Abstract
This narrative review describes up-to-date treatment options for peri-implantitis and proposes a treatment protocol and flowchart based on the current scientific evidence. Peri-implantitis treatment should be based on the phased treatment protocol for periodontitis, which is a continuous flow of decisions for extraction, nonsurgical and surgical treatments with step-by-step re-evaluation. The protocol’s goals are to fulfill the success criteria for peri-implantitis treatment (probing depth of ≤5 mm, and absence of bleeding on probing, suppuration, and progressive bone loss) and to halt disease progression. Fixtures with peri-implantitis can initially be classified as failed or failing. A failed implant needs to be removed. In contrast, nonsurgical and surgical treatments can be applied to a failing implant. Nonsurgical treatment should be the initial treatment for failing implants; however, sole nonsurgical treatment was regarded as inefficient for peri-implantitis. Recent studies have found that the adjunctive use of antibiotics to nonsurgical debridement increased the success of nonsurgical treatment for peri-implantitis. Surgical treatments can be classified into resective, access, and reconstructive surgeries. The technique should be selected according to the patient’s bone defect configuration, which relate to regenerative potential. Various combinations of decontamination methods (e.g., mechanical, chemical, and pharmacological approaches) are required to achieve absolute surface decontamination. Clinicians should select an appropriate surface decontamination strategy according to the purpose of surgery. After signs of disease disappear and its progression is halted through active peri-implantitis treatment, it is necessary to enroll patients into maintenance programs. Compliance of patients with the maintenance program reduces the recurrence of peri-implantitis and sustains clinical success after treatment. Maintenance visits should include professional plaque control and hygiene care reinforcement for patients, and their interval should be set according to individual peri-implantitis risk. Clinicians should remind that peri-implantitis treatment is not a single procedure, but rather a continuing cycle of treatment and re-evaluation.
Keywords: Clinical protocols, Dental implants, Flowchart, Peri-implantitis
Graphical Abstract
INTRODUCTION
Peri-implantitis was defined in the 2017 World Workshop consensus as “a plaque-associated pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri-implant mucosa and subsequent progressive loss of supporting bone” [1]. Contrary to patients’ expectations of implants lasting indefinitely, a Swedish study found that almost half of the implant recipients suffered peri-implantitis within 10 years [2]. Furthermore, the generalization of implant surgery has led to a dramatic increase in the number of patients suffering from peri-implantitis. Peri-implantitis tends to progress more rapidly and unpredictably than periodontitis, and its treatment outcome is less predictable [3]. In natural teeth, collagen fibers in the connective tissue area are embedded perpendicularly to the cementum and provide robust mucosal sealing, which operates as a biological barrier. In dental implants, however, collagen fibers around the transmucosal area run parallel to the abutment surface, which impairs mucosal sealing and its protective function [4]. The surface geometry of implant fixtures, such as their macro and micro thread structures and rough surfaces, also interferes with absolute surface decontamination and reduces the likelihood of successful treatment [5].
Various treatment protocols have been proposed for peri-implantitis based on experiences with periodontitis treatment. Mombelli and Lang proposed cumulative interceptive supportive therapy based on the diagnostic criteria for periodontitis, suggesting that peri-implantitis treatment is not a single procedure but rather a continuing sequence of therapeutic procedures like in periodontitis [6]. Since, various studies have investigated the factors that affect peri-implantitis, treatment protocols have been suggested to reflect these factors [7,8,9]. Sinjab et al. [8] highlighted the importance of keratinized gingiva around the implant and suggested that soft-tissue augmentation procedures are necessary for peri-implant disease management. Monje [9] proposed a decision tree that focused on solutions for failed implants and on detailed surgical approaches according to the configuration of the peri-implant defect. Volume 13 of the International Team for Implantology (ITI) Treatment Guide presents a peri-implantitis treatment protocol that considers nonsurgical and surgical treatments consecutively, combined with re-evaluation [7].
Treatment approaches for peri-implantitis need to be changed as the scientific evidences are updated. For example, nonsurgical therapy was formerly regarded as ineffective in peri-implantitis due to access restrictions [10]. However, recent studies have found higher success rates in treatment of peri-implantitis using nonsurgical therapy with the adjunctive use of antibiotics [11,12]. An assessment tool has also been suggested for the individual peri-implantitis risk based on the understanding of the key factors underlying peri-implantitis disease development [13]. The aims of the present narrative review were therefore to describe current treatment options for peri-implantitis and to propose an updated treatment flowchart based on the current scientific evidence.
FAILING IMPLANTS VERSUS FAILED IMPLANTS
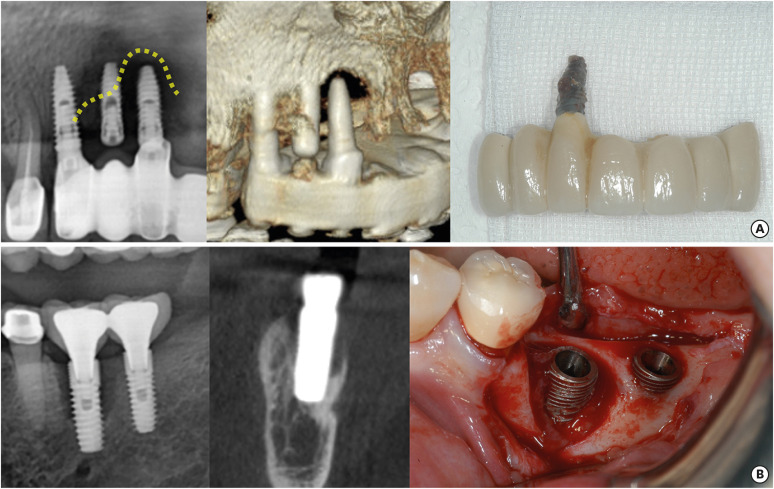
The phrase “ailing, failing, and failed” was first applied to dental implants by Meffert [14]. When this term is applied to the current diagnostic criteria of peri-implant disease, an ailing implant is one affected by peri-implant mucositis, and a failing implant is one affected by peri-implantitis whose prognosis is not yet hopeless. A failed implant is one with peri-implantitis so severe that it needs to be removed (Figure 1).
Figure 1. Examples of (A) failed and (B) failing implants.
It is obvious that mobile, which means complete loss of osseointegration, or fractured implants should be removed. A histological analysis of implant fixtures extracted because of mobility issues confirmed that bone–implant contact was completely lost when the implant stability quotient (ISQ) was 40 or less [15]. However, criteria for implant removal vary among clinicians. Volume 13 of the ITI Treatment Guide recommends that implant removal should be determined by defect extent and need to be done in cases where the esthetic outcome is expected to be severely compromised [7].
