilities were collected systematically at baseline (randomization, prior to surgery), and then at 3, 6, 9, 12, 18, 24, and 36 months after surgery using EQ-5D-3L [17], which was the survey tool available when this study was opened in 2012. EQ-5D-3L responses were converted to utilities using the Canadian tariffs [18]. For the purpose of this analysis, QALYs were calculated based on health utility scores at baseline, 12 months, 24 months, and 36 months, as there was not much difference in scores within 3-month intervals.
Costs were collected from various public sources, including the Patient Cost Estimator from the Canadian Institute for Health Information [19] for costs associated with surgery (abdominal, laparoscopic, or robotic), management of intraoperative complications including bowel and bladder injury, re-admission to hospital for complications including ileus, bowel obstruction, postoperative bleeding, thromboembolic events, the British Columbia Medical Services Plan for physician services including surgery, emergency medicine, outpatient clinics, urologic interventions including urodynamics and catheterization [20]. Rates of complications and readmission to hospital have been previously reported [15]. BC Cancer Provincial Pharmacy provided costs associated with adjuvant chemotherapy [21], and radiotherapy costs were extrapolated from public sources and indexed to 2023 costs [22,23]. Resource utilization data were collected (adjuvant therapy, emergency room visits, readmission to hospital, clinic visits) for Canadian patients in the study. As per recommendations of Canadian Agency for Drugs and Technologies in Health (CADTH), all costs and health outcomes were discounted by 1.5%, and a publicly funded health care payer perspective was primarily adopted for this model, but societal perspective was also considered [24]. We estimated opportunity costs related to productivity loss from temporary disability after surgery, postoperative complications, and adjuvant therapy based on event rates in SHAPE, and applied Canadian data on hourly wage and proportion of females in the labor force, according to age [25]. Table 1 summarizes selected data for the base case [15,19,20,21,22,23,25,26,27].
Table 1. Selected data for base case.
| Data inputs | Expected value (CAD) | Range | ||
|---|---|---|---|---|
| Cost inputs | ||||
| Radical hysterectomy (open) [19] | $7,732 | $5,412–$10,052 | ||
| Radical hysterectomy (MIS) [19] | $4,151 | $2,906–$5,396 | ||
| Robotic hysterectomy [26] | $13,012 | $9,108–$16,916 | ||
| Simple hysterectomy (open) [19] | $5,276 | $3,693–$6,859 | ||
| Simple hysterectomy (MIS) [19] | $4,151 | $2,906–$5,396 | ||
| Urinary tract complication requiring Urology [19] | $8,220 | $5,754–$10,686 | ||
| Intestinal complication requiring General Surgery [19] | $7,095 | $4,967–$9,224 | ||
| Postoperative complications requiring readmission to hospital [19] | ||||
| Bowel obstruction | $3,529 | $2,470–$4,588 | ||
| Pulmonary embolism | $5,098 | $3,568–$6,627 | ||
| Deep vein thrombosis/thrombophlebitis | $4,954 | $3,468–$6,440 | ||
| Outpatient visits [20] | ||||
| Initial consultation (gynecologic oncologist, radiation or medical oncologist) | $155 | $108–$202 | ||
| Follow-up visit | $85 | $60–$111 | ||
| Consultation in emergency department for postoperative complication | $130 | $91–$169 | ||
| CT scan abdomen and pelvis | $139 | $97–$181 | ||
| Biopsy vaginal vault for suspected recurrence | $52 | $36–$68 | ||
| Interventional radiology consultation for other biopsy | $103 | $72–$134 | ||
| Opportunity costs | ||||
| Median hourly wage for ages 25–54 [27] | 34 | 24–44 | ||
| Employment rate ages 25–54 [25] | 81.4% | 0.73–0.90 | ||
| Time lost after surgery | 6 weeks | 4–36 | ||
| Time lost after postoperative complication | 6 weeks | 4–36 | ||
