Abstract
Protein maldigestion and malabsorption lead to malnutrition and are a feature of exocrine pancreatic insufficiency (EPI). Although it is the current standard, measurement of nitrogen in stool to assess protease activity is indirect. Up to 80% of hydrolysed proteins appear in blood in the form of peptides, so we developed a method to measure peptide-derived amino acids in plasma as a relevant measure of proteolysis, verified its accuracy, precision, and linearity, and validated it in a porcine model. We modified a ninhydrin method. Large proteins were eliminated from plasma with 10 kDa-cut-off centrifugal filters. Free and total amino acids were measured in permeate before and after its hydrolysis. Peptide-derived amino acids were quantified by subtracting free amino acids from total amino acids. We verified the method in vitro and by comparing results in healthy and EPI pigs. The accuracy of the analysis was close to 100%, with excellent precision (mean relative standard deviation for low, medium, and high amino acid levels = 0.88%) and with stringent linearity (r2 = 0.986, %RE = 5.23). The high-throughput ninhydrin method detected levels of peptide-derived amino acids in vivo with maximal changes seen approximately 2 hours postprandially in young pigs. The AUC and Cmax were significantly higher in healthy compared to EPI pigs (P = .0026 and P = .0037, respectively). The high-throughput ninhydrin method is a sensitive, reliable, and practical method for the estimation of dietary peptide-derived amino acids. This assay endpoint could serve as a direct biomarker of protein digestion and absorption.
Keywords: peptide absorption, method, validation, healthy pigs, EPI pigs
Introduction
The digestion and absorption of dietary fats, proteins, and carbohydrates is a complex, multi-step process that involves the enzymatic breakdown of complex macronutrients to their smaller, absorbable digestion products. Fats are broken down to fatty acids and glycerol, proteins to peptides and amino acids, and starches to disaccharides and eventually monosaccharides such as glucose. The digestion of macronutrients is driven by enzymes secreted from the salivary glands, stomach, pancreas, and brush-border intestinal glands. Almost all absorption of macronutrient digestion products takes place through enterocytes that line the small intestine. With the aid of brush-border transporters, macronutrient digestion products traverse the enterocytes to reach the blood or lymph. In the case of fat absorption, enterocytes in particular modify the absorbable fat components: glycerol, free fatty acids, and monoglycerides, to fat molecules and transport them via the lymph [1].
The digestion of dietary proteins begins in the acidic pH conditions of the stomach through the action of pepsin. In the small intestine, pancreatic proteases further convert protein into free amino acids and peptides containing different numbers of amino acids. It has been suggested that the majority of dietary protein is absorbed in the form of peptides [2, 3]. It is important to note that in terms of protein metabolism, only di- and tripeptides should be considered as absorbable, since peptides containing a higher number of amino acids can exhibit biological activity, e.g., neuropeptides, CCK4 activity [4]. This phenomenon has been indirectly confirmed by the transport capacity of PepT1, which mediates the uptake of di- and tripeptides from the intestinal lumen, but not that of peptides with more than three amino acid residues [5].
Single amino acids are products of the action of exopeptidases, and peptides are first generated in the gut lumen as a direct result of the action of endopeptidases and the compensatory plastein reaction, where pepsin and trypsin play a main role [6]. It is suspected that enterocytes are able to synthesize di- and tripeptides from amino acids absorbed from the intestine and thus ensure the secondary generation of absorbable peptides [7]. At the same time, liver could be able to produce the third generation of peptides appearing in the blood postprandially [8].
Protein maldigestion and malabsorption occur when pancreatic proteases are not secreted or are secreted in an insufficient amount into the duodenum [9, 10]. Protein and fat malabsorption are conventionally treated with exogenous pancreatic enzyme replacement therapy (PERT) [11]. Calculation of the coefficient of fat absorption, a ratio of ingested to excreted fat, has been used to determine the success/performance of PERT, because of the emphasis on lipase activity and resulting control of steatorrhea [12]. The coefficient of nitrogen absorption (CNA) is determined using a similar concept (protein in: protein out), although CNA is not a true measure of pancreatic protease activity. In addition to the onerous requirements for stool collection, the measurement of faecal nitrogen losses also includes endogenous secreted proteins and dietary protein digested by colonic bacteria. The Kjeldahl method, which is generally used to assess the presence of nitrogen in organic matter, was developed in the 1880s and is still cited by most authors who report on CNA results [13]. Alternatively, the Dumas combustion method is used by one major commercial laboratory in the United States [14].