Some researchers have recommended fixture removal when bone loss affects more than half of the fixture [16,17]. With fixture removal, the existing inflammation and its source are removed, and further bone loss, especially in the vertical dimension, can be prevented, so that a favorable environment can be established for future prosthetic rehabilitation [18]. The failure rate of peri-implantitis fixture treatment was estimated to be 20 times higher in cases with ≥50% alveolar bone loss than with ≤25% bone loss [19]. Following implant removal, a second attempt at implant placement is practicable with a relatively high survival rate of around 88% [20,21]. The failure of the implant is affected by patient’s specific factors, so control of the risk factors should take place prior to surgery [21]. Additional ridge augmentation is common to compensate for alveolar bone loss around a fixture due to peri-implantitis or implant removal [20].
NONSURGICAL TREATMENT FOR PERI-IMPLANTITIS
Similar to the progression of gingivitis to periodontitis, peri-implant mucositis is regarded as the stage preceding peri-implantitis [22]. Based on the treatment concept of periodontal disease, managing peri-implant mucositis can be considered as the primary prevention of peri-implantitis [23]. It is widely accepted that nonsurgical treatment is effective in resolving peri-implant mucositis sites [10,24,25]. However, nonsurgical treatment alone showed limitations in most cases when the disease progressed to peri-implantitis [26]. In nonsurgical treatment, visual and instrumental access is often limited by the prosthetic structure and bony defects. Therefore, surgical interventions have been considered essential to treat peri-implantitis [10,27].
Nevertheless, attempts have been made to overcome the limitations of nonsurgical treatment by applying adjunctive measures to mechanical debridement, while taking advantage of nonsurgical treatment that are convenient for the clinician and less invasive for the patient [25]. Periodontal and peri-implant diseases have a shared etiology in that they are plaque-initiated inflammatory conditions; the levels of Gram-negative and anaerobic bacteria are high in both contexts [28,29]. Recent studies have found that the adjunctive use of antibiotics improves nonsurgical treatment for peri-implantitis, based on the success of its use for periodontal disease [11,12,30,31]. Liñares et al. [11] observed successful long-term follow-up outcomes from nonsurgical treatment with the adjunctive use of systemic metronidazole (500 mg, 3 times a day for 7 days) and 0.12% chlorhexidine (CHX) gel (twice a day for 14 days). The mean radiologic bone loss was 4.5 mm at baseline, and reductions in the mean radiological bone level and probing depth of 2.6 mm and 4.7 mm, respectively, were observed at a mean follow-up of 4.5 years. Narts et al. achieved radiologic bone gain and resolution of inflammation with nonsurgical treatment use of an ultrasonic scaler and glycine air abrasives, as well as supportive maintenance therapy every 3–6 months [31]. In their study, 500 mg of systemic metronidazole was prescribed 3 times a day for 7 days. Park et al. [12] found a significantly higher success rate of peri-implantitis treatment by administering local metronidazole and minocycline alongside nonsurgical treatment. Two types of ointment were used: minocycline ointment containing 10.0 mg of minocycline hydrochloride dehydrate, and minocycline-metronidazole ointment containing 10.0 mg of minocycline hydrochloride dehydrate and 201.0 mg of metronidazole benzoate. Repeated local application of either minocycline or metronidazole-minocycline was performed in follow-up visits. The adjunctive use of minocycline and metronidazole-minocycline with mechanical debridement led to treatment success rates of 20.5% and 31.6%, respectively, whereas mechanical debridement alone achieved a success rate of only 2.7%. However, because adjunctive antibiotic treatment has a temporary risk of antibiotic resistance in the subgingival microbiome, appropriate dosing and duration of antibiotic use are required [32].
Over-contoured restorations or those that hinder plaque control (i.e., both mesial and distal splinting) increase peri-implantitis risk [33]. Adjusting the implant restoration to a proper form that facilitates self-application of oral hygiene significantly improves the clinical outcome of nonsurgical treatment for peri-implant mucositis, and prevents inflammation rebound after the initial treatment [34]. Removing suprastructures to facilitate better instrumental access also significantly increases the efficacy of surface decontamination, compared to performing surface decontamination without prosthesis removal in nonsurgical treatment [35]. In a recent randomized controlled trial that adjusted the prosthesis to be favorable for self-application of hygiene control, removing the prosthesis before mechanical debridement and systemic metronidazole administration doubled the success rate relative to mechanical debridement alone [30].
SURGICAL TREATMENT FOR PERI-IMPLANTITIS
Surgical treatment is required for lesions where inflammation is not completely resolved after nonsurgical treatment. The surgical approach for peri-implantitis can be classified into 3 types. First, access surgery enables surface decontamination of fixture surfaces with direct visual and instrumental access without pocket reduction. Second, resective surgery, with or without implantoplasty, eliminates pathological pockets and facilitates access for hygiene control in bone defects with a lack of regenerative potential, such as supracrestal bone defects and 1- or 2-wall bone defects. Third, reconstructive surgery induces bone regeneration in the bony defect, supports re-osseointegration of the decontaminated fixture, and minimizes soft tissue recession in bone defects expected to be capable of regenerative potential, such as circumferential bone defects and 2- or 3-wall defects [36].
Access surgery overcomes the limitations of nonsurgical treatment through flap elevation, which facilitate more meticulous surface debridement with direct access, and is widely accepted as being effective for periodontitis. Likewise, its use in peri-implantitis has also been widespread and its long-term efficacy has been determined [37]. Applying various surface decontamination methods has improved the success rate of the surgery. A recent study found a higher success rate in access surgery with local delivery of minocycline and meticulous surface decontamination using titanium-coated curettes, a metallic copper-alloy ultrasonic scaler tip, a titanium brush, and an air-powder abrasive device [38]. Because access surgery does not involve apical positioning of the flap or ostectomy, it is a more conservative surgical approach than resective surgery. However, due to flap elevation, access surgery has a risk of subsequent mucosal recession [39].
Resective surgery is performed in areas where regeneration is not expected, such as in non-contained defects and suprcrestal defects, and it includes procedures such as bone recontouring, apically positioned flap, and implantoplasty (Figure 2). The difference between resective surgery for peri-implantitis and periodontitis is that implantoplasty is often considered in the former. In implantoplasty, a high-speed bur finely polishes the surface of the exposed implant thread outside the alveolar bone. Eliminating threads and making surfaces smooth can reduce biofilm accumulation and bacterial regrowth on exposed fixture surfaces without significant influence on biocompatibility [40]. However, thorough irrigation must also be performed, since the titanium particles generated in this process may cause biological complications [41]. The risk of fracture may also increase due to the thinner fixture walls, so caution is needed when applying to narrow-diameter implants with internal connections [42]. Englezos et al. [43] applied an apically positioned flap, osteoplasty, and implantoplasty in severe peri-implantitis cases with more than 3 mm of alveolar bone loss, and found favorable clinical outcomes during up to 2 years of follow-up.