| Time lost after adjuvant radiotherapy | 11 weeks | 9–26 | ||
| Time lost after adjuvant chemotherapy | 24 weeks | 24–52 | ||
| Chemotherapy [19,21] | ||||
| Chair time | $5,679 | $3,975–$7,383 | ||
| Adjuvant therapy if high risk disease (6 cycles of paclitaxel and carboplatin) | $16,260 | $11,382–$21,138 | ||
| Weekly cisplatin (5 cycles if intermediate risk disease) | $1,350 | $945–$1,755 | ||
| Radiotherapy [22,23] | ||||
| Adjuvant pelvic radiotherapy | $10,761 | $7,533–$13,989 | ||
| Brachytherapy | $6,467 | $4,527–$8,407 | ||
| Clinical inputs [15] | ||||
| Admission to hospital after surgery | ||||
| Radical hysterectomy | 0.116 | 0.10–0.128 | ||
| Simple hysterectomy | 0.044 | 0.040–0.048 | ||
| Urinary incontinence beyond 4 wk | ||||
| Radical hysterectomy | 0.11 | 0.10–0.12 | ||
| Simple hysterectomy | 0.047 | 0.042–0.052 | ||
| Urinary retention beyond 4 wk | ||||
| Radical hysterectomy | 0.099 | 0.090–0.11 | ||
| Simple hysterectomy | 0.006 | 0.005–0.007 | ||
| 3-yr event (recurrence or death) | ||||
| Radical hysterectomy | 0.022 | 0.02–0.024 | ||
| Simple hysterectomy | 0.037 | 0.033–0.041 | ||
| Health utility | ||||
| Baseline | ||||
| Radical hysterectomy | 0.83 | 0.75–0.91 | ||
| Simple hysterectomy | 0.81 | 0.73–0.89 | ||
| 12 mo | ||||
| Radical hysterectomy | 0.82 | 0.74–0.90 | ||
| Simple hysterectomy | 0.80 | 0.72–0.88 | ||
| 24 mo | ||||
| Radical hysterectomy | 0.82 | 0.74–0.90 | ||
| Simple hysterectomy | 0.82 | 0.74–0.90 | ||
| 36 mo | ||||
| Radical hysterectomy | 0.82 | 0.74–0.90 | ||
| Simple hysterectomy | 0.82 | 0.74–0.90 | ||
CAD, Canadian dollars; CT, computed tomography; MIS, minimally invasive surgery.
If a strategy was less costly and more effective, it would be considered the dominant strategy. However, if a strategy was more costly but more effective, the incremental cost-effectiveness ratio (ICER) would be calculated from the difference in average cost between the 2 treatment strategies, divided by the difference in effectiveness measured in QALYs [28]. By convention, $100,000/QALY was the willingness-to-pay (WTP) threshold [29] and an ICER less than this was considered cost-effective. We also calculated net monetary benefit (NMB) at a WTP of $100,000/QALY. A positive incremental NMB indicates that the strategy is cost-effective at that WTP threshold [30].
We conducted scenario-based sensitivity analyses to explore the effect of key parameters including recurrence-free survival (RFS) rates, health utility scores, health care costs including surgery, hospitalization, and adjuvant therapy. We also conducted probabilistic sensitivity analyses to characterize uncertainty in all model parameters, using Monte Carlo simulation with 1,000 samples. Probabilities and utility scores were varied according to 95% confidence intervals, or by ±10%. Costs were varied by ±30%. Probabilities and utilities were assumed to have a beta distribution, and costs were assumed to have a Gamma distribution [31]. Discounting rates were also varied between 0% and 3% [24].
Monte Carlo simulation was conducted to estimate the number of patients in the Canadian population who would experience postoperative complications as observed in the SHAPE trial. There were 1,550 women diagnosed with cervical cancer in Canada in 2023, and about 55% of them had Stage I disease who may have been eligible for less radical surgery [32]. Therefore, we modeled a hypothetical cohort of 800 women with early stage cervical cancer eligible for radical or simple hysterectomy with nodal assessment, and over 1,000 trials we estimated the number of patients who would experience postoperative complications. We then modeled the United States population of 11,500 women diagnosed with cervical cancer, but only 44% (approximately 5,000) with early stage disease who may have been eligible for less radical surgery [33,34].
