Abstract
Phyllodes tumors (PT) are fibroepithelial neoplasms that are treated by complete surgical excision. The effectiveness of adjuvant therapies, including radiotherapy and chemotherapy, for PT remains unclear, and the use of neoadjuvant chemotherapy (NAC) is yet to be established. We report a case of a 15-year-old girl with acute lymphatic leukemia (ALL) who was incidentally diagnosed with a 50-mm borderline PT in the left breast using computed tomography, ultrasonography, and histological examination following needle biopsy. Lumpectomy was performed after administration of anthracycline-based chemotherapy for ALL, resulting in tumor size reduction. Histopathological examination of the excised specimen demonstrated decreased mitotic activity and stromal cellularity post-chemotherapy. To our knowledge, this is the first study to report the histopathological differences in pre- and post-chemotherapy borderline PT samples. Our findings suggest that NAC may induce changes in borderline PT, potentially affecting diagnosis and treatment decisions. Hence, further investigation is warranted in this regard.
Keywords: Breast Neoplasms, Drug Therapy, Histological Types of Neoplasms, Phyllodes Tumor
INTRODUCTION
Phyllodes tumors (PT) are fibroepithelial neoplasms characterized by leaf-like biphasic growth patterns comprising predominantly stromal and epithelial cells [1,2]. This type of tumor is typically observed in women aged 40–50 years [2,3]. According to the 5th edition of the World Health Organization classification of human breast tumors, PT is categorized into three subtypes, namely benign, borderline, and malignant, based on several histological features, including mitotic activity, stromal cellularity, nuclear atypia, stromal overgrowth, and tumor-margin appearance [4]. Although complete surgical excision is usually recommended for PT, whether therapies such as radiotherapy and chemotherapy can improve prognosis remains unclear [5,6,7]. The use of neoadjuvant chemotherapy (NAC) for breast cancer is well-established. Although a previous study reported a reduction in tumor volume in patients with malignant PT who received neoadjuvant chemoembolization, it has not been widely applied, regardless of the PT subtype [8]. NAC causes tumor cell degeneration in breast cancer, characterized by nuclear enlargement, cell shrinkage and necrosis, and stromal fibrosis with the presence of inflammatory infiltrates [9]. However, histopathological changes in PTs after NAC have not been reported. Here, we present the case of a 15-year-old girl with borderline PT who received chemotherapy for acute lymphatic leukemia (ALL) and subsequently demonstrated a reduction in PT. To our knowledge, this is the first report comparing pre-chemotherapy core needle biopsy samples with post-chemotherapy excised specimens that demonstrates histopathological changes and a downgrading of PT post-chemotherapy.