Figure 2. Examples of resective surgery including bone recontouring and implantoplasty.
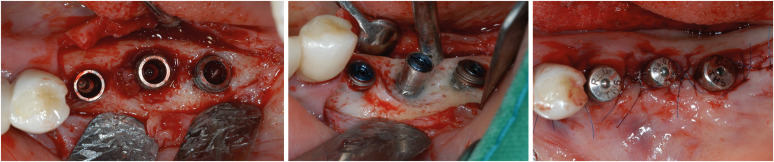
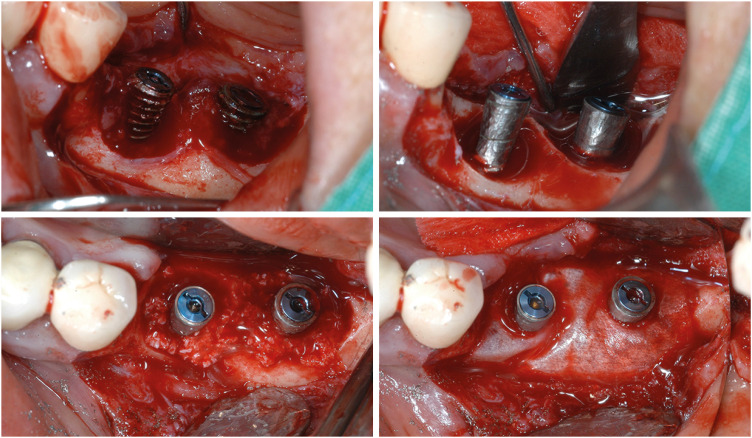
Reconstructive surgery shares the same surface decontamination process as access surgery, but it involves filling bony defects with graft materials instead of removing or leaving them (Figure 3). Whether the bony defect configuration is suitable for regeneration or not is important for applying reconstructive surgery. After using cone-beam computed tomography (CBCT) to analyze bone defects in patients diagnosed with peri-implantitis, Monje et al. [44] estimated that 58% of their bone defects (especially circumferential defects [3.2%] and 2- or 3-wall infraosseous defects [55%]) had regenerative potential for reconstructive surgery. Due to graft filling, the radiographic defect fill is significantly larger in reconstructive surgery than in access or resective surgery [25]. Additionally, the reduction in probing depth is substantial, and postoperative mucosal recession is minimized due to the graft materials’ support [39]. However, there was no significant difference from applying resective or access flap surgery regarding the resolution of inflammation [25]. Long-term stable bone gain was found after reconstructive surgery with the additional application of growth factors such as an enamel matrix derivative and platelet-derived growth factor [45].
Figure 3. Examples of reconstructive surgery. A pre-op photo of the implant is shown in Figure 1B. A glycine air abrasive and chlorhexidine were applied for surface decontamination.
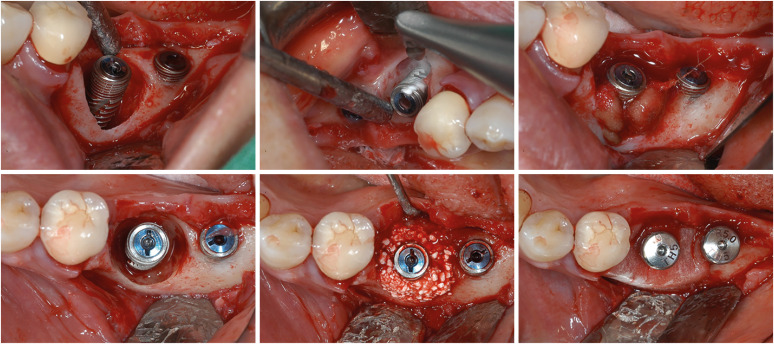
In the case of defects with combined intraosseous and supracrestal defect configurations, combined surgery consisting of resective surgery on the supracrestal part and regenerative surgery on the intraosseous part can be considered (Figure 4). In a recent study based on CBCT analysis, around 20% of the peri-implantitis defects had combined horizontal and intraosseous defect configurations [44]. Schwarz et al. [46] applied implantoplasty to the supracrestal area and a regenerative approach to the intraosseous area with natural bone mineral and collagen membrane. As a result, bleeding on probing (BOP) reduction of more than 85% and clinical attachment gain of more than 2 mm were sustained over 7 years of follow-up. Absence of BOP was also found in 60% of the patients. In a recent study, combined surgery with surface decontamination using a titanium brush significantly decreased the probing depth by 3–4 mm and the radiographical bone fill by around 80% after 12 months [47].
Figure 4. Examples of combined surgery. Implantoplasty was applied to the supracrestal part, and reconstructive surgery was applied to the infraosseous part. A titanium brush can be applied for surface decontamination of the infraosseous defect.
Also, soft tissue quality is a factor that can be improved with surgical intervention. Adequate peri-implant soft tissue condition is important for maintaining peri-implant health. Lack of keratinized mucosa, which is less than 2 mm, and shallow vestibular depth cause patient discomfort and disturb plaque removal [1,48]. Thin mucosal thickness offers limited mucosal seal and has a disadvantage in marginal bone stability [49,50]. Soft tissue augmentation using autogenous grafts or collagen substitutes can offer favorable peri-implant soft tissue conditions [51]. An apically positioned flap with a free gingival graft is regarded as the technique of choice in the sites with a lack of keratinized and attached mucosa. A connective tissue graft or use of collagen substitutes is usually applied to increase mucosal thickness. A recent systematic review observed that implants receiving soft tissue augmentation showed a low prevalence of peri-implantitis in long term [51].
SURFACE DECONTAMINATION OF THE IMPLANT FIXTURE SURFACE
The goal of proper surface decontamination is to remove the bacterial biofilm from the contaminated fixture surface and obtain a pristine surface, which induces re-osseointegration or minimizes bacterial recolonization [52]. Unlike natural teeth, the implant surface has rough surface and thread structure characterized by angulation and depth that makes accomplishing perfect surface decontamination a challenge. Restricted instrumental access to the fixture surface is also often observed due to the prosthetic’s features, which makes surface decontamination more difficult. Even with flap elevation, the bony defect’s configuration may constrain instrumental access [53]. Various surface decontamination strategies have been suggested to overcome these limitations [54].