There is therefore a need for a sensitive, practical, and rapid method that can directly measure relevant biomarkers of protein digestion followed by absorption after a meal, rather than measuring excretion in the stool. We therefore explored the measurement of peptide-derived amino acids, as well as free amino acids in the blood following an oral whey protein challenge, as a pharmacokinetic-like test that could be used in both research and clinical settings. Ninhydrin reactions are widely used to analyse and characterize amino acids, peptides, and proteins in biomedical, clinical, and nutritional studies [15]. This colorimetric method is based on the number of amine groups for a given number of amino acids. We adapted this method to allow for high throughput, by excluding plasma proteins using filters, and simultaneously analysing aliquots of unhydrolysed and hydrolysed permeate to allow for the estimation of peptide-derived amino acids, which was our primary endpoint. We hypothesize that our modified, high-throughput, ninhydrin method could be used as a direct measure of protein digestion and absorption.
Objective and aims of the study
The objective of this study was to describe the rapid measurement of biomarkers of protein digestion and absorption in the plasma, using a modified, high-throughput, ninhydrin method. We aimed to confirm the accuracy, precision, and linearity of the analysis in vitro. We also aimed to verify the results in vivo by applying the method in healthy and exocrine pancreatic insufficient (EPI) pigs.
Materials and methods
Ninhydrin method and its adaptation
The detailed protocol of the adapted ninhydrin method, as well as a list of the materials used, is provided in Supplementary File 1.
Briefly, the current assay was developed based on the concept that two molecules of ninhydrin (2,2-dihydroxyindane-1,3-dione) react with a free alpha-amino acid to produce a deep purple or blue colour, known as Ruhemann’s purple, which can be measured spectrophotometrically at a wavelength of 570 nm [16]. In this reaction, ninhydrin acts as an oxidizing agent and causes the deamination and decarboxylation of amino acids at an elevated temperature. By the end of the reaction, a diketohydrin complex is formed, which has a deep purple colour. Exposure to acetate buffer for 1 h at 100°C releases the amino acids from peptides, thus enabling measurement of all the nitrogen.
We adapted this method in two ways. We adapted the sample processing such that a microplate reader can be used, allowing for high throughput. Furthermore, centrifugal filters with a cut-off of 10 kDa were employed for mechanical deproteinization of the plasma to separate free amino acids and peptides from plasma proteins such as albumin and fibrinogen.
The intact (unhydrolysed) permeate was used to estimate the levels of free amino acids. The hydrolysed permeate (see Supplementary data) was used to estimate total amino acids. Peptide-derived amino acids, our primary endpoint, were quantified by subtraction as follows:
Peptide-derived amino acids = Total amino acids − Free amino acids
Determination of accuracy, precision, and linearity of the modified ninhydrin method
Accuracy was determined based on the percentage recovery of amino acids from the high, medium, and low amino acid concentration samples. All samples were run in quadruplicate. Percentage (%) recovery at each level was determined according to the equation below.
Accepted percentage recovery should be between 90 and 110% to demonstrate accuracy of the method.
Precision is here expressed as percentage Relative Standard Deviation (RSD). The target RSD should be ≤ 5%. Precision under the same operating conditions over a short interval of time is the intra-assay repeatability. To study the intra-assay repeatability, we used the data obtained for three samples with high, medium, and low concentrations of amino acids. Three concentrations with four replicates at each level were assessed. Intermediate precision expresses within-laboratory variations: different days, different analysts, different equipment, etc. To assess intermediate precision, samples with high, medium, and low concentrations of amino acids were re-analysed in quadruplicate on three different days, by two analysts.
To assess linearity, six standards of the amino acid mixture and a blank were used to construct a standard curve. The standard curve was then plotted as the amino acid concentration (µg/ml) of the standard vs. the obtained value of the experimental samples. The coefficient of determination (r2) and average relative errors of back-calculated concentrations (%RE) were calculated. The target r2 values for each of the standard curves should be ≥ 0.98, and the suggested limits for %RE deviation are between 15 and 20% [17, 18].
