Abstract
Objectives
To describe a case of spinocerebellar ataxia presenting with progressive apraxia of speech (AOS).
Methods
A 54-year-old man with progressive speech changes was seen clinically and referred to our observational research program on degenerative speech and language disorders. He underwent detailed speech-language and neurologic assessments and multimodal neuroimaging studies. Three board-certified speech-language pathologists, blinded to other study data, reached a consensus speech diagnosis.
Results
The patient reported 2 years of progressive speech changes against a background of mild imbalance. Speech alternating and sequential motion rates were regular but moderately slow. He segmented syllables, most prominently during repetition of multisyllabic words, and had decreased prosodic variation in connected speech. He was diagnosed with prosodic-predominant primary progressive AOS. He had mild extremity ataxia and difficulty with tandem gait on neurologic examination. MRI showed marked pontine-cerebellar atrophy. FDG-PET showed premotor area and posterior fossa hypometabolism. Genetic testing revealed cytosine-adenine-guanine repeat expansion in the ATXN2 gene, consistent with spinocerebellar ataxia type 2 (SCA2).
Discussion
SCA2 is an autosomal dominant, degenerative disease characterized by cerebellar ataxia, including ataxic dysarthria. Our case demonstrates that SCA2 can manifest with progressive AOS. Neuroimaging supported involvement of areas classically associated with AOS.
Introduction
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant, degenerative ataxic disorder characterized by expansion of cytosine-adenine-guanine (CAG) repeats in the ATXN2 gene.1 SCA2 is among the most prevalent subtypes of the heterogeneous group of 40+ spinocerebellar ataxias.2 The average age at onset correlates with the extent of gene expansion and is most common in the second or third decade but ranges from childhood to adulthood.2
SCA2 manifests in variable, progressive neurologic symptoms in addition to classic cerebellar ataxia and dysarthria, including oculomotor dysfunction, dysphagia, cognitive issues, peripheral neuropathy, muscle cramps, and sleep disturbance.3 ATXN2 gene expansions may result in phenotypes mimicking the extrapyramidal and pyramidal symptoms seen in other neurodegenerative disorders such as Parkinson disease, amyotrophic lateral sclerosis (ALS), and Huntington disease.3-5
Gait ataxia is the most common presenting symptom, reported in up to 97% of patients.3 Subtle speech changes, typically consistent with ataxic dysarthria and localizing to the cerebellum, arise in early disease and progress with time.6 The speech features associated with ataxic dysarthria vary; some speakers with ataxic dysarthria are characterized as having excessive instability (variability in loudness, irregular articulatory breakdowns) and others as inflexible (equal and excess stress patterns), and yet others have a mix of these presentations.7 In a study of hereditary ataxias, the patient with SCA2 was identified as having instable ataxic dysarthria.8
Distinct from dysarthria, apraxia of speech (AOS) is a motor speech disorder involving incorrect motor programming and planning, resulting in abnormal articulatory phonetics and prosody.9 AOS is often seen in neurodegenerative diseases such as corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP), differentiated by the presence of typical clinical features.10 To our knowledge, there are no reported cases of AOS in SCA. We present a patient with progressive speech changes most consistent with AOS, ultimately diagnosed with SCA2.
Case Report
A 54-year-old right-handed man presented to an outpatient neuromuscular disorders clinic with 2 years of progressive speech changes. He endorsed decades of slight imbalance, described as unsteadiness walking on the treadmill, climbing stairs without handrails, and performing patterned movements during jujitsu. He denied headache, changes in vision or hearing, dysphagia, weakness, sensory changes, bowel/bladder dysfunction, cognitive concerns, personality changes, difficulty with fine motor movements, tremor, and dream enactment behavior. Medical history was notable for medication-controlled hypertension and diet-controlled type 2 diabetes mellitus. His father was diagnosed with ALS at age 60. There were several other paternal family members with speech changes, imbalance, and tremor, but without formal diagnoses nor genetic testing.
He described his speech changes as slowed “cadence” and fluctuating hoarseness. He noted difficulty with long words, needing to “pause and think about how to pronounce them correctly.” A clinical speech evaluation revealed moderately slow speaking rate, segmentation of syllables between words and within multisyllabic words, and inconsistent articulatory errors including vowel distortions and prolongations (Video 1). Speech alternating and sequential motion rates were similarly slow but regular. He was, therefore, diagnosed with prosodic-predominant primary progressive AOS (PPAOS).