Mechanical debridement is primarily used to remove biofilm and calculus on contaminated fixture surfaces. Metal curettes and ultrasonic scalers, which were traditionally used in periodontal treatment, have the disadvantage of damaging the surface of titanium fixtures. Accordingly, various methods have been suggested to preserve surface characteristics during surface debridement, such as using a titanium curette, ultrasonic scalers with a plastic sleeve tip, air powder abrasive, and various types of titanium brush. However, there is still no standard of care for instrument selection [55]. The metal tips of conventional ultrasonic scalers are often larger than the inter-thread distance, so that it only able to access the top of the thread structure. Also, the tips are applied laterally and harder than the titanium, so that it damages the titanium surface and thread structures [56]. Ultrasonic scalers with plastic tips have the advantage of preserving the fixture surface, but such tips are not strong enough to completely remove the attached biofilm, and plastic remnants are left on the surface after debridement [56,57].
The rotating titanium brush has a bristle at the end that is thin enough to facilitate the cleaning of fixture thread valleys [58]. Air powder abrasive is a method of propelling particles smaller than 50 μm (i.e., sodium bicarbonate, amino acid glycine, or erythritol) together with compressed air and water. Its power is sufficient for plaque removal while not damaging the surface, and can therefore offer more precise surface decontamination by approaching every part of the thread while maintaining an implant’s surface characteristics [56]. Intrabony defects, which are often observed in cases of peri-implantitis, have areas where direct instrumental access is not possible. However, even in these areas, a rotating titanium brush and air powder abrasive have greater cleaning efficacy than ultrasonic scalers [53].
In clinical situations, the mechanical surface decontamination of fixtures affected by peri-implantitis is challenging due to their surface structure, prosthetic design, and the configuration of the osseous defects. Achieving absolute surface decontamination is therefore considered impossible with mechanical debridement alone. The additional use of chemical agents for surface decontamination has been applied to overcome the limitations of mechanical debridement, and this approach has improved the clinical results of peri-implantitis treatment [55]. Chemical adjuncts reduce or even completely remove bacteria on the fixture surface and eliminate its endotoxins. For chemical surface decontamination, Monje et al. [54] recommended a minimum of 2 minutes of chemical detoxification using citric acid, hydrogen peroxide, or ethylenediaminetetraacetic acid (EDTA) along with optional irrigation with 0.12% CHX. The additional use of pharmacological agents such as tetracycline may also reduce the bacterial burden on the surface. The efficacy of various chemical decontamination methods has been demonstrated, but there is no clearly superior method among chemical and pharmacological agents [59].
Complete surface decontamination is essential for ensuring a regenerative treatment. Numerous studies have attained successful long-term results. Roccuzzo et al. [60] found that a high success rate was maintained in reconstructive surgery even after 5 years when a titanium brush was used for mechanical debridement together with chemical detoxification using an EDTA and CHX gel. Froum et al. [45] used a bicarbonate air abrasive for mechanical debridement, with CHX rinsing and tetracycline or minocycline for chemical debridement, and found that high survival rates and improved clinical outcomes were maintained during the follow-up period of 2–10 years.
MAINTENANCE AND RE-EVALUATION OF TREATMENT
Because the progression of peri-implantitis is rapid and difficult to predict, clinicians often consider its recurrence after treatment to be highly likely. Just as continuous re-evaluation and maintenance treatment are important for maintaining the success of periodontitis treatment, an adequate maintenance program is also important for maintaining treatment success in the treatment of peri-implantitis. A recent study found that periodic supportive therapy consisting of supra- and submucosal biofilm removal using a plastic tip ultrasonic scaler and titanium curette at least once every 6 months, together with professional prophylaxis in other areas, effectively maintained the clinical success of access flap surgery [61]. In that study, treatment success was maintained in 63% of the patients, and the absence of BOP, meaning complete inflammation resolution, was maintained in 42%. The treatment success rate was significantly higher after regenerative surgery for peri-implantitis in the group with frequent maintenance visits (3 or more times annually) than in the group with sporadic maintenance visits (2 or fewer times annually) [19]. A recent systematic review found that patients who were continuously enrolled in a supportive care program that included professional biofilm removal had a high implant survival rate up to 7 years after peri-implantitis surgery [62].
Periodic maintenance therapy reduces the prevalence of peri-implantitis. In a retrospective study with a mean follow-up period of 6.4 years, peri-implantitis prevalence was significantly reduced in patients who complied with a maintenance program that included plaque control and adjunctive local antibiotic application every 3–6 months. The prevalence was only 29.4% in the compliant group, whereas it was 72.5% and 68.6% in the non-compliant and erratic-compliant groups, respectively [63]. In another retrospective study with 7 years of follow-up, the peri-implantitis risk in patients who regularly received encouragement for self-application of oral hygiene and plaque control was about 25% of the risk among those who did not [64]. Another cross-sectional study found that patient compliance with maintenance therapy was associated with peri-implantitis prevalence [65]. In the group with regular compliance, 72.7% of the patients were healthy, while 4.5% suffered from peri-implantitis. However, only 53.5% of the patients in the group with erratic compliance were healthy, and 23.9% suffered from peri-implantitis. Although the details of the maintenance programs differed slightly between the two studies, both included professional plaque control and reinforcement of the self-application of oral hygiene.
Evaluations of the peri-implantitis risk help clinicians to offer adequate maintenance programs and motivate patient participation. Implant disease risk assessment (IDRA) has been suggested as a tool for evaluating the patient-level risks of peri-implantitis [13]. Eight selected risk factors for peri-implantitis were presented in an octagonal diagram: history of periodontitis, percentage of sites with BOP, prevalence of probing depths of ≥5 mm, periodontal bone loss in relation to the patient’s age, periodontitis susceptibility, supportive periodontal therapy, distance from the restorative margin of the implant-supported prosthesis to the bone, and implant prosthesis-related factors. This octagonal diagram was used to classify patients as having low, moderate, or high risk. IDRA can be used as a checklist for preventing peri-implantitis after implant therapy and as a tool for communicating and encouraging patients to participate in maintenance programs. It is important to involve patients in maintenance programs that are designed according to their individualized risk assessments. Visits every 3–6 months are generally recommended, depending on individual risk. Visit intervals of more than 6 months decrease the preventive efficacy of maintenance therapy [66].