RESULTS
In the base case, simple hysterectomy was more effective and less costly than radical hysterectomy, therefore in economic analysis terms, simple hysterectomy is considered the dominant strategy. Table 2 summarizes the average lifetime costs and QALY gains associated with each strategy. Average lifetime costs were $11,022 and $12,533, and average QALY gains were 3.56 and 3.54, respectively for simple and radical hysterectomy. When a societal perspective was adopted and opportunity costs were included, average lifetime costs were $19,998 and $21,581 respectively for simple and radical hysterectomy. Health utilities changed over time. The average health utility score at baseline (at randomization, prior to surgery) was actually lower among those having simple hysterectomy (0.81) compared to radical hysterectomy (0.83). There was a decrease in health utility at 12 months after surgery (0.80 and 0.82 for simple hysterectomy and radical hysterectomy, respectively). By 24 months, there was an improvement in utility scores for both surgical strategies (0.82), which plateaued and remained stable at 36 months. However, the utility scores never recovered to baseline for those having radical hysterectomy, whereas they exceeded baseline for those having simple hysterectomy. For the Canadian population, the Monte Carlo simulation estimated 49 and 3 patients annually with prolonged urinary incontinence and retention (persisting beyond 4 weeks) respectively after simple hysterectomy, compared to 86 and 82 patients with those complications after radical hysterectomy. In the United States, the simulation estimated 240 and 590 patients annually with urinary incontinence, and 45 and 520 patients annually with urinary retention after simple and radical hysterectomy, respectively.
Table 2. Average lifetime costs and benefits of simple and radical hysterectomy.
| Strategy | Lifetime payer cost (societal cost) | Incremental payer cost (societal cost) | Effectiveness (QALY) | Incremental benefit (QALY) | ICER | INMB |
|---|---|---|---|---|---|---|
| Simple hysterectomy | $11,022 ($19,998) | 3.56 | N/A* | $3,583 | ||
| Radical hysterectomy | $12,533 ($21,581) | $1,511 ($1,583) | 3.54 | −0.02 |
ICER, incremental cost-effectiveness ratio; INMB, incremental net monetary benefit (positive value means cost-effective); N/A, not applicable; QALY, quality-adjusted life year.
*This strategy is less costly and more effective than the alternate strategy, therefore this strategy is “dominant”.
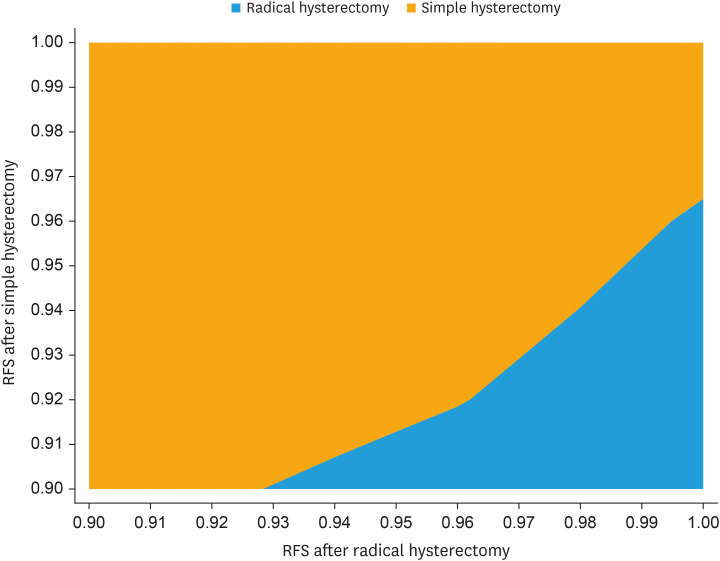
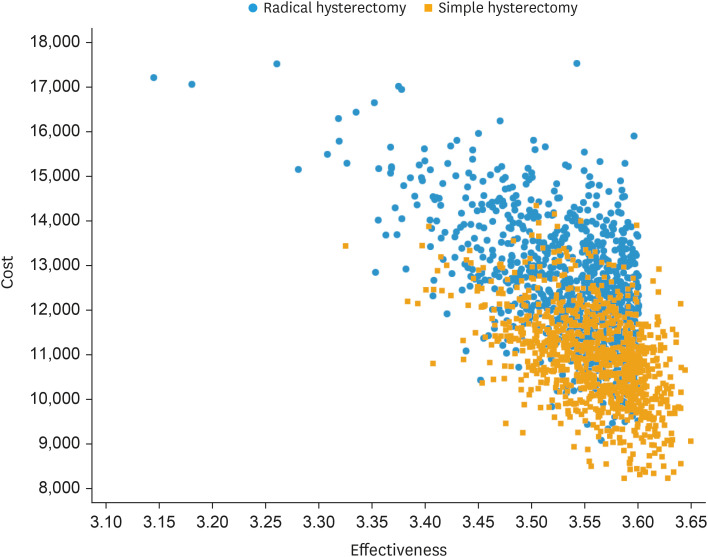
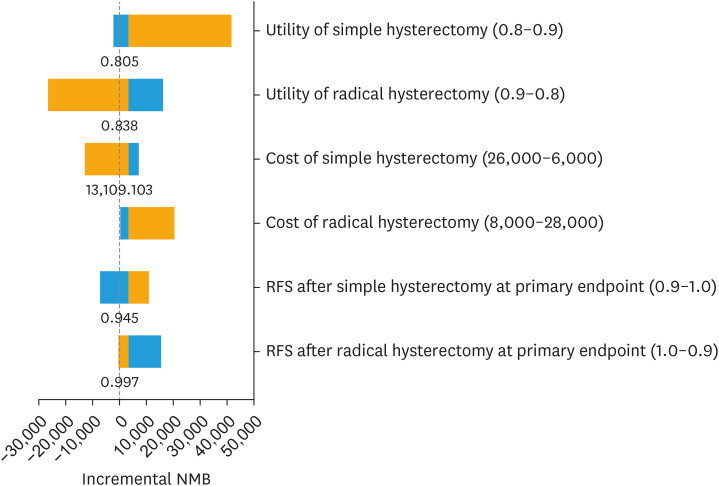
