Abstract
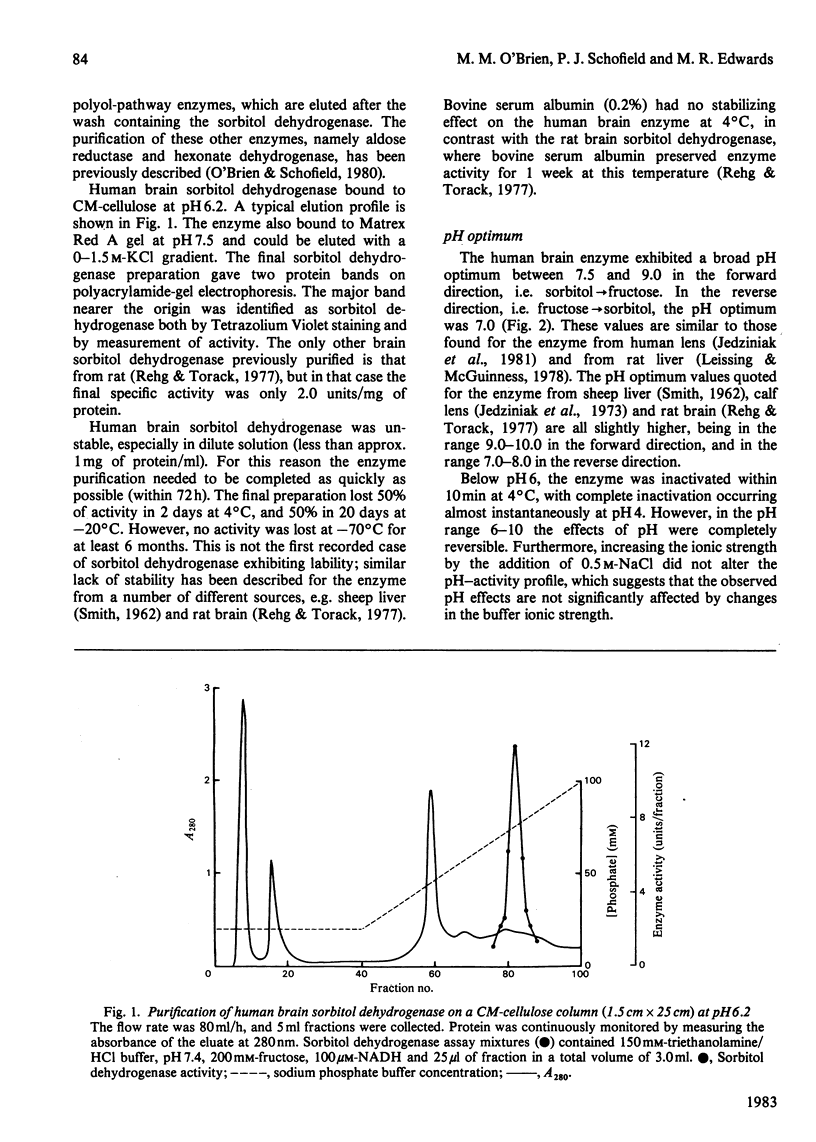
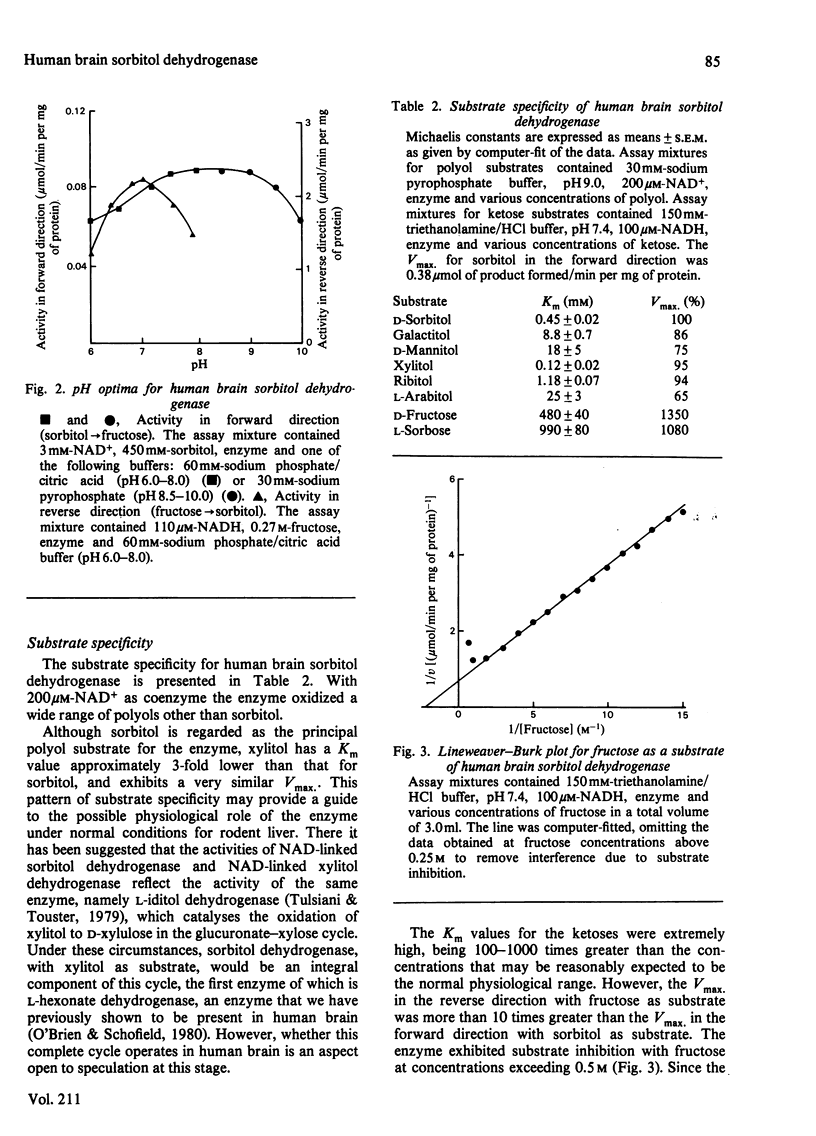
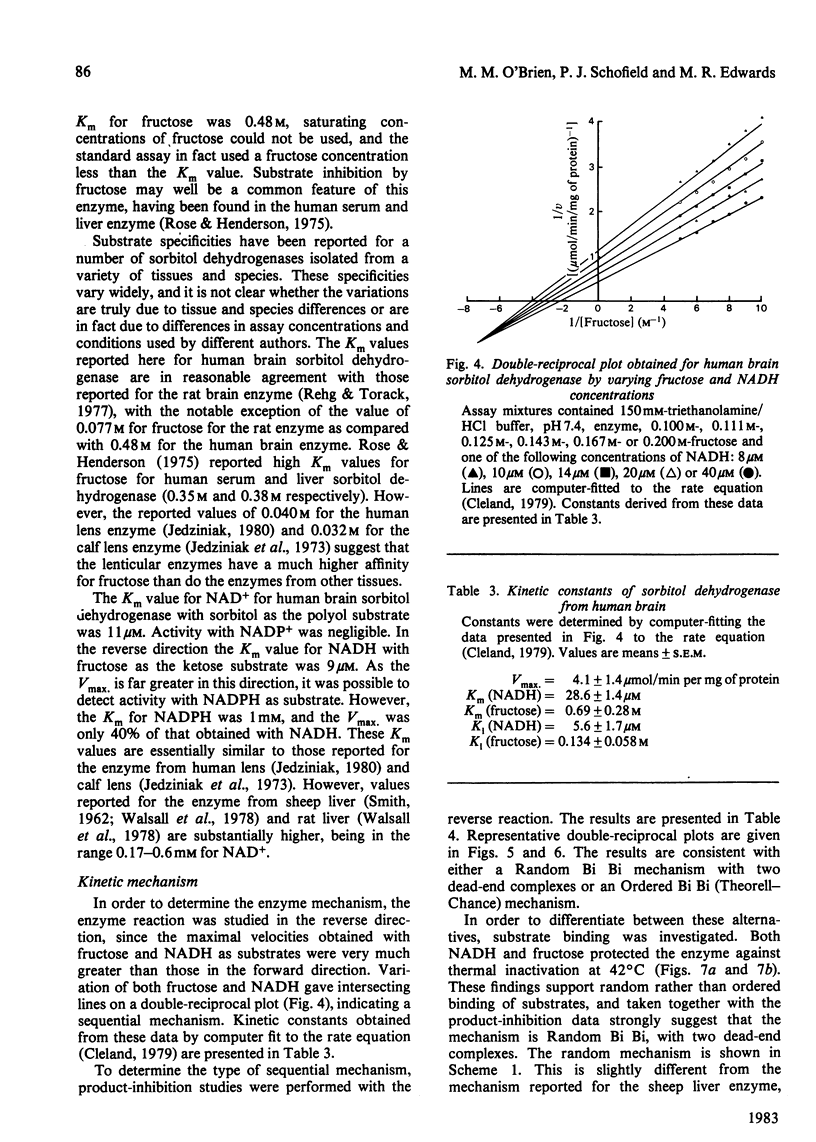
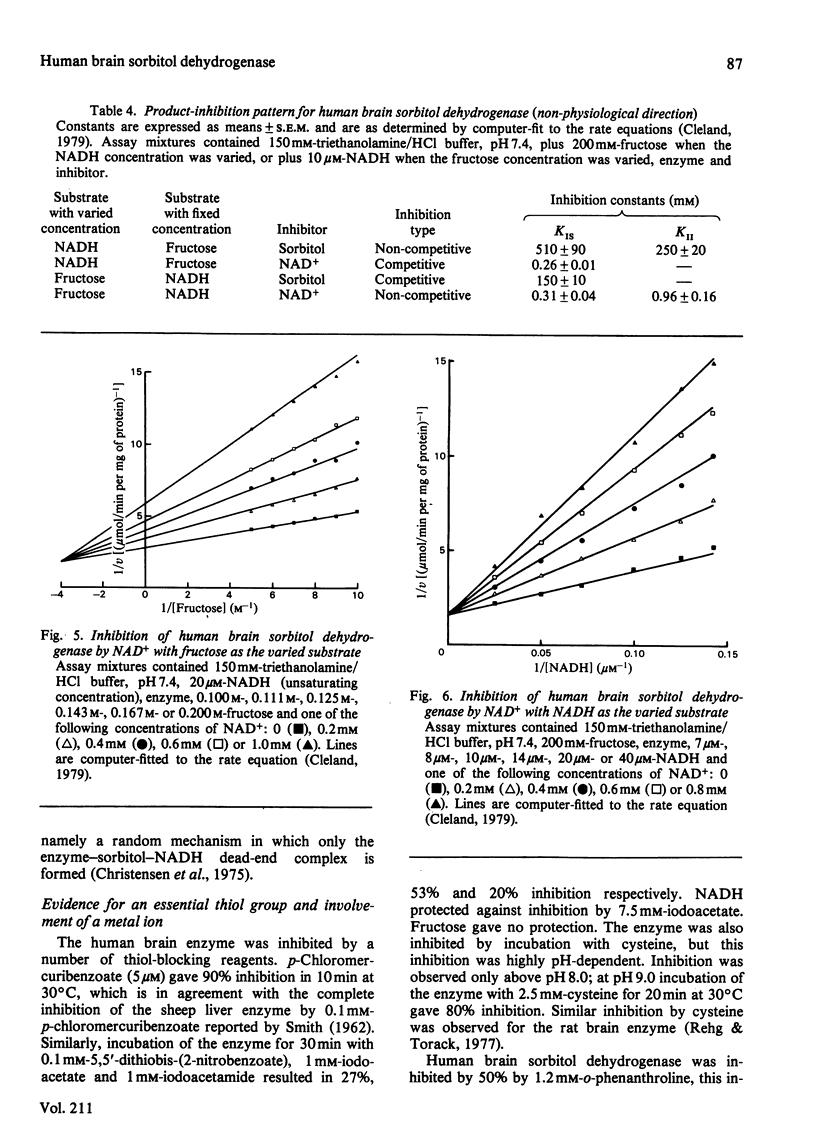
Sorbitol dehydrogenase was isolated from human brain and purified 690-fold, giving a final specific activity of 11.1 units/mg of protein. The enzyme preparation was nearly homogeneous, but was unstable at most temperatures. It exhibited a broad pH optimum of 7.5-9.0 in the forward reaction (i.e. sorbitol leads to fructose), and of 7.0 in the reverse reaction (i.e. fructose leads to sorbitol). Substrate-specificity studies demonstrated that the enzyme had the capability to oxidize a wide range of polyols and that the enzyme had a higher affinity for substrates in the forward reaction than in the reverse reaction, e.g. Km for sorbitol was 0.45 mM, and that for fructose was 480 mM. However, the Vmax. was 10 times greater in the reverse reaction. At high concentrations of fructose (500 mM) the enzyme exhibited substrate inhibition in the reverse reaction. The enzyme mechanism was sequential, as determined by the kinetic patterns arising from varying the substrate concentrations. In addition, both fructose and NADH protected the enzyme against thermal inactivation. These findings, together with product-inhibition data, suggested that the mechanism is