CASE REPORT
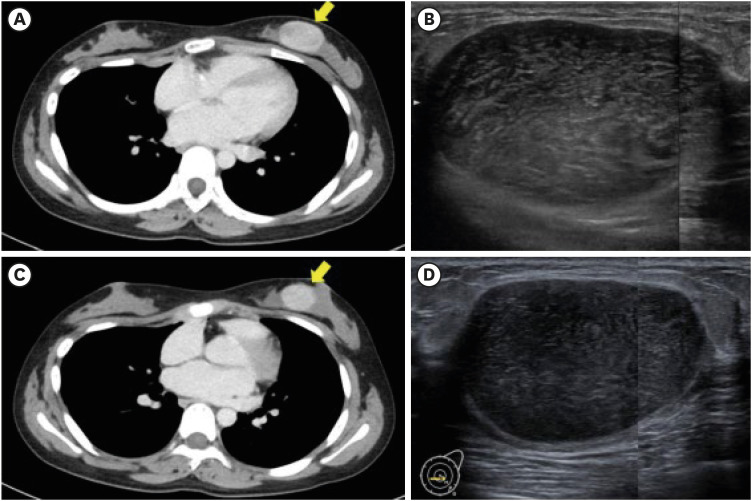
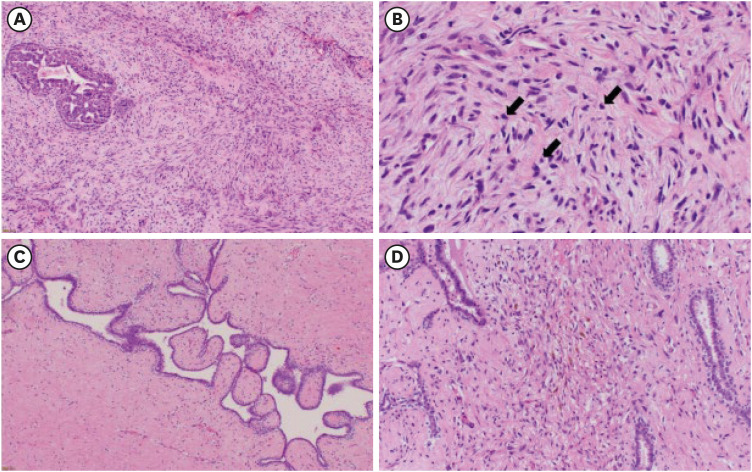
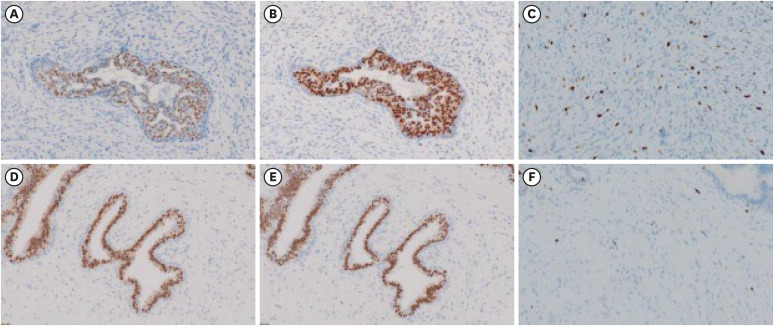
A 15-year-old girl presented to the hematology department of Nagasaki University Hospital with shortness of breath and palpitations accompanied by a marked increase in white blood cell count. Following further diagnostic tests, she was diagnosed with ALL, and a circumscribed left breast mass measuring 40 mm was incidentally detected on contrast-enhanced computed tomography (CT) in August (Figure 1A). The patient was referred to the breast and endocrine department for differentiation between an extranodal involvement of the ALL and a primary breast tumor. Notably, the patient did not take any medications and had no known family history of breast cancer. Physical examination revealed a circumscribed painless mass, measuring 50 mm, in the left lower medial quadrant. Ultrasonography (US) showed an oval-shaped mass measuring 50 mm with a heterogeneous interior and slit structures in the left lower medial quadrant (Figure 1B). A diagnosis of borderline PT was confirmed in the same month following a vacuum-assisted biopsy of the mass post-ALL diagnosis. Histological examination showed spindle-shaped atypical stromal cells with moderate proliferation. Before chemotherapy, the number of mitoses per 10 high-power fields (HPFs) was 5–10 (Figure 2). While the epithelial cells expressed the estrogen receptor (ER) and progesterone receptor (PR), very few of the stromal cells expressed PR (1–5%), and none expressed ER (0%) (Figure 3A and B). The Ki-67 labeling index was approximately 15% (Figure 3C).
Figure 1. Pre- and post-chemotherapy changes on contrast-enhanced computed tomography and ultrasonography. (A) Contrast-enhanced CT reveals a circumscribed mass measuring approximately 40 mm before chemotherapy. (B) US shows a circumscribed oval mass measuring approximately 50 mm before chemotherapy. (C, D) Post administration of chemotherapy, the mass is reduced in size without alterations in its properties and measures approximately 30 mm and 37 mm on contrast-enhanced CT and US, respectively.
CT = computed tomography; US = ultrasonography.