Each visit includes a connected speech sample, alternating motion rates (AMRs) and sequential motion rates (SMRs), and repetition of increasingly complex words. The connected speech samples are an excerpt of the patient's description of the picture from the Western Aphasia Battery-Revised at Visit 1 and a description of some of his communication difficulties at Visit 2. Note his slow overall speaking rate with segmentation between words and between syllables within some multisyllabic words (e.g., daugh-ter and ca-ta-stro-phe), decreased prosodic variation, and the slow but regular AMRs and SMRs.Download Supplementary Video 1 (446.1KB, mov) via http://dx.doi.org/10.1212/200202_Video_1
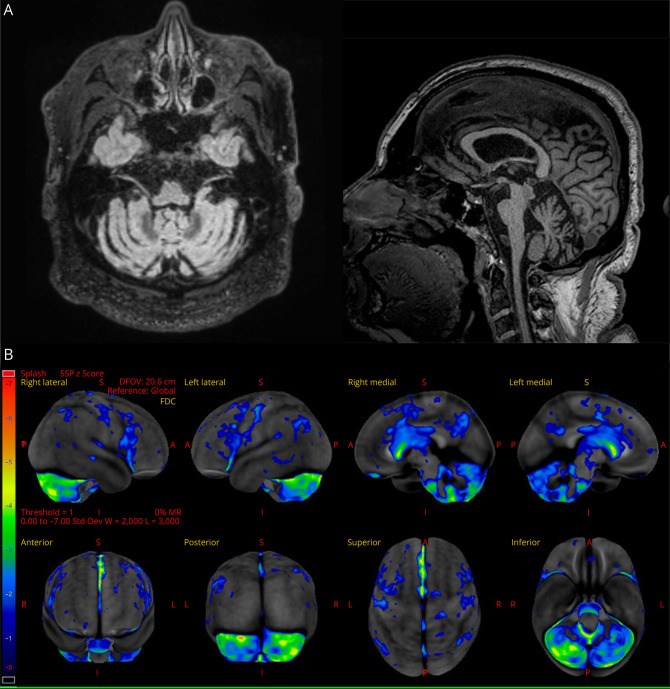
EMG and nerve conduction studies showed no evidence of motor neuron disease. Brain MRI showed mild, chronic small vessel ischemic changes. Owing to concern for PPAOS, [18F] fluorodeoxyglucose (FDG)-PET was pursued, revealing bilateral motor/premotor, bilateral cerebellar, and striatal hypometabolism, most prominent in the posterior fossa (Figure).
Figure. MRI and [18F] Fluorodeoxyglucose (FDG)-PET Imaging.
Research 3T volumetric MRI scan demonstrating pontine-cerebellar atrophy (A). [18F] fluorodeoxyglucose (FDG)-PET demonstrating bilateral motor/premotor, bilateral cerebellar, and striatal hypometabolism, most prominent in the posterior fossa (B).
The patient was referred to our research study of degenerative speech-language disorders, during which he underwent further evaluation with a behavioral neurologist. The cranial nerve examination revealed square wave jerks, saccadic pursuit, and oculomotor apraxia. Facial motor function was normal without tongue atrophy or weakness, and the palate was symmetric. Motor strength and tone were normal, without fasciculations. Reflexes were normal. There was no extremity apraxia. There were mild dysdiadochokinesis, dysmetria on finger-to-nose testing, and ataxia on heel-to-shin testing (Video 2). He was unable to perform tandem gait and swayed with eyes closed. The estimated score of the Scale for the Assessment and Rating of Ataxia was 9.5.11
Neurologic examination at Visit 2 demonstrating dysmetria on finger-to-nose testing and ataxia on heel-to-shin testing.Download Supplementary Video 1 (15.4MB, mp4) via http://dx.doi.org/10.1212/200202_Video_2
Neuropsychological testing demonstrated mild executive dysfunction. A comprehensive research speech-language battery revealed normal language (no aphasia) but, again, showed impaired motor speech consistent with previous findings. Three speech-language pathologists independently reviewed his recordings, blinded to other clinical and imaging data, and reached consensus that his speech features most closely aligned with prosodic-predominant PPAOS. His research 3T volumetric MRI scan showed pontine-cerebellar atrophy (Figure). Beta-amyloid and flortaucipir (tau) PET imaging were negative. Extensive laboratory evaluation, including a panel screening for autoimmune and paraneoplastic causes of dementia and ataxia, was normal.