In vivo verification of the modified ninhydrin method
Animals
This study was conducted strictly in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize animal suffering. The study received approval from the Second Local Ethical Committee for Animal Experiments in Warsaw (approval number WAW2/025/2022). The experiment was conducted on sixteen healthy pigs (Sus scrofa domesticus) ((Polish Spruce × Yorkshire) × Hampshire breed) of both sexes, whose body weight at the beginning of the study was 15 ± 2.3 kg. Six of the sixteen pigs underwent pancreatic duct ligation surgery to induce exocrine pancreatic insufficiency (EPI).
Surgical procedures
EPI was induced by pancreatic duct ligation as previously described [19]. Development of EPI was confirmed 4 weeks after surgery by external symptoms of maldigestion and malabsorption, manifested by growth arrest and the development of steatorrhea. Jugular vein catheters were inserted as previously described [20, 21] to allow for blood sampling (one week after arrival to the animal facility and then 3 weeks after EPI induction).
Feeding and experimental design
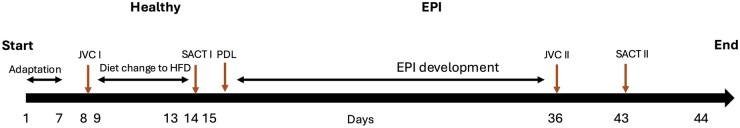
Upon arrival, pigs were fed a cereal-based, pelleted, standard low-fat diet (LFD) (18.0% (w/w) crude protein, 62.5% (w/w) carbohydrates, 5% (w/w) crude fat, and 7.2% (w/w) ash, 7.3% water) and were gradually (days 9 and 10—25%HFD + 75%LFD; day 11—50%HFD + 50%LFD, day12—75%HFD + 25%HFD; day 13–100%HFD; Fig. 1) changed to a high-fat diet (HFD), (17.5% (w/w) crude protein, 51.0% (w/w) carbohydrates, 20% (w/w) crude fat, and 5.2% (w/w) ash, 6.3% water). Both the LFD and HFD were supplemented with 5,000 IE/kg vitamin A, 500 IE/kg vitamin D, and 85 mg/kg vitamin E (Kcynia, Morawski Plant, Poland). The HFD was provided in an amount equivalent to 4% of the pigs’ body weight daily, with 1% (160 g) given at the morning meal (09:00-10:00 hr) and 3% (480 g) at the afternoon meal (17:00–18:00).
Figure 1.
Study design. EPI—exocrine pancreatic insufficiency, HFD—high-fat diet, JVC—jugular vein catheter insertion, PDL—pancreatic duct ligation surgery, SACT—substrate absorption challenge test. Healthy—intact pigs, n = 16, EPI—pigs with confirmed exocrine pancreatic insufficiency, n = 6.
On experimental days I and II, a substrate absorption challenge test (SACT) was performed in both healthy and EPI pigs. After an overnight fast, at 09:00 am, each pig received a meal consisting of 100 g of HFD enriched with 4 g docosahexaenoic and eicosapentaenoic acid triglycerides (Kinoko Life, Spain), 10 g milk whey protein, and 32 g potato starch.
The study design is presented in Fig. 1.
Blood sampling
Blood samples were collected via the jugular vein catheter one hour prior to SACT morning feeding and then at 60, 120, 180, and 240 minutes after feeding, and were transferred to BD Vacutainer® glass Aprotinin K2EDTA tubes (BD Diagnostics, New Jersey, USA). The blood samples were immediately placed on ice before they were centrifuged at 3000 × g for 15 minutes at 4°C, and plasma was separated and stored at −80°C until further analysis. The content of free, total, and peptide-derived amino acids in the plasma samples was analysed using the above-described method.
Statistical analysis
Statistical analysis was performed on the data generated from this study using the Student’s t-test with Welch correction for normally distributed data sets or Mann–Whitney test when data were not normally distributed. The data distribution was assessed using the Shapiro–Wilk normality test. Outliers within data sets were identified using the ROUT method of regression, using (Q = 0.05%). Baseline-adjusted area under the curve (AUC) values were calculated, and the total peak area values were compared. All the analyses were carried out using GraphPad Prism 10.0 (San Diego, CA, USA). Data were not corrected for multiple comparisons. Differences were considered significant if P ≤ .05; differences were considered as a trend when P ≤ .1; data with Gaussian distribution are expressed as mean ± standard deviation (± SD); data with non-Gaussian distribution are expressed as median ± interquartile range (± IQR).