TREATMENT PROTOCOL FOR PERI-IMPLANTITIS
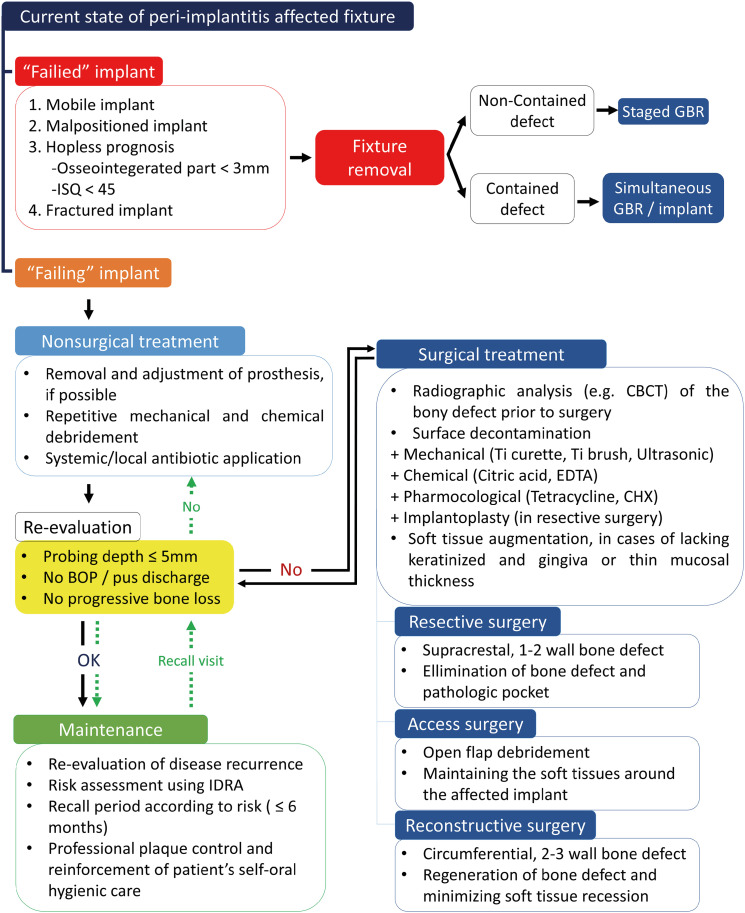
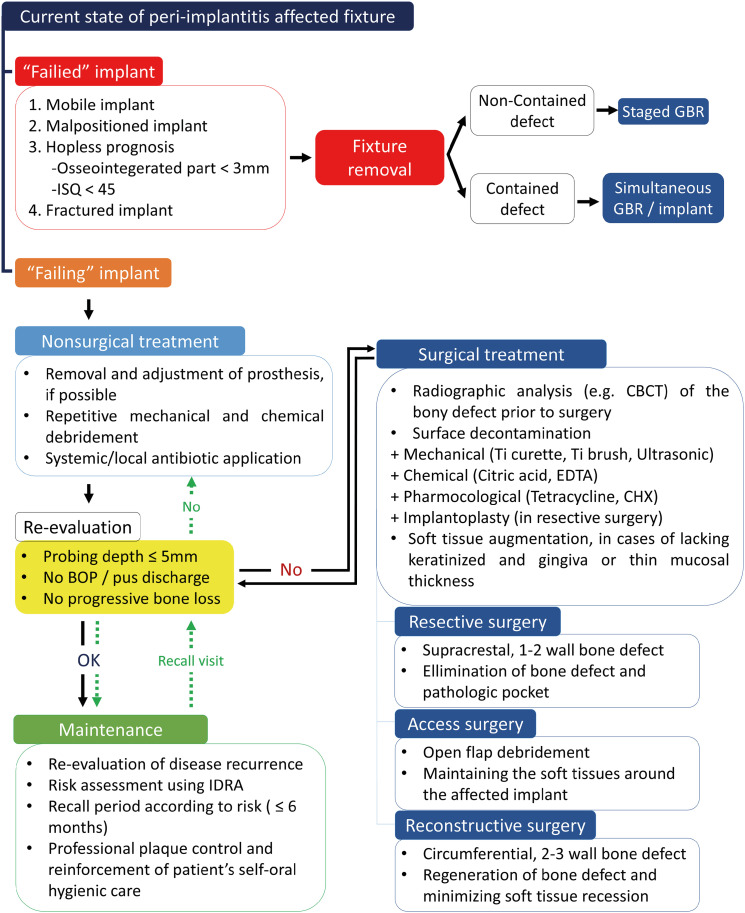
According to the current knowledge of peri-implantitis, the treatment flowchart in Figure 5 was suggested (Figure 5). First, it is necessary to determine whether the fixture involved in peri-implantitis is a failing or failed implant. If the fixture is mobile, malpositioned, fractured, or considered hopeless because less than 3 mm of the fixture is osseointegrated or because it has an ISQ value <45, it is classified as failed. In such cases, fixture removal is recommended because saving a failed implant is not possible. Subsequent treatment depends on the defect configuration after the fixture is removed. Staged guided bone regeneration (GBR) is recommended when the defect is not contained, but simultaneous GBR can be applied when it is contained. Furthermore, simultaneous fixture installation may be considered if sufficient residual bone is left to achieve adequate primary stability.
Figure 5. Comprehensive treatment protocol of peri-implantitis.
ISQ: implant stability quotient, GBR: guided bone regeneration, BOP: bleeding on probing, CBCT: cone-beam computed tomography, Ti: titanium, EDTA: ethylenediaminetetraacetic acid, CHX: chlorhexidine, IDRA: implant disease risk assessment.
Comprehensive peri-implantitis treatment is recommended for failing implants. Nonsurgical treatment should be applied prior to any surgical approach. Removing the prosthesis improves visual and instrumental access for facilitating surface decontamination, which is important in both nonsurgical and surgical treatments and is beneficial for healing after the latter. Nonsurgical treatment consists of repetitive mechanical debridement and chemical debridement, such as irrigation with CHX. Systemic metronidazole or local minocycline application is strongly recommended. Re-evaluation after nonsurgical treatment is necessary for decisions about subsequent procedures.
Surgical treatment is needed if the peri-implant conditions do not satisfy the re-evaluation criteria. In general, it is recommended to re-evaluate peri-implant conditions 3 months after nonsurgical treatment. Radiographical analysis using CBCT can confirm the defect configuration. Based on the defect configuration and esthetic concerns, a resective, access, or reconstructive surgical approach can be chosen. The clinician may choose combined surgery by incorporating surgical approaches in cases of combined defect configuration. Meticulous surface decontamination is essential regardless of the surgical method. Mechanical and chemical debridement should be applied together to achieve a pristine surface. Implantoplasty can also be an adequate method when using resective surgery.