Figure 2. Microscopic findings from pre-chemotherapy biopsy samples and post-chemotherapy excised specimen. Microscopic examination reveals moderate-to-marked stromal cellularity and moderate stromal atypia with mitotic events (arrows) ranging 5–10 per 10 high-power fields in pre-chemotherapeutic biopsy samples (A: ×100, B: ×400). Post chemotherapy, stromal cellularity and mitotic events are decreased, the stroma is degenerated, and the presence of histocytes and hemosiderin is evident in the excised specimen (C: ×100, D: ×200).
Figure 3. Pre- and post-chemotherapy immunohistochemical findings. (A) Prior to chemotherapy, epithelial cells in the biopsy samples express ERs, whereas stromal cells do not. (B) Prior to chemotherapy, epithelial cells in the biopsy samples express PRs whereas only a few stromal cells (1%–5%) do. (C) The Ki-67 labeling index is 15% in the pre-chemotherapy biopsy samples. (D) ER expression is positive in epithelial cells and negative in stromal cells post-chemotherapy. (E) Post chemotherapy, PR expression is positive in epithelial cells and low in stromal cells. (F) The Ki-67 labeling index decreases to 5% in the specimens excised post-chemotherapy. The magnification of microscopic all figures is ×200.
ER = estrogen receptor; PR = progesterone receptor.
Following consultation with a hematologist, surgical resection was performed for the PT during chemotherapy for ALL because of the long therapy duration. The patient received induction therapy in September (weeks 1–4) with prednisolone (days 1–28), vincristine (days 8, 15, 22, and 29), L-asparaginase (L-ASP) (days 12, 15, 18, 21, 24, 27, 30, and 33), and daunorubicin (DNR) (days 8, 15, 22, and 29), followed by consolidation therapy in October (weeks 5–8) with cyclophosphamide (days 1 and 29), L-ASP (days 3, 6, 10, 13, 17, 20, 24, and 27), cytarabine (days 3–6, 10–13, 17–20, and 24–27), and 6-mercaptopurine hydrate (days 1–28). The induction therapy involved four weekly DNR treatments (30 mg/m2), with a total dosage of 168 mg. Physical evaluation during the initial phase of the consolidation therapy revealed that the PT mass had reduced to approximately 40 mm. Concurrently, CT findings indicated a slight reduction in the mass, and US revealed shrinkage of the circumscribed oval mass, measuring 37 mm × 23 mm × 36 mm post-induction therapy, without any alteration in its properties (Figure 1C and D). These findings also demonstrate the suitability of imaging assessments for stable disease.
The patient subsequently developed chemotherapy-induced menopause. Lumpectomy was performed in December when consolidation therapy was discontinued due to fungal infections of the lungs and liver that required antibiotic treatment. Surgery was performed under general anesthesia using an inner inframammary fold incision to create a skin flap and remove the tumor. A margin of approximately 5 mm was maintained to preserve the cosmetic appearance of the nipple and continuity of the nipple–mammary duct. A gross examination of the specimen revealed a circumscribed mass measuring 33 × 22 mm2. Microscopic examination revealed the proliferation of epithelial and stromal cells exhibiting a leaf-like biphasic growth pattern. The number of mitoses per 10 HPFs ranged 0–5, and the stromal cellularity was lower than that observed in the biopsy specimens. Furthermore, shrunken cells indicating degenerative changes, and the presence of macrophages and hemosiderin in stroma were evident after chemotherapy for ALL (Figure 2C and D). The preoperative biopsy sample demonstrated epithelial cells that were positive for ER and PR, whereas stromal cells were weakly positive for PR and negative for ER (Figure 3D and E). The Ki-67 labeling index was 5% (Figure 3F) and surgical margins were negative. Thus, a final diagnosis of borderline PT, which was downgraded to benign PT post-chemotherapy was made.
During the eight-month postoperative follow-up, the girl exhibited no signs of recurrence on either physical examination or CT.
Ethical statement
Ethical approval was not required because this report outlines a single case. Written informed consent was obtained from the patient and her parents for the publication of this case report and the accompanying images.
