Abstract
Objective
To assess the cost-effectiveness of pembrolizumab in combination with chemotherapy compared to chemotherapy alone, based on the results of the NRG-GY018 trial, in patients with advanced or recurrent endometrial cancer (EC), stratified by mismatch repair-deficient (dMMR) and mismatch repair-proficient (pMMR) subgroups.
Methods
A Markov model was used to simulate patients receiving either pembrolizumab plus chemotherapy or chemotherapy alone. Lifetime costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER) were calculated using a willingness-to-pay (WTP) threshold of $150,000/QALY. Univariate and probabilistic sensitivity analyses were conducted to assess the robustness of our findings.
Results
The addition of pembrolizumab to chemotherapy led to an incremental gain of 4.05 QALYs at an additional cost of $167,224, resulting in an ICER of $41,305.09/QALY compared to chemotherapy alone in dMMR EC. Additionally, there were 0.93 additional QALYs at an additional cost of $83,661, which resulted in an ICER of $90,284.80/QALY in pMMR EC. Sensitivity analyses indicated that the cost of pembrolizumab, utility of progressed disease, and utility of progression-free survival had the greatest impact on the results. Probabilistic sensitivity analysis showed that pembrolizumab was considered cost-effective at a 100% probability at a WTP threshold of $150,000 per QALY.
Conclusion
Pembrolizumab, when combined with chemotherapy, was found to be cost-effective compared to chemotherapy alone both for patients with advanced or recurrent dMMR and pMMR EC from the perspective of a payer in the United States.
Keywords: Pembrolizumab; Endometrial Cancer; Economics, Pharmaceutical; Immunotherapy
INTRODUCTION
Endometrial cancer (EC) ranks among the most prevalent forms of gynecological malignancies [1]. According to estimates from the American Cancer Society, in 2023, there were approximately 66,200 newly diagnosed cases and 13,030 deaths attributed to EC in the United States [1]. Although the majority of patients are diagnosed with localized disease at an early stage, which results in a favorable 5-year survival rate of 95%, patients with metastatic or recurrent disease have lower treatment response rates and a poor prognosis. Specifically, patients who experience a recurrence of pelvic disease have a 5-year survival rate of 17% if the disease spreads beyond the pelvic region, compared to 55% for those with recurrent disease confined to the pelvis [2].
For a significant period of time, carboplatin/paclitaxel chemotherapy has been widely accepted as the standard treatment for patients with advanced or recurrent EC. However, approximately 50% of patients experience disease recurrence or progression. In the past 2 decades, scientific research in the field of immunobiology and the application of immune checkpoint blockade therapy for cancer treatment have significantly advanced the exploration of immunotherapy as a promising strategy for managing EC [3].
Pembrolizumab is a monoclonal and humanized anti-programmed cell death protein 1 (PD-1) antibody, specifically of the IgG4 kappa type. Its mechanism of action involves inhibiting the interaction between PD-1 on T-cells and programmed death-ligand 1 (PD-L1) or PD-L2 on tumor cells, thereby restoring the T-cell mediated anti-tumor immune response [4]. On March 21, 2022, pembrolizumab was approved by the U.S. Food and Drug Administration as a monotherapy for patients diagnosed with advanced mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) EC. This approval is beneficial for patients who have experienced disease progression after receiving previous systemic therapy in any situation and are not eligible for curative surgical or radiation treatment. Pembrolizumab in combination with chemotherapy has demonstrated superior progression-free survival (PFS) and overall survival (OS) among various solid tumors [5,6]. The NRG-GY018 trial, which was a phase III study with placebo control [7], revealed that the combination of pembrolizumab with carboplatin and paclitaxel led to a significant enhancement in PFS for patients with advanced or recurrent dMMR and mismatch repair-proficient (pMMR) EC. According to the 12-month analysis, the PFS rates in the dMMR cohort were 74% in the pembrolizumab and 38% in the placebo group (with a hazard ratio [HR] of 0.30; 95% confidence interval [CI]=0.19–0.48; p<0.001). In the pMMR cohort, the median PFS (mPFS) was 13.1 months with pembrolizumab and 8.7 months with placebo (with a HR of 0.54; 95% CI=0.41–0.71; p<0.001).