Patients should be enrolled in a maintenance program if the peri-implant conditions satisfy the re-evaluation criteria after nonsurgical or surgical treatment. IDRA can be used to assess the risk of peri-implantitis. The recall period should be set according to the individual level of risk. It is important that each recall visit include professional plaque control and reinforcement of patient self-application of oral hygiene. Re-evaluation should also be performed at every visit. If the peri-implant conditions do not satisfy the re-evaluation criteria, the peri-implantitis treatment process should be reactivated, starting with nonsurgical treatment. Clinicians should remind that peri-implantitis treatment is not a single procedure, but rather a continuous cycle of treatment and re-evaluation.
Footnotes
Funding: This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) & funded by Korean government (MSIT) (No. 2022M3A9F3016364).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Sang-Yoon Oh, Hwee Woong Park, Ki-Tae Koo, Jae-Kook Cha.
- Formal analysis: Inpyo Hong, Ignacio Sanz-Martin.
- Investigation: Inpyo Hong, Jae-Kook Cha.
- Methodology: Sang-Yoon Oh, HweeWoong Park, Ki-Tae Koo, Jae-Kook Cha.
- Project administration: Jae-Kook Cha, Ignacio Sanz-Martin.
- Writing - original draft: Inpyo Hong.
- Writing - review & editing: Sang-Yoon Oh, Hwee Woong Park, Ki-Tae Koo, Ignacio Sanz-Martin, Jae-Kook Cha.
References
- 1.Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45(Suppl 1):S286–S291. doi: 10.1111/jcpe.12957. [DOI] [PubMed] [Google Scholar]
- 2.Derks J, Schaller D, Håkansson J, Wennström JL, Tomasi C, Berglundh T. Effectiveness of implant therapy analyzed in a swedish population: prevalence of peri-implantitis. J Dent Res. 2016;95:43–49. doi: 10.1177/0022034515608832. [DOI] [PubMed] [Google Scholar]
- 3.Derks J, Schaller D, Håkansson J, Wennström JL, Tomasi C, Berglundh T. Peri-implantitis - onset and pattern of progression. J Clin Periodontol. 2016;43:383–388. doi: 10.1111/jcpe.12535. [DOI] [PubMed] [Google Scholar]
- 4.Araujo MG, Lindhe J. Peri-implant health. J Periodontol. 2018;89(Suppl 1):S249–S256. doi: 10.1002/JPER.16-0424. [DOI] [PubMed] [Google Scholar]
- 5.Carcuac O, Abrahamsson I, Albouy JP, Linder E, Larsson L, Berglundh T. Experimental periodontitis and peri-implantitis in dogs. Clin Oral Implants Res. 2013;24:363–371. doi: 10.1111/clr.12067. [DOI] [PubMed] [Google Scholar]
- 6.Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63–76. doi: 10.1111/j.1600-0757.1998.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 7.Heitz-Mayfield LJA, Salvi GE. ITI treatment guide volume 13- prevention and management of peri-implant diseases. Batavia: Quintessence Publishing; 2022. [Google Scholar]
- 8.Sinjab K, Garaicoa-Pazmino C, Wang HL. Decision making for management of periimplant diseases. Implant Dent. 2018;27:276–281. doi: 10.1097/ID.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 9.Monje A. Unfolding peri-implantitis. 1. Batavia: Quintessence Publishing; 2022. [Google Scholar]
- 10.Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35(Suppl):305–315. doi: 10.1111/j.1600-051X.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- 11.Liñares A, Pico A, Blanco C, Blanco J. Adjunctive systemic metronidazole to nonsurgical therapy of peri-implantitis with intrabony defects: a retrospective case series study. Int J Oral Maxillofac Implants. 2019;34:1237–1245. doi: 10.11607/jomi.7343. [DOI] [PubMed] [Google Scholar]
- 12.Park SH, Song YW, Cha JK, Lee JS, Kim YT, Shin HS, et al. Adjunctive use of metronidazole-minocycline ointment in the nonsurgical treatment of peri-implantitis: a multicenter randomized controlled trial. Clin Implant Dent Relat Res. 2021;23:543–554. doi: 10.1111/cid.13006. [DOI] [PubMed] [Google Scholar]
- 13.Heitz-Mayfield LJ, Heitz F, Lang NP. Implant disease risk assessment IDRA-a tool for preventing peri-implant disease. Clin Oral Implants Res. 2020;31:397–403. doi: 10.1111/clr.13585. [DOI] [PubMed] [Google Scholar]
- 14.Meffert RM. How to treat ailing and failing implants. Implant Dent. 1992;1:25–33. doi: 10.1097/00008505-199200110-00003. [DOI] [PubMed] [Google Scholar]
- 15.Scarano A, Carinci F, Quaranta A, Iezzi G, Piattelli M, Piattelli A. Correlation between implant stability quotient (ISQ) with clinical and histological aspects of dental implants removed for mobility. Int J Immunopathol Pharmacol. 2007;20(Suppl 1):33–36. doi: 10.1177/039463200702001s08. [DOI] [PubMed] [Google Scholar]
- 16.Okayasu K, Wang HL. Decision tree for the management of periimplant diseases. Implant Dent. 2011;20:256–261. doi: 10.1097/ID.0b013e3182263589. [DOI] [PubMed] [Google Scholar]
- 17.Decker AM, Sheridan R, Lin GH, Sutthiboonyapan P, Carroll W, Wang HL. A prognosis system for periimplant diseases. Implant Dent. 2015;24:416–421. doi: 10.1097/ID.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 18.Fu JH, Wang HL. Can periimplantitis be treated? Dent Clin North Am. 2015;59:951–980. doi: 10.1016/j.cden.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Ravidà A, Siqueira R, Di Gianfilippo R, Kaur G, Giannobile A, Galindo-Moreno P, et al. Prognostic factors associated with implant loss, disease progression or favorable outcomes after peri-implantitis surgical therapy. Clin Implant Dent Relat Res. 2022;24:222–232. doi: 10.1111/cid.13074. [DOI] [PubMed] [Google Scholar]
- 20.Zhou W, Wang F, Monje A, Elnayef B, Huang W, Wu Y. Feasibility of dental implant replacement in failed sites: a systematic review. Int J Oral Maxillofac Implants. 2016;31:535–545. doi: 10.11607/jomi.4312. [DOI] [PubMed] [Google Scholar]
- 21.Park YS, Lee BA, Choi SH, Kim YT. Evaluation of failed implants and reimplantation at sites of previous dental implant failure: survival rates and risk factors. J Periodontal Implant Sci. 2022;52:230–241. doi: 10.5051/jpis.2105020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Periodontol. 2018;89(Suppl 1):S267–S290. doi: 10.1002/JPER.16-0350. [DOI] [PubMed] [Google Scholar]