Results
In vitro validation of the high-throughput ninhydrin method
Accuracy
We obtained the following percentage recovery from the accuracy analysis: 109%, 94%, and 101% for the low, medium, and high amino acid concentration samples, respectively (Table 1).
Table 1.
Accuracy and precision.
| Sample/peptides level | Theoretical concentration (µg/ml) | Measured concentration (µg/ml) | Recovery (%) (Accuracy) | Intra-assay RSD (%) (Repeatability) | Average% RSD |
|---|---|---|---|---|---|
| Amino acids solution samples | |||||
| Low | 30 | 32.68 | 109 | 0.74 | 0.88 |
| Medium | 60 | 56.36 | 94 | 0.77 | |
| High | 105 | 106.36 | 101 | 1.14 | |
RSD—Relative standard deviation. All samples were run in quadruplicate.
Precision
Repeatability (intra-assay precision) is a measure of precision under the same conditions over a short interval of time. Repeatability measurements, reflecting within-laboratory variations for amino acid solutions, were 0.74%, 0.77%, and 1.14% for the low, medium, and high amino acid concentration levels, respectively. The average repeatability, reported as the average of the RSDs for the three amino acid levels, was 0.88% (Table 1).
Intermediate precision measurements, reflecting measurement on different days by different analysts, were 3.30, 0.99 and 0.37%, for the low, medium, and high amino acid concentration levels, respectively. The average RSD was 1.55% (Table 2).
Table 2.
Intermediate precision: mean concentration measured on different days by two analysts.
| Amino acid content level | Mean concentration |
Intermediate %RSD | Average %RSD | ||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |||
| Low (30 µg/ml) | 31.68 | 27.61 | 33.18 | 3.30 | 1.55 |
| Medium (60 µg/ml) | 67.27 | 59.29 | 57.89 | 0.99 | |
| High (105 µg/ml) | 105.32 | 102.69 | 106.34 | 0.37 | |
RSD—Relative standard deviation. All samples were run in quadruplicate.
Linearity
Linearity, which is an important performance characteristic of any analytical method, was assessed using the coefficient of determination r2 and relative errors of back-calculated concentrations (%RE), for three independent measurements. The average value of r2 was 0.986 and the average % RE value was 5.23 from 3 days (Table 3).
Table 3.
Coefficient of determination (r2) and average relative errors of back-calculated concentrations (%RE) for standard curves on different days.
| Day 1 | Day 2 | Day 3 | Mean r2 | |
|---|---|---|---|---|
| r2 | 0.987 | 0.992 | 0.979 | 0.986 |
| %RE | 7.49 | 4.44 | 3.76 | 5.23 |
%RE—Relative error of back-calculated concentrations. All samples were run in quadruplicate.
In vivo verification of the modified ninhydrin method in healthy and exocrine pancreatic insufficient pigs
Free amino acids
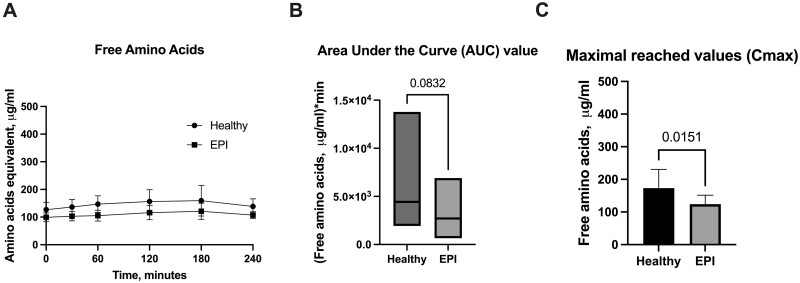
Results on the levels of free amino acids in porcine plasma are provided in Fig. 2 A–C. The levels in healthy and EPI pigs were similar, ranging between 100 and 200 µg/ml. In both groups, the levels of free amino acids were stable, without strong postprandial fluctuations, even despite the observed significant difference in Cmax (Fig. 2 C).
Figure 2.