Because simple hysterectomy was more effective and less costly than radical hysterectomy, ICER was not relevant. Incremental NMB at a WTP of $100,000/QALY was $3,583, indicating that simple hysterectomy is cost-effective relative to radical hysterectomy at that threshold. Extensive sensitivity analyses were conducted around parameters of uncertainty, including costs of treatment and RFS estimates. Fig. 1 illustrates a 2-way sensitivity analysis on RFS for both surgical strategies. It demonstrates that the RFS associated with simple hysterectomy (on the Y axis) must be within about 3.5% of that of radical hysterectomy (on the X axis), for simple hysterectomy to remain a cost-effective strategy given a WTP threshold of $100,000 per QALY. In the SHAPE trial, the observed 3-year RFS rates were 96.3% and 97.8% for simple and radical hysterectomy, respectively, or a difference of 1.5%, which falls below the 3.5% threshold difference as described above. However, if RFS rates after simple and radical hysterectomy were 93% and 98%, respectively, then radical hysterectomy becomes the cost-effective strategy (data points intersect in the blue section of the figure), because this absolute difference of 5% is too high for simple hysterectomy to be cost-effective. Fig. 2 illustrates a cost-effectiveness scatterplot, in which costs and effectiveness estimates are randomly drawn from a Monte Carlo simulation using parameter distributions instead of mean estimates. Costs are plotted on the Y axis and effectiveness in QALYs on the X axis. In 75% of simulations, simple hysterectomy is the dominant strategy, because it has lower costs and higher effectiveness (depicted by the red points in the lower right quadrant of the figure) compared to radical hysterectomy (depicted by the blue points in the figure). In the remaining 25% of simulations, there is overlap between treatment strategies with respect to cost and effectiveness, but the ICER for simple hysterectomy is still less than the WTP threshold of $100,000/QALY, or the ICER associated with radical hysterectomy exceeds this threshold, which means that simple hysterectomy remains the cost-effective strategy. Fig. 3 is a tornado diagram illustrating the variables with the greatest impact on outcome. It demonstrates that the model is sensitive to variations in health utility and RFS rates associated with simple hysterectomy and radical hysterectomy. The average utility scores associated with simple and radical hysterectomy had to be lower than 0.805 and higher than 0.838, respectively, for radical hysterectomy to be a cost-effective strategy. The 3-year RFS rates after simple and radical hysterectomy had to be lower than 94.5% or higher than 99.7%, respectively, for radical hysterectomy to be a cost-effective strategy. The observed 3-year RFS rates in the SHAPE trial were 96.3% and 97.8% for simple and radical hysterectomy, respectively, well within the threshold rates as defined by the tornado diagram.
Fig. 1. Two-way sensitivity analysis on RFS.
RFS, recurrence-free survival.
Fig. 2. Cost-effectiveness scatterplot.
Fig. 3. Tornado diagram.
RFS, recurrence-free survival.