Given the relatively high cost of pembrolizumab, it is crucial to conduct further research to assess its cost-effectiveness attributes. Additionally, performing a cost-effectiveness evaluation of medical interventions could assist decision-makers and healthcare providers in optimizing the allocation of limited healthcare resources. Regarding United States payers, our study focused on evaluating the cost-effectiveness of the combination of pembrolizumab with chemotherapy for patients with advanced or recurrent EC, stratified by dMMR and pMMR subgroups.
MATERIALS AND METHODS
1. Participants and interventions
The primary clinical data utilized in our study were obtained from the NRG-GY018 trial [7]. Our model focuses on patients with advanced-stage, metastatic, or recurrent EC, stratified by dMMR and pMMR subgroups, with a median age of 66. Individuals in each cohort were assigned in a 1:1 ratio randomly and allocated to 2 separate therapeutic regimens.
Pembrolizumab Group: patients in this group received intravenous administration of pembrolizumab (200 mg), carboplatin (area under the curve [AUC]=5), and paclitaxel (175 mg/m2). This treatment was administered every 3 weeks for the initial 6 cycles. Following that, patients continued to receive pembrolizumab (400 mg) intravenously every 6 weeks, for up to 14 cycles or until disease progression occurred.
Placebo group: patients in this group received intravenous administration of a placebo, carboplatin (AUC=5), and paclitaxel (175 mg/m2). Similar to the experimental group, this treatment was also given every 3 weeks for 6 cycles, followed by regular follow-up.
In case of disease recurrence in this model, due to the absence of a detailed subsequent therapeutic regimen from the NRG-GY018 trial, we have assumed 2 scenarios as follows: for the experimental group, doxorubicin (60 mg/m2) is administered every 3 weeks for 6 cycles as subsequent anticancer therapy, whereas the control group receives pembrolizumab monotherapy upon disease recurrence.
2. Model construction
The construction of the Markov model to evaluate the economic and clinical implications of pembrolizumab involved the use of TreeAge Pro 2022 software. Subsequently, we conducted statistical analysis using R 4.2.1 software. Our model framework comprised 3 distinct health states: PFS, progressive disease (PD), and death (Fig. S1). PFS and death were designated as the initial and terminal states, respectively. Patients in the PFS state may transition to PD or death following the initial treatment, and those who received subsequent treatments in the PD state may deteriorate towards death. Patients may also remain in the same health state after each cycle. However, irrespective of the effectiveness of salvage therapy, patients cannot return to the former state once the disease progresses. Each model cycle represented a duration of 3 weeks, and the model horizon was set as a lifetime. The primary outcomes of our analysis included overall costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs). Half-cycle correction was applied and a 3% annual discount rate was used in the calculation of costs and life expectancy [8] (Table S1).
3. Costs estimates
The evaluation of costs was conducted from the perspective of American public healthcare payers who act as third-party entities. We took into account the utilization of direct medical costs and health resources, including drug procurement, disease management, drug administration, and treatment-induced adverse events (AEs) (Table S1). The drug dosage was established using the average body surface area of women in the United States, which measures 1.84 m2 [8].
We obtained drug prices from the Centers for Medicare & Medicaid Services [9], while expenses related to medication administration, supportive care, palliative care at end-of-life, and disease management (including laboratory examinations, computed tomography, and hospitalization) were gathered from published databases [8,10,11,12,13]. Following the NRG-GY018 trial protocol and clinical practice, computed tomography scans were performed every 9 weeks during the initial treatment period until the first 9 months, and then every 12 weeks until PD was detected. Laboratory testing and administration costs were recorded in each treatment cycle. The cost of mismatch repair (MMR)-microsatellite instability (MSI) status testing was a one-time expense only recorded in the first treatment cycle [10].
In order to adjust for inflation and reflect 2023 U.S. dollar values, we incorporated the American Consumer Price Index for cost adjustments. Specifically, we used Tom's Inflation Calculator to inflate the costs in line with the year 2023 [14]. For the analysis of outcomes, we used the same willingness-to-pay (WTP) threshold as previous literature, which is $150,000 per QALY [15].