Levels of free amino acids in porcine plasma. A - Postprandial changes in free amino acid plasma levels over time; B - Area under the Curve values (AUC) for free amino acids; C - Maximal reached values (Cmax) for free amino acids. Healthy—intact pigs, n = 16, EPI—pigs with confirmed exocrine pancreatic insufficiency, n = 6. Data on AUC are presented with a line indicating the mean. AUCs and Cmax data are presented as mean±SD, and data on amino acid levels at separate timepoints are given as mean±SD. Differences were considered significant if P ≤ .05; differences were considered as a trend when P ≤ 0.1. P-values are given with the results bars.
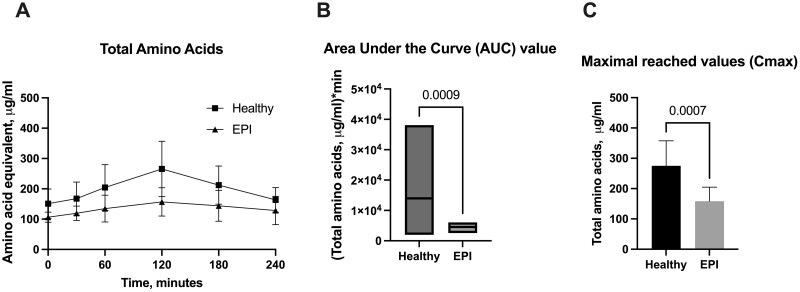
Total amino acids
Results for the levels of total amino acids (peptide derived + free amino acids) from porcine plasma are presented in Fig. 3 A–C. The initial fasted levels of total amino acids in both healthy and EPI pigs were similar, but the postprandial increase in total amino acids differed (Fig. 3 A). Healthy pigs had significantly higher levels of total amino acids for up to 3 hours after the meal, with Tmax at 120 min after feeding. Both the AUC and Cmax values were significantly higher in the healthy pigs compared to the EPI pigs (Fig. 3 B and C).
Figure 3.
Levels of total amino acids in porcine plasma. A - Postprandial changes in total amino acid plasma levels over time; B - Area under the Curve values (AUC) for total amino acids; C - Maximal reached values (Cmax) for total amino acids. Healthy—intact pigs, n = 16, EPI—pigs with confirmed exocrine pancreatic insufficiency, n = 6. Data on AUC are presented with a line indicating the mean, Cmax, and data on amino acid levels at separate timepoints are given as mean±SD. Differences were considered significant if P ≤ .05; differences were considered as a trend when P ≤ .1. P-values are given with the results bars.
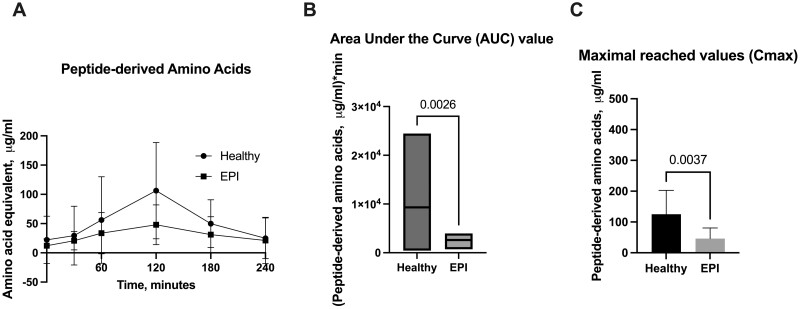
Peptide-derived amino acids
Results for the estimation of peptide-derived amino acids from porcine plasma are shown in Fig. 4 A–C. The initial fasted amounts of peptide-derived amino acids in both healthy and EPI pigs were similar, but the postprandial increase observed differed between the two groups (Fig. 4 A). Healthy pigs had significantly higher levels of peptide-derived amino acids for up to 3 hours after the meal, with Tmax at 120 min after feeding. Both the AUC and Cmax values were significantly higher in the healthy pigs compared to the EPI pigs (Fig. 4 B and C).
Figure 4.
Levels of peptide-derived amino acids in porcine plasma. A - Postprandial changes in peptide-derived amino acids plasma levels over time; B - Area under the Curve values (AUC) for peptide-derived amino acids; C - Maximal reached values (Cmax) for peptide-derived amino acids. Healthy—intact pigs, n = 16, EPI—pigs with confirmed exocrine pancreatic insufficiency, n = 6. Data on AUC are presented with a line indicating the mean, Cmax, and data on amino acid levels at separate timepoints are given as mean ± SD. Differences were considered significant if P ≤ .05; differences were considered as a trend when P ≤ .1. P-values are given with the results bars.