DISCUSSION
Cervical cancer is still a leading cause of morbidity and mortality in women worldwide. The World Health Organization (WHO) estimates that there were 660,000 new cases and 350,000 deaths in 2022, with rates highest in low and middle-income countries, particularly in Asia, Africa, and Latin America [35]. The WHO has launched a global strategy to eliminate cervical cancer, with targets of 90-70-90 (rates of HPV vaccination, screening between ages 35–45, and treatment of pre-cancer and cancer) to be achieved by the year 2030 [36]. Until this is realized, there are still hundreds of thousands of women who will be diagnosed with cervical cancer annually and could be eligible for surgery. Offering simple hysterectomy with nodal assessment instead of standard radical hysterectomy to eligible women will not only improve quality of life, but it will decrease overall health care costs associated with treatment. Although CADTH recommended a publicly funded health care payer perspective, we also modeled the societal perspective. Opportunity costs in this patient population are high. These are young women in the prime of their lives, and many of them would have had to take time away from work or care of families because of surgery and management of postoperative complications such as urinary incontinence or retention. Inclusion of opportunity costs did not change the outcome of this analysis. Simple hysterectomy remained the dominant strategy (less costly but more effective). Therefore, the SHAPE trial has the potential to revolutionize cervical cancer treatment worldwide.
Our results were stable across a wide range of plausible estimates for probabilities (recurrence, complications, adjuvant therapy) and costs, but sensitive to variations in health utility scores. Our model predicted that the average health utility associated with simple hysterectomy had to be 0.033 points lower than radical hysterectomy, for radical hysterectomy to be the better treatment strategy. However, this does not seem to be a plausible scenario, in which the overall health utility of simple hysterectomy would be worse than that of radical hysterectomy. Having said that, the average health utility at baseline in the SHAPE trial was actually lower prior to simple hysterectomy (by 0.02) than radical hysterectomy. The reasons for this are unclear, as the treatment groups by process of randomization were comparable in terms of age, ethnicity, performance status, body mass index, stage distribution [15]. We do not know if the groups were different with respect to comorbidities prior to surgery, however these were young women (median age 42 and 45 for simple and radical hysterectomy, respectively), with excellent performance status (over 95% in both groups with Eastern Cooperative Oncology Group performance score of 0), and therefore comorbidities were unlikely to be significantly different between groups. One conceivable explanation is that simple hysterectomy was the “experimental” treatment, possibly with a higher likelihood of adjuvant therapy or recurrence, and this uncertainty could have translated into a lower utility score. The health utility associated with surgery followed by adjuvant chemoradiotherapy is lower than that after surgery alone for early cervical cancer, indicating a clear preference for single modality treatment [37]. The lower baseline health utility in the simple hysterectomy group may not be clinically meaningful (0.81 vs. 0.83 for radical hysterectomy), but what is notable is that the average health utility score improved past baseline for simple hysterectomy, whereas it never recovered to baseline for radical hysterectomy. This reflects a deterioration in quality of life in the radical hysterectomy group which persists up to 3 years after surgery. The impact of radical hysterectomy on quality of life and sexual health is the subject of a separate manuscript [16].
The strength of this study is that it uses empirical data from a large prospective phase III randomized trial, including health utilities collected directly from patients at specific time points. This study also had a few limitations. First, although we had health utility data on the majority of patients, there was an attrition in completed EQ-5D-3L surveys over time, such that there were very few surveys completed after 36 months. Therefore, health utility scores after 36 months may not be as robust as the earlier scores, and potentially biased towards those with better health, performance status, and quality of life who may have been able and willing to complete the surveys. Although the results were sensitive to variations in health utility scores, the difference between the 2 groups had to exceed 0.033 at baseline, with the higher utility attributed to radical hysterectomy, and we did not think this was plausible. Second, Canadian tariffs were used to calculate the EQ-5D-3L utilities. It is possible that the preferences of Canadian participants may not align with the preferences of non-Canadian patients. Although scores were fairly consistent among the 4 countries that contributed health utilities in this study, there is uncertainty if these scores would be generalizable to the other countries that participated in this trial (notably South Korea and the other European countries) but did not complete the health utility surveys. Resource utilization data were collected from Canadian centers participating in the trial, and cost data were obtained from Canadian sources, and these may not be generalizable to other countries although our results were stable when costs were varied over a wide range. Third, we did not model recurrent disease, as the recurrence rates were low and comparable between both treatment groups, and there were very few utility scores collected from these patients so that we could not reliably calculate QALYs.