DISCUSSION
The prognostic effects of chemotherapy in PT is unclear, and anthracycline-based regimens are recommended in metastatic settings according to recommended guidelines for the management of soft tissue sarcoma (STS) [10,11]. Doxorubicin (DXR) exerts antitumor effects in both in vitro and ex vivo malignant PT cell culture models [12]. However, the efficacy of NAC in malignant PT remains unknown and only a few case reports are available in the literature [8,13]. The DXR-equivalent dose for DNR is 0.75 [14], and the prescribed dose for DXR in STS clinical trials is 75 mg/m2 per cycle [15,16]. In this case, the dose of 168 mg of DNR was equivalent to 126 mg of DXR; thus, the girl patient received a higher dose of DXR than that typically administered in a single cycle of STS. This treatment resulted in a reduction in size and downgrading of the tumor. However, chemotherapy regimens for ALL and STS differ in dosage and dosing intervals, and whether DNR was truly responsible for tumor shrinkage in this case remains unclear.
PT is more commonly found in middle-aged women, with < 10% of cases reported in women < 20 years of age [2,3,17]. Most younger women have benign PT, and malignant PT is extremely rare [18]. Alipour and Eskandari [19] reported that patients with PT during pregnancy were younger and had larger and more malignant tumors than those typically observed in PT cases, suggesting the role of sex hormones in PT progression. Notably, while most epithelial cells in PT express hormone receptors, only a few stromal cells do so [4,20], and there is no evidence for the use of endocrine therapy in PT. Since only a few stromal cells expressed PR in the present case, it is unlikely that changes in baseline sex hormone levels caused tumor shrinkage despite the onset of chemotherapy-induced menopause.
Vacuum-assisted biopsy samples exhibited moderate stromal cellularity with a Ki-67 labeling index of approximately 15%. Shubham et al. [21] reported that 67% of patients with borderline PT exhibited Ki-67 positivity in 10%–20% of their stromal cells, whereas those with benign and malignant PT had Ki-67 positivity in < 10% and 15% of their stromal cells, respectively.
The surgically excised specimen in this case showed decreased stromal cellularity and a Ki-67 labeling index of 5%. Hormone receptor expression patterns were similar in biopsy and surgically-excised specimens. Additionally, the stroma exhibited shrinkage and degeneration with the presence of macrophages and hemosiderin. These changes, indicative of downgrading of the PT from borderline to benign, were attributed to the chemotherapy administered for ALL. To the best of our knowledge, this is the first documented instance of histopathological changes in a borderline PT after chemotherapy (Table 1).
Table 1. Changes in histopathological characteristics between pre-chemotherapy biopsy samples and post-chemotherapy excised specimens.
| Histopathological feature | Pre-chemotherapy (biopsy sample) | → | Post-chemotherapy (excised specimen) |
|---|---|---|---|
| Tumor border | Well defined | → | Well defined |
| Stromal cellularity | Moderate to marked | → | Mild |
| Stromal atypia | Moderate | → | Mild |
| Mitotic activity | 5 to 9 per 10 HPFs | → | < 5 per HPFs |
| Ki-67 labeling index | 15% | → | 5% |
| Stromal overgrowth | Absent | → | Absent |
| Diagnosis | Borderline PT | → | Benign PT (post-chemotherapeutic status) |
HPF = high-power field; PT = phyllodes tumor.
Ward et al. [22] reported a 63% sensitivity for core needle biopsy in PT diagnosis, with individual sensitivities of 57%, 90%, and 100% for benign, borderline, and malignant lesions, respectively. Although it is difficult to distinguish fibroadenomas from benign PT on the basis of histopathological features and genetic findings [23], differentiating fibroadenomas from borderline PT may be relatively simple. Although the distinction between benign and malignant PT is well-established, the distinction between borderline and benign or malignant PT is less clear [4]. Furthermore, needle biopsies sample only a portion of the tumor; however, evaluating the entire lesion is crucial in cases of heterogeneous tumors.