Discussion
We studied the evaluation of peptide-derived amino acids in plasma in vitro and in vivo following ingestion of a standardized whey substrate as a new endpoint to measure pancreatic protease activity. Biologically active peptides, both synthesized in vivo and of dietary origin, can induce numerous physiological effects [2, 3, 8, 22, 23]. The absorption of dietary di-, tripeptides, and even oligopeptides has been proven in several previous studies [3, 24, 25]. The absorbed nutritional peptides may play a key role in growth and even affect overall metabolism. Thus, a method to calculate their absorption after a meal may be a more clinically relevant measure of proteolysis than measuring excreted (unabsorbed) nitrogen. Recently, it was clearly shown that oligopeptides relating to hydrophobic, aromatic, and acidic amino acids are readily absorbed through the intestinal epithelium and preserve their bioactivity [25]. However, both the mechanisms of oligopeptides absorption and the question concerning the preservation of their biological activity after entering the circulation remain unclear. Thus, the current study focused on the measurement of nutritional (because of their absorbed amounts) di- and tripeptides which is far better proven and described in the literature.
Having developed new methodology, we wanted to ensure that it was accurate and precise and that the results were linear. A test method is said to be accurate when the test value approaches the absolute 'true' value of the substance being measured and is said to be precise when repeated analyses on the same sample give similar results. When a test method is precise, the amount of random variation is small. Our in vitro tests of these parameters indicate that the whey protein SACT is a reliable and repeatable measurement.
Our data confirm that levels of free amino acids in the blood of healthy and EPI pigs does not reflect feeding, while levels of total amino acids and peptide-derived amino acids change postprandially. This is consistent with findings in humans in which free plasma amino acid levels after an intact protein meal do not parallel the relative amino acid composition of the ingested food [26, 27]. Our data support the contention that the measurement of free amino acids would not be an appropriate measure of postprandial protease activity.
The high-throughput ninhydrin method we developed shows clear differences between protein absorption in healthy compared to EPI pigs. Maximal changes were seen approximately 2 hours after a meal, and the AUC and Cmax were significantly higher in healthy pigs compared to EPI pigs, suggesting that peptide-derived amino acids reflect the activity of pancreatic protease. A method to determine the most efficacious dose of protease could be helpful clinically. Current commercially available PERTs for treatment of EPI are porcine extracts and contain a higher proportion of protease per unit of lipase than is seen in humans [28]. In pigs, the proportion of protease to lipase is 3:1, in contrast to that seen in humans, which is approximately 0.2–1 [29, 30]. The availability of a test of protease activity could be valuable in the rational development of new PERTs.
The current study has limitations. The assessment of the plasma concentration of amino acids was performed taking into consideration only the Ruhemann’s purple measurement (570 nm), as the porcine samples demonstrated negligible absorbance at 440 nm which could be explained by the standardized pig diet. However, for human samples, a wavelength of 520 nm, which is common for both aminoacids and iminoacids, is recommended [31]. Thus, generalization of this method to humans would need to be verified before accepting it as a new research or clinical endpoint, which is our ultimate goal. Likewise, although portal vein catheters would provide a more direct assessment of absorption, we used jugular catheters because only systemic blood could be used in humans outside of highly limited research settings. The absorption of proteins by pigs varies based on age and diet, although both experimental and control pigs were of the same age and were fed the same diet [32]. Additionally, some peptides can be resistant to the hydrolysis used in this method, and that will lower the number of amine groups recognized by the Ruhemann’s purple reaction. Catabolic disease processes can lead to additional plasma amine groups derived from muscle wasting rather than dietary protein. Thus, it is possible that our measurements might have been affected by the malnourished state caused by the development of EPI. Whether this might affect the validity of the whey protein SACT, as a measure of protease activity, could be verified by studying this method when EPI pigs are given exogenous protease to treat protein maldigestion.
However, despite all the above-mentioned limitations, we have demonstrated that the modified, high-throughput ninhydrin method described in the current study is a sensitive, reliable, and practical method for the measurement of post prandial changes in dietary peptide-derived amino acids levels. Other methods (e.g. LC- MS/MS) [33], which are conventionally used, also make use of the same principle as we did using the ninhydrin method when processing the blood samples (sample deproteinization and hydrolysis). However, the chemical deproteinization was shown to affect the assay outcomes [34], so the choice of agent and protocols can lead to reproducibility issues. Measurement of labelled amino acids after ingestion has contributed insights to the field but is not relevant to understanding the role of protease on a mixed meal.