4. Survival and progression transition estimates
The transition probability in the NRG-GY018 trial [7] was estimated by extrapolating the PFS and OS curves using GetData Graph Digitizer software 2.22. To generate the simulated patient data, we utilized the algorithm developed by Hoyle and Henley [16]. The survival functions, such as exponential, log-normal, log-logistic, gengamma, gamma, Gompertz, and Weibull, were fitted to the curve. This fitting process aimed to achieve the best fit by considering both the Akaike and Bayesian information criteria. To represent the total number of patients in the preprogression health state over time, we use the concept of the cumulative area under the PFS curve. Similarly, the area above the OS curve is used to measure the total number of patients in the death health state. Furthermore, the region between the OS and PFS curves characterizes the cumulative number of patients in the postprogression health state. To calculate time-dependent transition probabilities for the 2 groups of patients, we utilized Microsoft Excel software and incorporated data from the NRG-GY018 trial. These probabilities were then extrapolated to cover a lifetime horizon. The formula used to calculate the transition probability values for each model cycle was as follows: transition probabilities (tu) = 1 − exp[λ(t − u)γ − λtγ], λ> 0, γ>0), where λ>0 and γ>0. Here, u represents the model cycle, and tu represents the arrival at state t following u cycles. For each age group, the background death rates were assessed using the life tables for females in the United States (Fig. S2 and Table S2) [17].
5. Health-state utilities
The health utility values used in our study for PFS, PD, and death were obtained from previously published investigations, with the values of 0.817, 0.779, and 0, respectively [18]. Consistent with traditional research methodologies, our primary focus was on severe treatment-related AEs that occur at an incidence rate of 5% or higher [19]. This emphasis is based on the understanding that mild AEs typically do not require treatment or result in significant treatment costs. The reduction in QALYs associated with all AEs was recorded for in the initial cycle of our models [20] (Table S1).
6. Univariate and probabilistic sensitivity analyses
We conducted a rigorous sensitivity analysis by systematically adjusting clinical parameters within a range that accounted for possible deviations of up to 20% from their baseline values. The tornado diagram visually illustrates variations resulting from these adjustments. We also conducted 1,000 Monte Carlo simulations. In these simulations, preset parameters were randomly and simultaneously varied according to specific distribution patterns. Specifically, costs were modeled using gamma distributions, while proportions and utilities followed beta distributions (Table S1).
RESULTS
1. Model validation
The mPFS and median OS (mOS) values obtained in our simulation were consistent with those reported in the NRG-GY018 trial. Since the mOS data for the NRG-GY018 trial has not been disclosed, only the mPFS can be evaluated for the error. The fidelity of the simulation is above 98% (Table S3).
2. Base case results
In the dMMR cohort, the pembrolizumab + chemotherapy group led to total costs of $373,136, whereas the placebo + chemotherapy group spent $205,912. The pembrolizumab + chemotherapy group achieved 8.31 QALYs, while the placebo + chemotherapy group achieved 4.26 QALYs. As a result, patients receiving pembrolizumab + chemotherapy gained an additional 4.05 QALYs at an extra cost of $167,224 compared to the placebo + chemotherapy group. The resulting ICER was $41,305.09/QALY, which is below the predetermined WTP threshold of $150,000/QALY (Table 1).
Table 1. The results of the model's base-case evaluation.
| Groups | Costs ($*) | ΔCosts ($*) | QALYs | ΔQALYs | ICER ($*/QALY) |
|---|---|---|---|---|---|
| dMMR placebo | 205,912 | - | 4.26 | - | - |
| dMMR pembrolizumab | 373,136 | 167,224 | 8.31 | 4.05 | 41,305.09 |
| pMMR placebo | 184,766 | - | 2.31 | - | - |
| pMMR pembrolizumab | 268,427 | 83,661 | 3.24 | 0.93 | 90,284.80 |
dMMR, mismatch repair-deficient; ICER, incremental cost-effectiveness ratio; pMMR, mismatch repair-proficient; QALYs, quality-adjusted life-years.
*US dollar.
In the pMMR cohort, the pembrolizumab + chemotherapy group had total costs of $268,427, while the placebo + chemotherapy group had costs of $184,766. The pembrolizumab + chemotherapy group achieved 3.24 QALYs, whereas the placebo + chemotherapy group achieved 2.31 QALYs. Consequently, patients receiving pembrolizumab + chemotherapy gained an additional 0.93 QALYs at an extra cost of $83,661 compared to the placebo + chemotherapy group. This resulted in an ICER of $90,284.80/QALY, which is also below the predetermined WTP threshold of $150,000/QALY (Table 1).