In this case, the young woman successfully underwent surgical resection while maintaining her cosmetic appearance. Massive PT may be unresectable or may require skin grafting in operable cases. Furthermore, complete excision is generally the preferred approach for both borderline and malignant PT since surgical margins remain unclear [5,24]. NAC may reduce tumor size and allow curative resection in patients with borderline or malignant PT that are unresectable or require skin grafting, and thus serve as a potential treatment option in such cases.
In summary, NAC, particularly anthracycline-based regimens, result in histopathological changes in PT lesions. Understanding these changes may assist in accurate pathological diagnosis and treatment decisions. However, this study was based on a single case report and hence subject to sampling bias. The histopathological findings of the biopsy samples and excised specimens were homogenous, thereby minimizing the impact of sampling bias.
In this study, we report the case of a patient with borderline PT who received chemotherapy for ALL. Chemotherapy resulted in the shrinkage and downgrading of the lesion from borderline to benign. To our knowledge, this is the first study to document the microscopic changes in borderline PT following chemotherapy. Further investigations using larger sample sizes or in vitro models to explore the influence of NAC on borderline PT are warranted.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for their support with the English language editing.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
Data Availability: In accordance with the ICMJE data sharing policy, the authors have agreed to make the data available upon request.
- Project administration: Hara Y, Otsubo R, Fukushima A, Inamasu E, Akashi M, Morita M, Kuba S.
- Supervision: Eguchi S, Matsumoto K.
- Writing - original draft: Hara Y, Yamaguchi R.
- Writing - review & editing: Yamaguchi R, Otsubo R, Fukushima A, Inamasu E, Akashi M, Morita M, Kuba S.
References
- 1.Panda KM, Naik R. A clinicopathological study of benign phyllodes tumour of breast with emphasis on unusual features. J Clin Diagn Res. 2016;10:EC14–EC17. doi: 10.7860/JCDR/2016/18025.8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strode M, Khoury T, Mangieri C, Takabe K. Update on the diagnosis and management of malignant phyllodes tumors of the breast. Breast. 2017;33:91–96. doi: 10.1016/j.breast.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Rowell MD, Perry RR, Hsiu JG, Barranco SC. Phyllodes tumors. Am J Surg. 1993;165:376–379. doi: 10.1016/s0002-9610(05)80849-9. [DOI] [PubMed] [Google Scholar]
- 4.Tse G, Koo JS, Thike AA. In: World Health Organization Classification of Tumours of the Breast. 5th ed. Lokuhetty D, White VA, Watanabe R, Cree IA, editors. Lyon: IARC; 2019. Phyllodes tumour; pp. 172–176. [Google Scholar]
- 5.Ofri A, Stuart KE, Chan B, Mak C, Warrier S, Bhadri V, et al. Diagnosis and management of phyllodes tumours for the surgeon: an algorithm. Surgeon. 2022;20:e355–e365. doi: 10.1016/j.surge.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Chao X, Chen K, Zeng J, Bi Z, Guo M, Chen Y, et al. Adjuvant radiotherapy and chemotherapy for patients with breast phyllodes tumors: a systematic review and meta-analysis. BMC Cancer. 2019;19:372. doi: 10.1186/s12885-019-5585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales-Vásquez F, Gonzalez-Angulo AM, Broglio K, Lopez-Basave HN, Gallardo D, Hortobagyi GN, et al. Adjuvant chemotherapy with doxorubicin and dacarbazine has no effect in recurrence-free survival of malignant phyllodes tumors of the breast. Breast J. 2007;13:551–556. doi: 10.1111/j.1524-4741.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K, Mimura H, Arai Y, Doi M, Kojima Y, Tsugawa K, et al. Successful preoperative chemoembolization in the treatment of a giant malignant phyllodes tumor. Cardiovasc Intervent Radiol. 2016;39:1070–1075. doi: 10.1007/s00270-016-1311-8. [DOI] [PubMed] [Google Scholar]