This novel endpoint, the determination of postprandial changes in peptide-derived amino acids using a modified ninhydrin high-throughput method, could serve as a direct, quick, and economical biomarker of protein digestion and absorption. If verified in humans, it has the potential to be a useful tool in drug development and clinical care.
Supplementary Material
Contributor Information
Kateryna Pierzynowska, Department of Biology, Lund University, Sölvegatan 35, Lund, 22362, Sweden; Department of Animal Physiology, The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Instytucka 3, Jabłonna, 05110, Poland; Anagram Therapeutics Inc, 10 Speen Street, Framingham, MA, 01701, United States; Anara AB, Alfågelgränden 24, Trelleborg, 23132, Sweden.
Kamil Zaworski, Department of Animal Physiology, The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Instytucka 3, Jabłonna, 05110, Poland; Anara AB, Alfågelgränden 24, Trelleborg, 23132, Sweden.
Piotr Wychowański, Anara AB, Alfågelgränden 24, Trelleborg, 23132, Sweden; Fondazione Policlinico Universitatio A. Gemelli IRCCS-Universita Largo Agostino Gemelli 8, Rome, 00168, Italy.
Janine Donaldson, Anara AB, Alfågelgränden 24, Trelleborg, 23132, Sweden; School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, Princess of Wales Terrace Parktown, Johannesburg, 2050, South Africa.
Jarosław Woliński, Anara AB, Alfågelgränden 24, Trelleborg, 23132, Sweden; Large Animal Models Laboratory, The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Instytucka 3, Jabłonna, 05110, Poland.
Drucy Borowitz, Department of Pediatrics, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, 1001 Main Street, Buffalo, NY, 14203, United States.
Robert Gallotto, Anagram Therapeutics Inc, 10 Speen Street, Framingham, MA, 01701, United States.
Stefan Pierzynowski, Department of Biology, Lund University, Sölvegatan 35, Lund, 22362, Sweden; Anara AB, Alfågelgränden 24, Trelleborg, 23132, Sweden; Department of Medical Biology, Witold Chodźki Institute of Rural Medicine, Doktora Kazimierza Jaczewskiego 2, Lublin, 20090, Poland.
Author contributions
Kateryna Pierzynowska (Conceptualization [equal], Data curation [equal], Formal analysis [lead], Investigation [lead], Methodology [lead], Project administration [lead], Supervision [equal], Visualization [lead], Writing—original draft [equal], Writing—review & editing [equal]), Kamil Zaworski (Formal analysis [supporting], Investigation [equal], Methodology [equal], Writing—original draft [supporting], Writing—review & editing [supporting]), Piotr Wychowański (Investigation [equal], Methodology [equal]), Janine Donaldson (Methodology [equal], Writing—review & editing [equal]), Jarosław Woliński (Investigation [supporting], Methodology [supporting], Project administration [supporting], Resources [supporting]), Drucy Borowitz (Writing—original draft [equal], Writing—review & editing [equal]), Robert Gallotto (Conceptualization [equal], Project administration [equal], Writing—review & editing [equal]), and Stefan Pierzynowski (Conceptualization [lead], Investigation [lead], Methodology [lead], Supervision [equal], Writing—review & editing [equal])
Supplementary data
Supplementary data are available at Biology Methods and Protocols online.
Conflict of interest statement. Kateryna Pierzynowska and Stefan Pierzynowski are owners of Anara AB and have a consultancy agreement with Anagram Therapeutics. Drucy Borowitz receives compensation as a member of the Anagram Scientific Advisory Board and has stock options in Anagram Therapeutics. Robert Gallotto is the President and CEO of Anagram Therapeutics.
Funding
This manuscript was written by the authors without external support.
Data availability
Data are available in supplementary data.