3. Sensitivity analysis
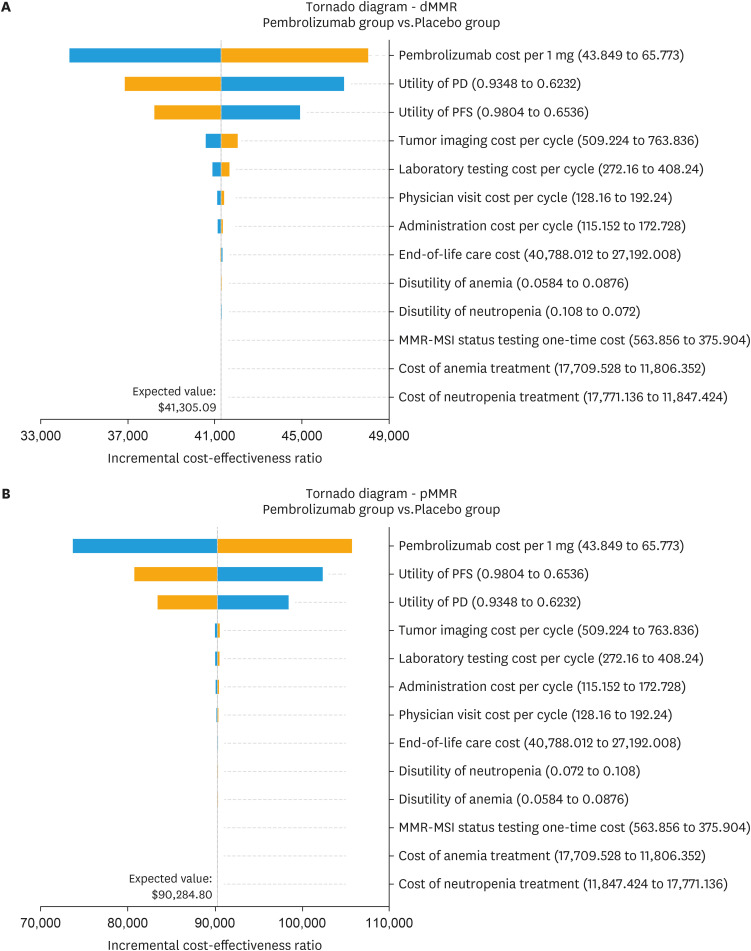
The tornado diagram in Fig. 1 demonstrates the significant impact of specific parameters on the ICER, including the cost of pembrolizumab and the utility of PD/PFS. Other variables exert minimal influence on the outcome. When all parameters vary within their respective ranges, the absence of any intersection between the generated ICER and the WTP values confirms the robustness of our model outcomes.
Fig. 1. Tornado diagram illustrating the results of univariate sensitivity analyses for dMMR EC (A) and pMMR EC (B).
dMMR, mismatch repair-deficient; EC, endometrial cancer; MMR-MSI, mismatch repair-microsatellite instability; PD, progressive disease; PFS, progression-free survival; pMMR, mismatch repair-proficient.
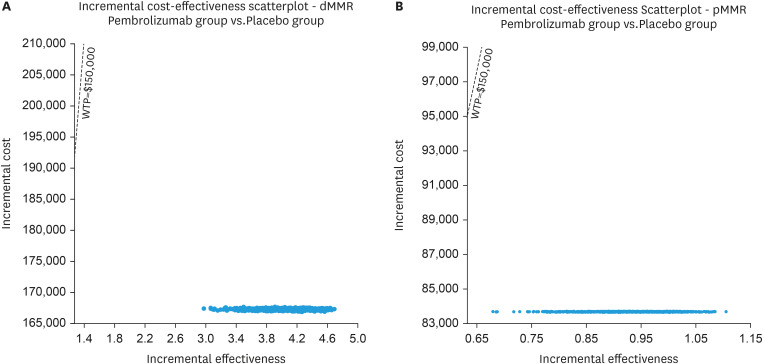
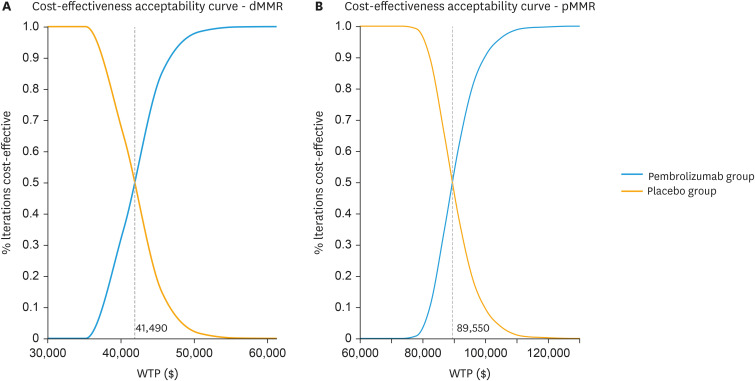
To investigate the spatial distribution of data points, a Monte Carlo simulation was conducted with a sample size of 1,000 individuals. The findings indicate that all scattered points are positioned in the first quadrant of the coordinate axis and lie below the WTP line. Although this increase in QALYs is accompanied by higher costs, these costs still remain below the WTP threshold of $150,000 per QALY (Fig. 2). Furthermore, the probability sensitivity analysis reveals a 100% chance for pembrolizumab to be deemed cost-effective for patients using a WTP threshold of $150,000/QALY (Fig. 3).