Genetic testing for hereditary ataxias revealed a pathogenic CAG repeat expansion in the ATXN2 gene associated with autosomal dominant SCA2. There were an expanded allele of 41 CAG repeats and 1 normal allele of 22 CAG repeats. At 1-year follow-up, his speech was subjectively worse, which was observed on repeat examination.
Discussion
This case report details a 54-year-old man who presented with prosodic-predominant PPAOS in the context of subtle ataxia on neurologic examination, ultimately found to have SCA2. Patients with SCA2 most often have ataxic dysarthria, consistent with cerebellar degeneration.
AOS can be difficult to differentiate from ataxic dysarthria because both disorders predominantly affect articulation and prosody, resulting in overlapping perceptual features.9 In this case, the alternative diagnosis of inflexible ataxic dysarthria was considered and could not be entirely excluded. However, the only ataxic dysarthria features he had (vowel distortions) were overlapping features that could be accounted for by AOS. In addition to the clinical gestalt that he had PPAOS, the diagnosis was supported by features that are not characteristic of ataxic dysarthria (delayed initiation, increased difficulty for phonetically complex words, report of having to pause and think about pronouncing longer words). A diagnosis of ataxia with oculomotor apraxia could have been considered, given his examination and EMG findings; however, this group of disorders is associated with ataxic dysarthria, rather than AOS, and pathogenic variants in the SETX or APTX genes.12 Imaging findings were consistent with his mixed presentation, with cerebellar hypometabolism seen in SCA and motor/premotor hypometabolism reported in progressive AOS. Of note, the cerebellar hypometabolism was predominantly posterior, as can be seen in cerebellar cognitive affective syndrome, in which language and motor planning dysfunction can occur.13
Progressive AOS as a manifestation of a neurodegenerative diseases is usually associated with underlying 4-repeat tauopathies, namely CBD and PSP.10 Patients initially diagnosed with PPAOS often later meet criteria for a Parkinson-plus syndrome and, at that point, are no longer considered to have PPAOS. The prognostic significance of AOS in our patient with SCA2 is unclear although it may declare itself with time. Patients who present with PPAOS are not typically tested for hereditary ataxias, making it uncertain how common this combination is.
This patient's father was diagnosed with ALS, and other paternal relatives had similar speech changes. The extent of CAG repeats in the ATXN2 gene correlates with phenotypes resembling other neurodegenerative diseases.14 Intermediate length expansions (27–33 repeats) increase the risk of ALS and PSP-like phenomena, and expansions <39 repeats with CAA interruptions increase the risk of levodopa-responsive parkinsonism.3-5,14 Our patient had an abnormal allele of 41 repeats, and it could be presumed that with increased expansion with subsequent generations, his father may have had 27–33 repeats leading to an ALS phenotype.
Patients with SCA2 may have speech changes as early as 15 years before onset of ataxia, during a period termed the preataxic stage.6 These changes are subtle and easily missed without formal, repeated evaluation during progression to the ataxic stage of disease, at which point patients often present because of gait changes. It is possible that with close characterization of speech features in SCA2, more cases of AOS may be identified.
Appendix. Authors
| Name | Location | Contribution |
| Audrey M. Blazek, MD | Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Gabriela Meade, PhD | Speech Pathology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Lauren M. Jackson, MD | Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Ralitza Gavrilova, MD | Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Julie Stierwalt, PhD | Speech Pathology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Jennifer M. Martinez-Thompson | Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Joseph R. Duffy, MD | Speech Pathology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Heather Clark, MD | Speech Pathology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Mary M. Machulda, PhD, LP | Psychology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Jennifer L. Whitwell, PhD | Radiology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Keith A. Josephs, MD, MST, MSc | Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Rene L. Utianski, PhD | Speech Pathology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Hugo Botha, MB, ChB | Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
The NIH supported this work (grant numbers R01 DC014942 [PIs Josephs/Utianski], R01 DC010367 [PI Josephs], R01 DC012519 [PI Whitwell]).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NG for full disclosures.