There are 2 other prospective trials that have evaluated conservative surgery (without removal of parametrium as required in a radical hysterectomy or fertility-sparing radical trachelectomy) for early stage cervical cancer. Both trials have demonstrated excellent oncologic outcomes and minimal adverse events [38,39]. These results along with the SHAPE trial provide compelling evidence that conservative surgery should be offered instead of radical hysterectomy for appropriately selected patients with low-risk early-stage cervical cancer [40].
In summary, simple hysterectomy with lymph node assessment is less costly and more effective in terms of quality-adjusted life expectancy compared to radical hysterectomy for low-risk early-stage cervical cancer. In the context of our health care system, we would recommend that simple hysterectomy replace radical hysterectomy as the standard of care.
Footnotes
Funding: Canadian Cancer Trials Group (CCTG) for operational support and the Canadian Institutes of Health Research (CIHR) for research funding.
Presentation: This study was presented at the European Society of Gynecological Oncology 25th Congress in Barcelona, Spain on March 9, 2024.
Conflict of Interest: JSK discloses research funding from Michael Smith Health Research BC (formerly Michael Smith Foundation for Health Research), ownership of shares from Hexamer Therapeutics, and speaker fee from Astra Zeneca. MP discloses research funding from Astra Zeneca, royalties from UpToDate, and serving as an Advisory Board member for Serono-Merck.
- Conceptualization: K.J.S., S.L.E., P.M.
- Data curation: K.J.S., F.S.E., S.V., L.E., G.F., T.J., W.K., G.N., D.C., V.W., M.S., H.L., G.F., B.R., E.B., K.J.W., B.L.A., Y.S.S.T., U.J., P.M.
- Formal analysis: K.J.S., M.H., F.S.E., S.V., B.L.A., P.R., C.K.K., C.M.C., T.D., P.M.
- Funding acquisition: K.J.S., S.L.E., P.M.
- Investigation: K.J.S., F.S.E., S.V., G.F., T.J., B.L.A., P.M.
- Methodology: K.J.S., M.H., P.R., C.K.K., C.M.C., T.D., P.M.
- Project administration: K.J.S., S.L.E., P.M.
- Resources: K.J.S., M.H., T.D., S.L.E., P.M.
- Software: K.J.S., M.H., T.D., P.M.
- Supervision: K.J.S., P.M.
- Validation: K.J.S., P.M.
- Visualization: K.J.S., P.M.
- Writing - original draft: K.J.S., P.M.
- Writing - review & editing: K.J.S., F.S.E., S.V., L.E., G.F., T.J., W.K., G.N., D.C., V.W., M.S., H.L., G.F., B.R., E.B., K.J.W., B.L.A., P.R., C.K.K., C.M.C., T.D., S.L.E., P.M.
References
- 1.National Library of Medicine. Radical versus simple hysterectomy and pelvic node dissection with low-risk early stage cervical cancer (SHAPE) [Internet] Bethesda, MD: National Library of Medicine; 2024. [cited 2024 Mar 5]. Available from: https://clinicaltrials.gov/study/NCT01658930. [Google Scholar]
- 2.Covens A, Rosen B, Murphy J, Laframboise S, DePetrillo AD, Lickrish G, et al. How important is removal of the parametrium at surgery for carcinoma of the cervix? Gynecol Oncol. 2002;84:145–149. doi: 10.1006/gyno.2001.6493. [DOI] [PubMed] [Google Scholar]
- 3.Frumovitz M, Sun CC, Schmeler KM, Deavers MT, Dos Reis R, Levenback CF, et al. Parametrial involvement in radical hysterectomy specimens for women with early-stage cervical cancer. Obstet Gynecol. 2009;114:93–99. doi: 10.1097/AOG.0b013e3181ab474d. [DOI] [PubMed] [Google Scholar]
- 4.Stegeman M, Louwen M, van der Velden J, ten Kate FJ, den Bakker MA, Burger CW, et al. The incidence of parametrial tumor involvement in select patients with early cervix cancer is too low to justify parametrectomy. Gynecol Oncol. 2007;105:475–480. doi: 10.1016/j.ygyno.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Wright JD, Grigsby PW, Brooks R, Powell MA, Gibb RK, Gao F, et al. Utility of parametrectomy for early stage cervical cancer treated with radical hysterectomy. Cancer. 2007;110:1281–1286. doi: 10.1002/cncr.22899. [DOI] [PubMed] [Google Scholar]