Fig. 2. Scatter plot diagrams showing the incremental cost-effectiveness of pembrolizumab plus chemotherapy compared to chemotherapy alone in dMMR EC (A) and pMMR EC (B).
dMMR, mismatch repair-deficient; EC, endometrial cancer; pMMR, mismatch repair-proficient; WTP, willingness-to-pay.
Fig. 3. The cost-effectiveness acceptability curves were generated through probabilistic sensitivity analyses for dMMR EC (A) and pMMR EC (B).
dMMR, mismatch repair-deficient; EC, endometrial cancer; pMMR, mismatch repair-proficient; WTP, willingness-to-pay.
DISCUSSION
Based on our model's findings, the base case analysis indicates superior health outcomes for the combination of pembrolizumab and chemotherapy. Specifically, this combination results in 8.31 QALYs compared to 4.26 QALYs in dMMR EC, and 3.24 QALYs compared to 2.31 QALYs in pMMR EC. In terms of cost-effectiveness, the combination demonstrates an ICER of $41,305.09/QALY in dMMR EC and $90,284.80/QALY in pMMR EC, both within the WTP threshold of $150,000/QALY. When compared to chemotherapy alone, the sensitivity analysis results further confirm that pembrolizumab, combined with chemotherapy, represents an effective and cost-efficient alternative for patients with advanced or recurrent EC, regardless of their dMMR or pMMR status.
Recent studies assessing the cost-effectiveness of pembrolizumab-based treatment options in patients with advanced dMMR/MSI-H EC have demonstrated a notable cost-effectiveness of pembrolizumab monotherapy when compared to chemotherapy [18,21]. In contrast, the combined use of lenvatinib and pembrolizumab does not exhibit the same level of cost-effectiveness as chemotherapy in patients with advanced EC [10,15,22].
The NRG-GY018 trial has shown promising results in treating EC patients. Specifically, for those with dMMR EC, the trial reported a 70% decrease in the risk of disease progression or death compared to the placebo group. For the pMMR EC cohort, there was a 46% reduced risk [7]. This heterogeneity of treatment outcomes is also reflected in our cost-effectiveness analysis. Specifically, the ICER for dMMR EC was found to be lower than that for pMMR EC, indicating that the combination of pembrolizumab with chemotherapy is more cost-effective in patients with dMMR EC. However, in pMMR EC, ICER remains below the predetermined WTP threshold of $150,000/QALY, meaning that it still possesses cost-effectiveness characteristics. MMR status has emerged as a critical factor in determining treatment strategies for patients with EC. EC can be classified into 4 distinct subgroups based on genetic markers: Polymerase epsilon (POLE) ultramutated, MSI hypermutated, copy-number (CN) low, and CN high. These subgroups exhibit varying prognosis, recurrence risks, and mortality rates [23]. Typically, patients exhibiting ultra-mutated (POLE) and hyper-mutated (MSI-H) profiles tend to respond favorably to immune checkpoint inhibitors. However, it is worth noting that only approximately 30% of EC cases exhibit the dMMR/MSI-H phenotype [24]. Recent research has revealed a promising correlation between LAG-3 and PD-L1 expressions. This discovery suggests that immunotherapy targeted at LAG-3 may potentially confer therapeutic advantages to patients with pMMR/MSS EC, particularly those who may not respond favorably to standard immune checkpoint inhibitors [25]. Another attempt involves the ongoing ultrasonographic image analysis, which will deepen the understanding of the correlation between radiomic signals and molecular/genetic profiling. This knowledge will facilitate the identification of novel radiogenomic signals that can accurately predict patients' risk. Furthermore, it is hoped that the utilization of radiomic features as a cost-effective alternative to molecular/genomic profiling will significantly alleviate the financial burden on the healthcare system [26].