- 23.Jepsen S, Berglundh T, Genco R, Aass AM, Demirel K, Derks J, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015;42(Suppl 16):S152–S157. doi: 10.1111/jcpe.12369. [DOI] [PubMed] [Google Scholar]
- 24.Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29(Suppl):325–345. doi: 10.11607/jomi.2014suppl.g5.3. [DOI] [PubMed] [Google Scholar]
- 25.Ramanauskaite A, Fretwurst T, Schwarz F. Efficacy of alternative or adjunctive measures to conventional non-surgical and surgical treatment of peri-implant mucositis and peri-implantitis: a systematic review and meta-analysis. Int J Implant Dent. 2021;7:112. doi: 10.1186/s40729-021-00388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwarz F, John G, Hegewald A, Becker J. Non-surgical treatment of peri-implant mucositis and peri-implantitis at zirconia implants: a prospective case series. J Clin Periodontol. 2015;42:783–788. doi: 10.1111/jcpe.12439. [DOI] [PubMed] [Google Scholar]
- 27.Klinge B, Meyle J Working Group 2. Peri-implant tissue destruction. The Third EAO Consensus Conference 2012. Clin Oral Implants Res. 2012;23(Suppl 6):108–110. doi: 10.1111/j.1600-0501.2012.02555.x. [DOI] [PubMed] [Google Scholar]
- 28.Salvi GE, Stahli A, Imber JC, Sculean A, Roccuzzo A. Physiopathology of peri-implant diseases. Clin Implant Dent Relat Res. 2023;25:629–639. doi: 10.1111/cid.13167. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Ahn DH, Yu Y, Han H, Kim SY, Joo JY, et al. Microbial profiling of peri-implantitis compared to the periodontal microbiota in health and disease using 16S rRNA sequencing. J Periodontal Implant Sci. 2023;53:69–84. doi: 10.5051/jpis.2202080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco C, Pico A, Dopico J, Gándara P, Blanco J, Liñares A. Adjunctive benefits of systemic metronidazole on non-surgical treatment of peri-implantitis. A randomized placebo-controlled clinical trial. J Clin Periodontol. 2022;49:15–27. doi: 10.1111/jcpe.13564. [DOI] [PubMed] [Google Scholar]
- 31.Nart J, Pons R, Valles C, Esmatges A, Sanz-Martin I, Monje A. Non-surgical therapeutic outcomes of peri-implantitis: 12-month results. Clin Oral Investig. 2020;24:675–682. doi: 10.1007/s00784-019-02943-8. [DOI] [PubMed] [Google Scholar]
- 32.Ardila CM, Bedoya-Garcia JA, Arrubla-Escobar DE. Antibiotic resistance in periodontitis patients: a systematic scoping review of randomized clinical trials. Oral Dis. 2023;29:2501–2511. doi: 10.1111/odi.14288. [DOI] [PubMed] [Google Scholar]
- 33.Yi Y, Koo KT, Schwarz F, Ben Amara H, Heo SJ. Association of prosthetic features and peri-implantitis: a cross-sectional study. J Clin Periodontol. 2020;47:392–403. doi: 10.1111/jcpe.13251. [DOI] [PubMed] [Google Scholar]
- 34.de Tapia B, Mozas C, Valles C, Nart J, Sanz M, Herrera D. Adjunctive effect of modifying the implant-supported prosthesis in the treatment of peri-implant mucositis. J Clin Periodontol. 2019;46:1050–1060. doi: 10.1111/jcpe.13169. [DOI] [PubMed] [Google Scholar]
- 35.Korello K, Eickholz P, Zuhr O, Ratka C, Petsos H. In vitro efficacy of non-surgical and surgical implant surface decontamination methods in three different defect configurations in the presence or absence of a suprastructure. Clin Implant Dent Relat Res. 2023;25:549–563. doi: 10.1111/cid.13198. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz F, Jepsen S, Obreja K, Galarraga-Vinueza ME, Ramanauskaite A. Surgical therapy of peri-implantitis. Periodontol 2000. 2022;88:145–181. doi: 10.1111/prd.12417. [DOI] [PubMed] [Google Scholar]
- 37.Berglundh T, Wennström JL, Lindhe J. Long-term outcome of surgical treatment of peri-implantitis. A 2-11-year retrospective study. Clin Oral Implants Res. 2018;29:404–410. doi: 10.1111/clr.13138. [DOI] [PubMed] [Google Scholar]
- 38.Cha JK, Lee JS, Kim CS. Surgical therapy of peri-implantitis with local minocycline: a 6-month randomized controlled clinical trial. J Dent Res. 2019;98:288–295. doi: 10.1177/0022034518818479. [DOI] [PubMed] [Google Scholar]
- 39.Sanz-Martín I, Cha JK, Sanz-Sánchez I, Figuero E, Herrera D, Sanz M. Changes in peri-implant soft tissue levels following surgical treatment of peri-implantitis: a systematic review and meta-analysis. 2021;32:230–244. doi: 10.1111/clr.13840. [DOI] [PubMed] [Google Scholar]
- 40.Burgueño-Barris G, Camps-Font O, Figueiredo R, Valmaseda-Castellón E. The influence of implantoplasty on surface roughness, biofilm formation, and biocompatibility of titanium implants: a systematic review. Syst Rev. 2021;36:e111–e119. doi: 10.11607/jomi.8785. [DOI] [PubMed] [Google Scholar]
- 41.Mombelli A, Hashim D, Cionca N. What is the impact of titanium particles and biocorrosion on implant survival and complications? Crit Rev. 2018;29:37–53. doi: 10.1111/clr.13305. [DOI] [PubMed] [Google Scholar]
- 42.Bertl K, Isidor F, von Steyern PV, Stavropoulos A. Does implantoplasty affect the failure strength of narrow and regular diameter implants? A laboratory study. Clin Oral Investig. 2021;25:2203–2211. doi: 10.1007/s00784-020-03534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Englezos E, Cosyn J, Koole S, Jacquet W, De Bruyn H. Resective treatment of peri-implantitis: clinical and radiographic outcomes after 2 years. Int J Periodont Restor Dent. 2018;38:729–735. doi: 10.11607/prd.3386. [DOI] [PubMed] [Google Scholar]
- 44.Monje A, Pons R, Insua A, Nart J, Wang HL, Schwarz F. Morphology and severity of peri-implantitis bone defects. Clin Implant Dent Relat Res. 2019;21:635–643. doi: 10.1111/cid.12791. [DOI] [PubMed] [Google Scholar]
- 45.Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodont Restor Dent. 2015;35:857–863. doi: 10.11607/prd.2571. [DOI] [PubMed] [Google Scholar]