References
- 1.Sullivan R, Yau WY, O'Connor E, Houlden H. Spinocerebellar ataxia an update. J Neurol. 2019;266(2):533-544. doi: 10.1007/s00415-018-9076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velázquez-Pérez L, Rodríguez-Labrada R, González-Garcés Y, Vázquez-Mojena Y, Pérez-Rodríguez R, Ziemann U. Neurophysiological features in spinocerebellar ataxia type 2 Prospects for novel biomarkers. Clin Neurophysiol. 2022;135:1-12. doi: 10.1016/j.clinph.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 3.Velázquez-Pérez LC, Rodríguez-Labrada R, Fernandez-Ruiz J. Spinocerebellar ataxia type 2 clinicogenetic aspects, mechanistic insights, and management approaches. Front Neurol. 2017;8:472. doi: 10.3389/fneur.2017.00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Primers. 2019;5(1):24. doi: 10.1038/s41572-019-0074-3 [DOI] [PubMed] [Google Scholar]
- 5.Auburger GW. Spinocerebellar ataxia type 2. Handb Clin Neurol. 2012;103:423-436. doi: 10.1016/B978-0-444-51892-7.00026-7 [DOI] [PubMed] [Google Scholar]
- 6.Vogel AP, Magee M, Torres-Vega R, et al. . Features of speech and swallowing dysfunction in pre-ataxic spinocerebellar ataxia type 2. Neurology. 2020;95(2):e194-e205. doi: 10.1212/WNL.0000000000009776 [DOI] [PubMed] [Google Scholar]
- 7.Spencer KA, Dawson M. Dysarthria profiles in adults with hereditary ataxia. Am J Speech Lang Pathol. 2019;28(2S):915-924. doi: 10.1044/2018_AJSLP-MSC18-18-0114 [DOI] [PubMed] [Google Scholar]
- 8.Spencer KA, France AA. Perceptual ratings of subgroups of ataxic dysarthria. Int J Lang Commun Disord. 2016;51(4):430-441. doi: 10.1111/1460-6984.12219 [DOI] [PubMed] [Google Scholar]
- 9.Duffy JR, Utianski RL, Josephs KA. Primary progressive apraxia of speech from recognition to diagnosis and care. Aphasiology. 2021;35(4):560-591. doi: 10.1080/02687038.2020.1787732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utianski RL, Josephs KA. An update on apraxia of speech. Curr Neurol Neurosci Rep. 2023;23(7):353-359. doi: 10.1007/s11910-023-01275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grobe-Einsler M, Amin AT, Faber J, Völkel H, Synofzik M, Klockgether T. Scale for the assessment and rating of ataxia (SARA) development of a training tool and certification program. Cerebellum 2024;23(3):877-880. doi: 10.1007/s12311-023-01543-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandford E, Burmeister M. Genes and genetic testing in hereditary ataxias. Genes (Basel). 2014;5(3):586-603. doi: 10.3390/genes5030586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argyropoulos GPD, van Dun K, Adamaszek M, et al. . The cerebellar cognitive affective/schmahmann syndrome a task force paper. Cerebellum. 2020;19(1):102-125. doi: 10.1007/s12311-019-01068-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antenora A, Rinaldi C, Roca A, et al. . The multiple faces of spinocerebellar ataxia type 2. Ann Clin Transl Neurol. 2017;4(9):687-695. doi: 10.1002/acn3.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each visit includes a connected speech sample, alternating motion rates (AMRs) and sequential motion rates (SMRs), and repetition of increasingly complex words. The connected speech samples are an excerpt of the patient's description of the picture from the Western Aphasia Battery-Revised at Visit 1 and a description of some of his communication difficulties at Visit 2. Note his slow overall speaking rate with segmentation between words and between syllables within some multisyllabic words (e.g., daugh-ter and ca-ta-stro-phe), decreased prosodic variation, and the slow but regular AMRs and SMRs.Download Supplementary Video 1 (446.1KB, mov) via http://dx.doi.org/10.1212/200202_Video_1
Neurologic examination at Visit 2 demonstrating dysmetria on finger-to-nose testing and ataxia on heel-to-shin testing.Download Supplementary Video 1 (15.4MB, mp4) via http://dx.doi.org/10.1212/200202_Video_2