In our model, the most influential factors taken into consideration include the cost of pembrolizumab as well as the value of PD and PFS. The current price of combined treatment with pembrolizumab has already demonstrated cost-effectiveness. The used PD and PFS health utility data are from published studies of EC patients [18]. To ensure the reliability of our findings, sensitivity analyses were performed using a wide range of utility values obtained from various research sources. The results indicated that variations in utility values did not significantly affect the conclusions, and the combination treatment with pembrolizumab remained cost-effective when considering both the upper and lower boundaries of utility values.
The ideal duration of immunotherapy maintenance continues to spark debate and concern. A comprehensive meta-analysis, encompassing nearly 23,000 solid tumor patients, has revealed that administering immunotherapy until disease progression does not result in superior outcomes compared to a fixed treatment course lasting up to 2 years [27]. Presently, considerable variation exists in treatment durations among different drugs. For instance, pembrolizumab is administered for a maximum of 2 years [7], dostarlimab therapy may be extended up to 3 years [28], and atezolizumab and durvalumab are typically administered until disease progression or unacceptable toxicity occurs [29,30]. This inconsistency underscores the urgent need for further exploration to determine the most suitable treatment duration for each drug. By establishing the optimal treatment durations, we can not only maximize survival benefits for patients but also minimize unnecessary side effects, enhance cost-effectiveness, and improve patients' quality of life.
Our analysis evaluates the cost-effectiveness of combining pembrolizumab with chemotherapy in patients stratified by dMMR and pMMR subgroups, providing valuable data to inform health system policy-making and clinical practice. However, it is imperative to acknowledge the limitations present in this research. Firstly, in line with most previous studies, our focus was limited to AEs of grade ≥3 with an occurrence rate of ≥5%. This approach may potentially lead to an underestimation of the ICER. However, it is worth noting that the treatment costs and disutility linked to low-grade and low-frequency AEs exert minimal influence on the overall outcomes. Secondly, the exclusion of certain treatments such as local lesion radiotherapy and surgeries imposed constraints on treatment decisions for patients in PD status. This exclusion limits the real-world applicability of the treatment decisions derived from this study. Future real-world research has the potential to address or overcome these limitations. Thirdly, since this economic evaluation is grounded in the phase 3 NRG-GY018 randomized clinical trial, potential biases in the trial, including the absence of intention-to-treat data, may have implications for our study. However, it is important to emphasize that these factors have minimal impact on the final ICER results presented in this study.
Our study provides insights into the cost-effectiveness of combining pembrolizumab with chemotherapy for advanced or recurrent EC. From the perspective of payers in the United States, we have found evidence that supports the cost-effectiveness of this treatment combination. As the range of cancer treatment options continues to expand and combination immunotherapy regimens are developed, the strategies that achieve good therapeutic effects without significantly increasing the financial burden on patients or the overall healthcare insurance burden on society will be more widely accepted by patients and third-party payers [31,32,33]. Moreover, future research should tackle the mentioned limitations and assess the long-term cost-effectiveness of this combination therapy in the real world.
In conclusion, from the perspective of a payer in the United States, the combination of pembrolizumab with chemotherapy was found to be cost-effective compared to chemotherapy alone for patients with advanced or recurrent EC, regardless of dMMR or pMMR status. To provide additional evidence for physicians and medical decision-making departments, it is desirable to conduct further real-world studies on pembrolizumab and assess its health outcomes.
Footnotes
Funding: This work was funded by China anti-cancer association HER2 target Chinese research fund (No. CETSDSSCORP239018), the key project of science and technology development fund of Tianjin education commission for higher education, China (No. 2022ZD064), and Tianjin key medical discipline (specialty) construction project (No. TJYXZDXK-010A).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: C.P.
- Data curation: S.Y.
- Formal analysis: H.G.
- Investigation: S.Y.
- Methodology: H.G.
- Project administration: C.P.
- Resources: S.Y.
- Software: H.G.
- Supervision: C.P.
- Validation: S.Y.
- Writing - original draft: H.G.
- Writing - review & editing: S.Y.
SUPPLEMENTARY MATERIALS
Model parameters and distributions
Background mortality rate
NRG-GY018 trial outcomes and cost-effectiveness model estimations
The Markov model simulated with 3 health states: PFS, PD and death.