- 9.Sethi D, Sen R, Parshad S, Khetarpal S, Garg M, Sen J. Histopathologic changes following neoadjuvant chemotherapy in various malignancies. Int J Appl Basic Med Res. 2012;2:111–116. doi: 10.4103/2229-516X.106353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, et al. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:815–833. doi: 10.6004/jnccn.2022.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samii E, Hurni Y, Huber D. Management and outcomes of metastatic and recurrent malignant phyllodes tumors of the breast: a systematic literature review. Eur J Breast Health. 2023;19:191–200. doi: 10.4274/ejbh.galenos.2023.2023-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urbaniak A, Jousheghany F, Yuan Y, Piña-Oviedo S, Huczyński A, Delgado M, et al. The response of phyllodes tumor of the breast to anticancer therapy: an in vitro and ex vivo study. Oncol Lett. 2019;18:5097–5106. doi: 10.3892/ol.2019.10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugie T, Takeuchi E, Kunishima F, Yotsumoto F, Kono Y. A case of ductal carcinoma with squamous differentiation in malignant phyllodes tumor. Breast Cancer. 2007;14:327–332. doi: 10.2325/jbcs.14.327. [DOI] [PubMed] [Google Scholar]
- 14.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 15.Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 16.Seddon B, Strauss SJ, Whelan J, Leahy M, Woll PJ, Cowie F, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1397–1410. doi: 10.1016/S1470-2045(17)30622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makar GS, Makar M, Ghobrial J, Bush K, Gruner RA, Holdbrook T. Malignant phyllodes tumor in an adolescent female: a rare case report and review of the literature. Case Rep Oncol Med. 2020;2020:1989452. doi: 10.1155/2020/1989452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafeez S, Balarezo F, Ricci A., Jr Benign phyllodes tumor in children: a study of 8 cases and review of the literature. J Pediatr Hematol Oncol. 2020;42:e388–e391. doi: 10.1097/MPH.0000000000001501. [DOI] [PubMed] [Google Scholar]
- 19.Alipour S, Eskandari A. Phyllodes tumor of the breast in pregnancy and lactation. Adv Exp Med Biol. 2020;1252:137–142. doi: 10.1007/978-3-030-41596-9_19. [DOI] [PubMed] [Google Scholar]
- 20.Tse GM, Lee CS, Kung FY, Scolyer RA, Law BK, Lau TS, et al. Hormonal receptors expression in epithelial cells of mammary phyllodes tumors correlates with pathologic grade of the tumor: a multicenter study of 143 cases. Am J Clin Pathol. 2002;118:522–526. doi: 10.1309/D206-DLF8-WDNC-XJ8K. [DOI] [PubMed] [Google Scholar]
- 21.Shubham S, Ahuja A, Bhardwaj M. Immunohistochemical expression of Ki-67, p53, and CD10 in phyllodes tumor and their correlation with its histological grade. J Lab Physicians. 2019;11:330–334. doi: 10.4103/JLP.JLP_106_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward ST, Jewkes AJ, Jones BG, Chaudhri S, Hejmadi RK, Ismail T, et al. The sensitivity of needle core biopsy in combination with other investigations for the diagnosis of phyllodes tumours of the breast. Int J Surg. 2012;10:527–531. doi: 10.1016/j.ijsu.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Yang C, Pfeifer JD, Caprioli RM, Judd AM, Patterson NH, et al. Histopathologic, immunophenotypic, and proteomics characteristics of low-grade phyllodes tumor and fibroadenoma: more similarities than differences. NPJ Breast Cancer. 2020;6:27. doi: 10.1038/s41523-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutnik L, Ren Y, Thomas SM, Plichta JK, Greenup RA, Fayanju OM, et al. Malignant phyllodes tumor and primary breast sarcoma; distinct rare tumors of the breast. J Surg Oncol. 2022;125:947–957. doi: 10.1002/jso.26820. [DOI] [PMC free article] [PubMed] [Google Scholar]