random rapid equilibrium with two dead-end complexes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arieff A. I., Kleeman C. R. Studies on mechanisms of cerebral edema in diabetic comas. Effects of hyperglycemia and rapid lowering of plasma glucose in normal rabbits. J Clin Invest. 1973 Mar;52(3):571–583. doi: 10.1172/JCI107218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto O. C., Beutler E. The sorbitol-oxidizing enzyme of red blood cells. J Lab Clin Med. 1975 Apr;85(4):645–649. [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Christensen U., Tüchsen E., Andersen B. Initial velocity and product inhibition studies on L-iditol:NAD oxidoreductase. Acta Chem Scand B. 1975;29(1):81–87. doi: 10.3891/acta.chem.scand.29b-0081. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Prockop L. D., Winegrad A. I. Acute cerebral oedema during treatment of hyperglycaemia. An experimentsl model. Lancet. 1968 Aug 17;2(7564):384–386. doi: 10.1016/s0140-6736(68)90597-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Jedziniak J. A., Chylack L. T., Jr, Cheng H. M., Gillis M. K., Kalustian A. A., Tung W. H. The sorbitol pathway in the human lens: aldose reductase and polyol dehydrogenase. Invest Ophthalmol Vis Sci. 1981 Mar;20(3):314–326. [PubMed] [Google Scholar]