Estimated best-fitting OS curves for dMMR EC (A), pMMR EC (B). Estimated best-fitting PFS curves for dMMR EC (C), pMMR EC (D).
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Burmeister C, Hanna RK, Munkarah A, Elshaikh MA. Predictors of survival after recurrence in women with early-stage endometrial carcinoma. Int J Gynecol Cancer. 2016;26:1137–1142. doi: 10.1097/IGC.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 3.Mahdi H, Chelariu-Raicu A, Slomovitz BM. Immunotherapy in endometrial cancer. Int J Gynecol Cancer. 2023;33:351–357. doi: 10.1136/ijgc-2022-003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scapin G, Yang X, Prosise WW, McCoy M, Reichert P, Johnston JM, et al. Structure of full-length human anti-PD1 therapeutic IgG4 antibody pembrolizumab. Nat Struct Mol Biol. 2015;22:953–958. doi: 10.1038/nsmb.3129. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 6.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 7.Eskander RN, Sill MW, Beffa L, Moore RG, Hope JM, Musa FB, et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388:2159–2170. doi: 10.1056/NEJMoa2302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K, Zhu Y, Zhou Y, Zhang Y, Zhu H. Pembrolizumab plus lenvatinib as first-line therapy for patients with mismatch repair-proficient advanced endometrial cancer: a United States-based cost-effectiveness analysis. Gynecol Oncol. 2022;166:582–588. doi: 10.1016/j.ygyno.2022.06.015. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Centers for Medicare & Medicaid Services. The Centers for Medicare & Medicaid Services [Internet] Baltimore, MD: U.S. Centers for Medicare & Medicaid Services; 2023. [cited 2023 Aug 14]. Available from: https://www.cms.gov. [Google Scholar]
- 10.Barrington DA, Haight PJ, Calhoun C, Tubbs C, Cohn DE, Bixel KL. Lenvatinib plus pembrolizumab in advanced recurrent endometrial cancer: a cost-effectiveness analysis. Gynecol Oncol. 2021;162:626–630. doi: 10.1016/j.ygyno.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Barrington DA, Tubbs C, Smith HJ, Straughn JM, Jr, Senter L, Cohn DE. Niraparib maintenance in frontline management of ovarian cancer: a cost effectiveness analysis. Int J Gynecol Cancer. 2020;30:1569–1575. doi: 10.1136/ijgc-2020-001550. [DOI] [PubMed] [Google Scholar]
- 12.Ding D, Hu H, Li S, Zhu Y, Shi Y, Liao M, et al. Cost-effectiveness analysis of durvalumab plus chemotherapy in the first-line treatment of extensive-stage small cell lung cancer. J Natl Compr Canc Netw. 2021;19:1141–1147. doi: 10.6004/jnccn.2020.7796. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Centers for Medicare & Medicaid Services. 2023 Medicare physician fee schedule [Internet] Baltimore, MD: U.S. Centers for Medicare & Medicaid Services; 2023. [cited 2023 Jul 17]. Available from: https://www.cms.gov/medicare/medicare-fee-for-service-payment/feeschedulegeninfo. [Google Scholar]
- 14.Medical-care-inflation. Tom's inflation calculator [Internet] place unknown: publisher unknown; 2022. [cited 2022 Dec 29]. Available from: https://halfhill.com/inflation_js.html. [Google Scholar]
- 15.Ackroyd SA, Huang ES, Kurnit KC, Lee NK. Pembrolizumab and lenvatinib versus carboplatin and paclitaxel as first-line therapy for advanced or recurrent endometrial cancer: a Markov analysis. Gynecol Oncol. 2021;162:249–255. doi: 10.1016/j.ygyno.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139. doi: 10.1186/1471-2288-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arias E, Xu J. United States life tables, 2019. Natl Vital Stat Rep. 2022;70:1–59. [PubMed] [Google Scholar]
- 18.Thurgar E, Gouldson M, Matthijsse S, Amonkar M, Marinello P, Upadhyay N, et al. Cost-effectiveness of pembrolizumab compared with chemotherapy in the US for women with previously treated deficient mismatch repair or high microsatellite instability unresectable or metastatic endometrial cancer. J Med Econ. 2021;24:675–688. doi: 10.1080/13696998.2021.1917140. [DOI] [PubMed] [Google Scholar]