- 46.Schwarz F, John G, Schmucker A, Sahm N, Becker J. Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: a 7-year follow-up observation. J Clin Periodontol. 2017;44:337–342. doi: 10.1111/jcpe.12648. [DOI] [PubMed] [Google Scholar]
- 47.de Tapia B, Valles C, Ribeiro-Amaral T, Mor C, Herrera D, Sanz M, et al. The adjunctive effect of a titanium brush in implant surface decontamination at peri-implantitis surgical regenerative interventions: a randomized controlled clinical trial. J Clin Periodontol. 2019;46:586–596. doi: 10.1111/jcpe.13095. [DOI] [PubMed] [Google Scholar]
- 48.Perussolo J, Souza AB, Matarazzo F, Oliveira RP, Araújo MG. Influence of the keratinized mucosa on the stability of peri-implant tissues and brushing discomfort: a 4-year follow-up study. Clin Oral Implants Res. 2018;29:1177–1185. doi: 10.1111/clr.13381. [DOI] [PubMed] [Google Scholar]
- 49.Garaicoa-Pazmino C, Mendonca G, Ou A, Chan HL, Mailoa J, Suárez-López Del Amo F, et al. Impact of mucosal phenotype on marginal bone levels around tissue level implants: a prospective controlled trial. J Periodontol. 2021;92:771–783. doi: 10.1002/JPER.20-0458. [DOI] [PubMed] [Google Scholar]
- 50.Gharpure AS, Latimer JM, Aljofi FE, Kahng JH, Daubert DM. Role of thin gingival phenotype and inadequate keratinized mucosa width (<2 mm) as risk indicators for peri-implantitis and peri-implant mucositis. J Periodontol. 2021;92:1687–1696. doi: 10.1002/JPER.20-0792. [DOI] [PubMed] [Google Scholar]
- 51.Stefanini M, Barootchi S, Sangiorgi M, Pispero A, Grusovin MG, Mancini L, et al. Do soft tissue augmentation techniques provide stable and favorable peri-implant conditions in the medium and long term? A systematic review. Clin Oral Implants Res. 2023;34(Suppl 26):28–42. doi: 10.1111/clr.14150. [DOI] [PubMed] [Google Scholar]
- 52.Koo KT, Khoury F, Keeve PL, Schwarz F, Ramanauskaite A, Sculean A, et al. Implant surface decontamination by surgical treatment of periimplantitis: a literature review. Implant Dent. 2019;28:173–176. doi: 10.1097/ID.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 53.Luengo F, Sanz-Esporrín J, Noguerol F, Sanz-Martín I, Sanz-Sánchez I, Sanz M. In vitro effect of different implant decontamination methods in three intraosseous defect configurations. Clin Oral Implants Res. 2022;33:1087–1097. doi: 10.1111/clr.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monje A, Amerio E, Cha JK, Kotsakis G, Pons R, Renvert S, et al. Strategies for implant surface decontamination in peri-implantitis therapy. Int J Oral Implantol (Berl) 2022;15:213–248. [PubMed] [Google Scholar]
- 55.Francis S, Iaculli F, Perrotti V, Piattelli A, Quaranta A. Titanium surface decontamination: a systematic review of in vitro comparative studies. Int J Oral Maxillofac Implants. 2022;37:76–84. doi: 10.11607/jomi.8969. [DOI] [PubMed] [Google Scholar]
- 56.Cha JK, Paeng K, Jung UW, Choi SH, Sanz M, Sanz-Martín I. The effect of five mechanical instrumentation protocols on implant surface topography and roughness: a scanning electron microscope and confocal laser scanning microscope analysis. Clin Oral Implants Res. 2019;30:578–587. doi: 10.1111/clr.13446. [DOI] [PubMed] [Google Scholar]
- 57.Sirinirund B, Garaicoa-Pazmino C, Wang HL. Effects of mechanical instrumentation with commercially available instruments used in supportive peri-implant therapy: an in vitro study. Int J Oral Maxillofac Implants. 2019;34:1370–1378. doi: 10.11607/jomi.7409. [DOI] [PubMed] [Google Scholar]
- 58.Giffi R, Pietropaoli D, Mancini L, Tarallo F, Sahrmann P, Marchetti E. The efficacy of different implant surface decontamination methods using spectrophotometric analysis: an in vitro study. J Periodontal Implant Sci. 2023;53:295–305. doi: 10.5051/jpis.2203500175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suarez F, Monje A, Galindo-Moreno P, Wang HL. Implant surface detoxification: a comprehensive review. Implant Dent. 2013;22:465–473. doi: 10.1097/ID.0b013e3182a2b8f4. [DOI] [PubMed] [Google Scholar]
- 60.Roccuzzo M, Mirra D, Pittoni D, Ramieri G, Roccuzzo A. Reconstructive treatment of peri-implantitis infrabony defects of various configurations: 5-year survival and success. Clin Oral Implants Res. 2021;32:1209–1217. doi: 10.1111/clr.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heitz-Mayfield LJ, Salvi GE, Mombelli A, Loup PJ, Heitz F, Kruger E, et al. Supportive peri-implant therapy following anti-infective surgical peri-implantitis treatment: 5-year survival and success. Clin Oral Implants Res. 2018;29:1–6. doi: 10.1111/clr.12910. [DOI] [PubMed] [Google Scholar]
- 62.Roccuzzo M, Layton DM, Roccuzzo A, Heitz-Mayfield LJ. Clinical outcomes of peri-implantitis treatment and supportive care: a systematic review. Clin Oral Implants Res. 2018;29(Suppl 16):331–350. doi: 10.1111/clr.13287. [DOI] [PubMed] [Google Scholar]
- 63.Lee SB, Lee BA, Choi SH, Kim YT. Long-term outcomes after peri-implantitis treatment and their influencing factors: a retrospective study. J Periodontal Implant Sci. 2022;52:194–205. doi: 10.5051/jpis.2103020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frisch E, Vach K, Ratka-Krueger P. Impact of supportive implant therapy on peri-implant diseases: a retrospective 7-year study. J Clin Periodontol. 2020;47:101–109. doi: 10.1111/jcpe.13206. [DOI] [PubMed] [Google Scholar]
- 65.Monje A, Wang HL, Nart J. Association of preventive maintenance therapy compliance and peri-implant diseases: a cross-sectional study. J Periodontol. 2017;88:1030–1041. doi: 10.1902/jop.2017.170135. [DOI] [PubMed] [Google Scholar]
- 66.Monje A, Aranda L, Diaz KT, Alarcón MA, Bagramian RA, Wang HL, et al. Impact of maintenance therapy for the prevention of peri-implant diseases: a systematic review and meta-analysis. J Dent Res. 2016;95:372–379. doi: 10.1177/0022034515622432. [DOI] [PubMed] [Google Scholar]