- Jedziniak J. A., Yates E. M., Kinoshita J. H. Lens polyol dehydrogenase. Exp Eye Res. 1973 Jun;16(2):95–104. doi: 10.1016/0014-4835(73)90304-7. [DOI] [PubMed] [Google Scholar]
- Jeffery J., Cummins L., Carlquist M., Jörnvall H. Properties of sorbitol dehydrogenase and characterization of a reactive cysteine residue reveal unexpected similarities to alcohol dehydrogenases. Eur J Biochem. 1981 Nov;120(2):229–234. doi: 10.1111/j.1432-1033.1981.tb05693.x. [DOI] [PubMed] [Google Scholar]
- Leissing N., McGuinness E. T. Rapid affinity purification and properties of rat liver sorbitol dehydrogenase. Biochim Biophys Acta. 1978 Jun 9;524(2):254–261. doi: 10.1016/0005-2744(78)90162-6. [DOI] [PubMed] [Google Scholar]
- O'Brien M. M., Schofield P. J. Polyol-pathway enzymes of human brain. Partial purification and properties of aldose reductase and hexonate dehydrogenase. Biochem J. 1980 Apr 1;187(1):21–30. doi: 10.1042/bj1870021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop L. D. Hyperglycemia, polyol accumulation, and increased intracranial pressure. Arch Neurol. 1971 Aug;25(2):126–140. doi: 10.1001/archneur.1971.00490020044005. [DOI] [PubMed] [Google Scholar]
- Rehg J. E., Torack R. M. Partial purification and characterization of sorbitol dehydrogenase from rat brain. J Neurochem. 1977 Mar;28(3):655–660. doi: 10.1111/j.1471-4159.1977.tb10438.x. [DOI] [PubMed] [Google Scholar]