- 19.Kuznik A, Smare C, Chen CI, Venkatachalam M, Keeping S, Atsou K, et al. Cost-effectiveness of cemiplimab versus standard of care in the United States for first-line treatment of advanced non-small cell lung cancer with programmed death-ligand 1 expression ≥50. Value Health. 2022;25:203–214. doi: 10.1016/j.jval.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. 2021;4:e210037. doi: 10.1001/jamanetworkopen.2021.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrington DA, Dilley SE, Smith HJ, Straughn JM., Jr Pembrolizumab in advanced recurrent endometrial cancer: a cost-effectiveness analysis. Gynecol Oncol. 2019;153:381–384. doi: 10.1016/j.ygyno.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Feng M, Chen Y, Yang Y, Li Q. Lenvatinib plus pembrolizumab vs. chemotherapy in pretreated patients with advanced endometrial cancer: a cost-effectiveness analysis. Front Public Health. 2022;10:881034. doi: 10.3389/fpubh.2022.881034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Dio C, Bogani G, Di Donato V, Cuccu I, Muzii L, Musacchio L, et al. The role of immunotherapy in advanced and recurrent MMR deficient and proficient endometrial carcinoma. Gynecol Oncol. 2023;169:27–33. doi: 10.1016/j.ygyno.2022.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Di Donato V, Giannini A, Bogani G. Recent advances in endometrial cancer management. J Clin Med. 2023;12:2241. doi: 10.3390/jcm12062241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong JH, Cho HW, Ouh YT, Lee JK, Chun Y. Lymphocyte activation gene (LAG)-3 is a potential immunotherapeutic target for microsatellite stable, programmed death-ligand 1 (PD-L1)-positive endometrioid endometrial cancer. J Gynecol Oncol. 2023;34:e18. doi: 10.3802/jgo.2023.34.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogani G, Chiappa V, Lopez S, Salvatore C, Interlenghi M, D’Oria O, et al. Radiomics and molecular classification in endometrial cancer (the ROME study): a step forward to a simplified precision medicine. Healthcare (Basel) 2022;10:2464. doi: 10.3390/healthcare10122464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogani G, Cinquini M, Signorelli D, Pizzutilo EG, Romanò R, Bersanelli M, et al. A systematic review and meta-analysis on the optimal treatment duration of checkpoint inhibitoRS in solid tumors: the OTHERS study. Crit Rev Oncol Hematol. 2023;187:104016. doi: 10.1016/j.critrevonc.2023.104016. [DOI] [PubMed] [Google Scholar]
- 28.Mirza MR, Chase DM, Slomovitz BM, dePont Christensen R, Novák Z, Black D, et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388:2145–2158. doi: 10.1056/NEJMoa2216334. [DOI] [PubMed] [Google Scholar]
- 29.Colombo N, Harano K, Hudson E, Galli F, Antill Y, Choi CH, et al. LBA40 - Phase III double-blind randomized placebo controlled trial of atezolizumab in combination with carboplatin and paclitaxel in women with advanced/recurrent endometrial carcinoma. Ann Oncol. 2023;34:S1281–S1282. [Google Scholar]
- 30.Westin SN, Moore K, Chon HS, Lee JY, Thomes Pepin J, Sundborg M, et al. Durvalumab plus carboplatin/paclitaxel followed by maintenance durvalumab with or without olaparib as first-line treatment for advanced endometrial cancer: the phase III DUO-E trial. J Clin Oncol. 2024;42:283–299. doi: 10.1200/JCO.23.02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parekh N, McClellan M, Shrank WH. Payment reform, medication use, and costs: can we afford to leave out drugs? J Gen Intern Med. 2019;34:473–476. doi: 10.1007/s11606-018-4794-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68:153–165. doi: 10.3322/caac.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith GL, Banegas MP, Acquati C, Chang S, Chino F, Conti RM, et al. Navigating financial toxicity in patients with cancer: a multidisciplinary management approach. CA Cancer J Clin. 2022;72:437–453. doi: 10.3322/caac.21730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Model parameters and distributions
Background mortality rate
NRG-GY018 trial outcomes and cost-effectiveness model estimations
The Markov model simulated with 3 health states: PFS, PD and death.
Estimated best-fitting OS curves for dMMR EC (A), pMMR EC (B). Estimated best-fitting PFS curves for dMMR EC (C), pMMR EC (D).