- 6.Steed H, Capstick V, Schepansky A, Honore L, Hiltz M, Faught W. Early cervical cancer and parametrial involvement: is it significant? Gynecol Oncol. 2006;103:53–57. doi: 10.1016/j.ygyno.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Frumovitz M, Sun CC, Schover LR, Munsell MF, Jhingran A, Wharton JT, et al. Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol. 2005;23:7428–7436. doi: 10.1200/JCO.2004.00.3996. [DOI] [PubMed] [Google Scholar]
- 8.Kadar N, Saliba N, Nelson JH. The frequency, causes and prevention of severe urinary dysfunction after radical hysterectomy. Br J Obstet Gynaecol. 1983;90:858–863. doi: 10.1111/j.1471-0528.1983.tb09328.x. [DOI] [PubMed] [Google Scholar]
- 9.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 10.Low JA, Mauger GM, Carmichael JA. The effect of Wertheim hysterectomy upon bladder and urethral function. Am J Obstet Gynecol. 1981;139:826–834. doi: 10.1016/0002-9378(81)90551-2. [DOI] [PubMed] [Google Scholar]
- 11.Sood AK, Nygaard I, Shahin MS, Sorosky JI, Lutgendorf SK, Rao SS. Anorectal dysfunction after surgical treatment for cervical cancer. J Am Coll Surg. 2002;195:513–519. doi: 10.1016/s1072-7515(02)01311-x. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Byun HK, Im SH, Son WJ, Roh YH, Kim YB. Risk factors for lower extremity lymphedema after surgery in cervical and endometrial cancer. J Gynecol Oncol. 2023;34:e28. doi: 10.3802/jgo.2023.34.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drouin E, Classe JM, Hautecoeur P. 125 years of the Wertheim operation. What next? J Med Life. 2023;16:341–343. doi: 10.25122/jml-2022-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zullo MA, Manci N, Angioli R, Muzii L, Panici PB. Vesical dysfunctions after radical hysterectomy for cervical cancer: a critical review. Crit Rev Oncol Hematol. 2003;48:287–293. doi: 10.1016/s1040-8428(03)00125-2. [DOI] [PubMed] [Google Scholar]
- 15.Plante M, Kwon JS, Ferguson S, Samouëlian V, Ferron G, Maulard A, et al. Simple versus radical hysterectomy in women with low-risk cervical cancer. N Engl J Med. 2024;390:819–829. doi: 10.1056/NEJMoa2308900. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson SE, Brotto LA, Kwon J, Samouelian V, Ferron G, Maulard A, et al. Sexual health and quality of life in patients with low-risk early-stage cervical cancer: results from GCIG/CCTG CX.5/SHAPE trial comparing simple versus radical hysterectomy. J Clin Oncol. 2024 doi: 10.1200/JCO.24.00440. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 17.EuroQol. EQ-5D-3L [Internet] Rotterdam: EuroQol; 2024. [cited 2023 Oct 26]. Available from: https://euroqol.org/information-and-support/euroqol-instruments/eq-5d-3l. [Google Scholar]
- 18.Bansback N, Tsuchiya A, Brazier J, Anis A. Canadian valuation of EQ-5D health states: preliminary value set and considerations for future valuation studies. PLoS One. 2012;7:e31115. doi: 10.1371/journal.pone.0031115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canadian Institute for Health Information. Patient cost estimator [Internet] Ottawa: Canadian Institute for Health Information; 2023. [cited 2023 Oct 26]. Available from: https://www.cihi.ca/en/patient-cost-estimator. [Google Scholar]
- 20.Ministry of Health British Columbia. Medical services commission (MSC) payment schedule [Internet] Victoria: Ministry of Health British Columbia; 2024. [cited 2023 Nov 6]. Available from: https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/medical-services-plan/msc_payment_schedule_oct_31_2023.pdf. [Google Scholar]
- 21.BC Cancer Provincial Pharmacy . Wholesale prices for pharmaceuticals. Vancouver, BC: BC Cancer Provincial Pharmacy; 2023. [Google Scholar]
- 22.Provincial Health Services Agency. Charges for radiotherapy services. Vancouver: BC Cancer; 2023. [Google Scholar]