- Rose C. I., Henderson A. R. Reaction-rate assay of serum sorbitol dehydrogenase activity of 37 degrees C. Clin Chem. 1975 Oct;21(11):1619–1626. [PubMed] [Google Scholar]
- SMITH M. G. Polyol dehydrogenases. 4. Crystallization of the L-iditol dehydrogenase of sheep liver. Biochem J. 1962 Apr;83:135–144. doi: 10.1042/bj0830135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. Studies on dehydrogenases of the glucuronate-xylulose cycle in the livers of diabetic mice and rats. Diabetes. 1979 Sep;28(9):793–798. doi: 10.2337/diab.28.9.793. [DOI] [PubMed] [Google Scholar]
- Varma S. D., Kinoshita J. H. Inhibition of lens aldose reductase by flavonoids--their possible role in the prevention of diabetic cataracts. Biochem Pharmacol. 1976 Nov 15;25(22):2505–2513. doi: 10.1016/0006-2952(76)90457-3. [DOI] [PubMed] [Google Scholar]
- Varma S. D., Mikuni I., Kinoshita J. H. Flavonoids as inhibitors of lens aldose reductase. Science. 1975 Jun 20;188(4194):1215–1216. doi: 10.1126/science.1145193. [DOI] [PubMed] [Google Scholar]
- Walsall E. P., Lyons S. A., Metzger R. P. A comparison of selected physical properties of hepatic sorbitol dehydrogenases [L-iditol: NAD oxidoreductases] from four mammalian species. Comp Biochem Physiol B. 1978;59(3):213–218. doi: 10.1016/0305-0491(78)90249-3. [DOI] [PubMed] [Google Scholar]