- 23.Mittmann N, Liu N, Cheng SY, Seung SJ, Saxena FE, Look Hong NJ, et al. Health system costs for cancer medications and radiation treatment in Ontario for the 4 most common cancers: a retrospective cohort study. CMAJ Open. 2020;8:E191–E198. doi: 10.9778/cmajo.20190114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CADTH. Guidelines for the economic evaluation of health technologies: Canada. 4th ed. Ottawa: CADTH; 2017. [Google Scholar]
- 25.Statistics Canada. Employee wages by job permanency and union coverage, monthly, unadjusted for seasonality [Internet] Ottawa: Statistics Canada; 2024. [cited 2024 Apr 17]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410006501. [Google Scholar]
- 26.Halliday D, Lau S, Vaknin Z, Deland C, Levental M, McNamara E, et al. Robotic radical hysterectomy: comparison of outcomes and cost. J Robot Surg. 2010;4:211–216. doi: 10.1007/s11701-010-0205-z. [DOI] [PubMed] [Google Scholar]
- 27.Statistics Canada. Employee wages by job permanency and union coverage, annual. Ottawa: Statistics Canada; 2023. [Google Scholar]
- 28.Cohen DJ, Reynolds MR. Interpreting the results of cost-effectiveness studies. J Am Coll Cardiol. 2008;52:2119–2126. doi: 10.1016/j.jacc.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Institute for Clinical and Economic Review. ICER 2019 perspectives on cost-effectiveness threshold ranges. Boston, MA: Institute for Clinical and Economic Review; 2019. [Google Scholar]
- 30.York Health Economics Consortium. Net monetary benefit [Internet] York: York Health Economics Consortium; 2016. [cited 2024 Apr 17]. Available from: https://yhec.co.uk/glossary/net-monetary-benefit. [Google Scholar]
- 31.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM modeling good research practices task force--6. Value Health. 2012;15:835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Canadian Cancer Society. Canadian cancer statistics 2023. Toronto: Canadian Cancer Society; 2023. [Google Scholar]
- 33.U.S. Cancer Statistics Working Group. Stage distribution (%) of new cancer cases, all ages, all races and ethnicities, cervix, United States, 2016–2020 [Internet] [place unknown]: U.S. Cancer Statistics Working Group; 2024. [cited 2024 Apr 3]. Available from: https://gis.cdc.gov/Cancer/USCS/#/StageatDiagnosis/ [Google Scholar]
- 34.Centers for Disease Control and Prevention. Cervical cancer statistics [Internet] Atlanta, GA: Centers for Disease Control and Prevention; 2023. [cited 2024 Apr 29]. Available from: https://www.cdc.gov/cancer/cervical/statistics/index.htm. [Google Scholar]
- 35.World Health Organization. Cervical cancer [Internet] Geneva: World Health Organization; 2024. [cited 2024 Apr 3]. Available from: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer. [Google Scholar]
- 36.World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem [Internet] Geneva: World Health Organization; 2020. [cited 2024 Apr 3]. Available from: https://www.who.int/publications/i/item/9789240014107. [Google Scholar]
- 37.Jewell EL, Smrtka M, Broadwater G, Valea F, Davis DM, Nolte KC, et al. Utility scores and treatment preferences for clinical early-stage cervical cancer. Value Health. 2011;14:582–586. doi: 10.1016/j.jval.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Carneiro VCG, Batista TP, Andrade MR, Barros AV, Câmara LHLD, Ramalho NM, et al. Proof-of-concept randomized phase II non-inferiority trial of simple versus type B2 hysterectomy in early-stage cervical cancer ≤2 cm (LESSER) Int J Gynecol Cancer. 2023;33:498–503. doi: 10.1136/ijgc-2022-004092. [DOI] [PubMed] [Google Scholar]
- 39.Schmeler KM, Pareja R, Lopez Blanco A, Humberto Fregnani J, Lopes A, Perrotta M, et al. ConCerv: a prospective trial of conservative surgery for low-risk early-stage cervical cancer. Int J Gynecol Cancer. 2021;31:1317–1325. doi: 10.1136/ijgc-2021-002921. [DOI] [PubMed] [Google Scholar]
- 40.Naumann RW. The SHAPE trial: is good is good enough? J Gynecol Oncol. 2024;35:e107. doi: 10.3802/jgo.2024.35.e107. [DOI] [PMC free article] [PubMed] [Google Scholar]