References
- 1. Hussain MM. Intestinal lipid absorption and lipoprotein formation. Curr Opin Lipidol 2014;25:200–6. 10.1097/MOL.0000000000000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shen W, Matsui T.. Intestinal absorption of small peptides: a review. Int J of Food Sci Tech 2019;54:1942–8. 10.1111/ijfs.14048 [DOI] [Google Scholar]
- 3. Miner-Williams WM, Stevens BR, Moughan PJ.. Are intact peptides absorbed from the healthy gut in the adult human? Nutr Res Rev 2014;27:308–29. 10.1017/S0954422414000225 [DOI] [PubMed] [Google Scholar]
- 4. Jerabek I, Boulenger JP, Bradwejn J. et al. CCK4-induced panic in healthy subjects II: neurochemical correlates. Eur Neuropsychopharmacol 1999;9:157–64. 10.1016/s0924-977x(98)00021-2 [DOI] [PubMed] [Google Scholar]
- 5. Adibi SA. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology 1997;113:332–40. 10.1016/s0016-5085(97)70112-4 [DOI] [PubMed] [Google Scholar]
- 6. Northrop JH. Plastein from pepsin and trypsin. J Gen Physiol 1947;30:377–8. 10.1085/jgp.30.5.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rohm F, Skurk T, Daniel H. et al. Appearance of di- and tripeptides in human plasma after a protein meal does not correlate with PEPT1 substrate selectivity. Mol Nutr Food Res 2019;63:e1801094. 10.1002/mnfr.201801094 [DOI] [PubMed] [Google Scholar]
- 8. Pierzynowski SG, Kruszewska D, Weström BW.. Chapter 3 The quality of dietary protein digestion affects animal performance and regulates gut bacteria growth: hypotheses and facts. In: Mosenthin R, Zentek J, Żebrowska T (eds), Biology of Growing Animals. Amsterdam, the Netherlands: Elsevier, 2006, 65–79. [Google Scholar]
- 9. Somaraju URR, Solis-Moya A.. Pancreatic enzyme replacement therapy for people with cystic fibrosis. Cochrane Database Syst Rev 2020;8:CD008227. 10.1002/14651858.CD008227.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Price HL, Gazzard BG, Dawson AM.. Steatorrhoea in the elderly. Br Med J 1977;1:1582–4. 10.1136/bmj.1.6076.1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fieker A, Philpott J, Armand M.. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin Exp Gastroenterol 2011;4:55–73. 10.2147/CEG.S17634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaskin KJ, Durie PR, Hill RE. et al. Colipase and maximally activated pancreatic lipase in normal subjects and patients with steatorrhea. J Clin Invest 1982;69:427–34. 10.1172/jci110466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kjeldahl J. A new method for the determination of nitrogen in organic matter Z. Anal Chem 1883;22:366–82. [Google Scholar]
- 14. Marcó A, Rubio R, Compañó R. et al. Comparison of the Kjeldahl method and a combustion method for total nitrogen determination in animal feed. Talanta 2002;357:1019–26. 10.1016/s0039-9140(02)00136-4 [DOI] [PubMed] [Google Scholar]
- 15. Friedman M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J Agric Food Chem 2004;52:385–406. 10.1021/jf030490p [DOI] [PubMed] [Google Scholar]
- 16. Ruhemann S. Cyclic di- and tri-ketones. J Chem Soc, Trans 1910;97:1438–99. 10.1039/CT9109701438 [DOI] [Google Scholar]
- 17. Raposo F. Evaluation of analytical calibration based on least-squares linear regression for instrumental techniques: a tutorial review. TrAC Trends in Analytical Chemistry 2016;77:167–85. 10.1016/j.trac.2015.12.006. [DOI] [Google Scholar]
- 18. Jurado JM, Alcázar A, Muñiz-Valencia R. et al. Some practical considerations for linearity assessment of calibration curves as function of concentration levels according to the fitness-for-purpose approach. Talanta 2017;172:221–9. 10.1016/j.talanta.2017.05.049 [DOI] [PubMed] [Google Scholar]
- 19. Lozinska L, Prykhodko O, Sureda EA. et al. Monitoring changes in plasma levels of pancreatic and intestinal enzymes in a model of pancreatic exocrine insufficiency—induced by pancreatic duct-ligation—in young pigs. Adv Med Sci 2015;60:112–7. 10.1016/j.advms.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 20. Pierzynowski SG, Weström BR, Karlsson BW. et al. Pancreatic cannulation of young pigs for long-term study of exocrine pancreatic function. Can J Anim Sci 1988;68:953–9. 10.4141/cjas88-105 [DOI] [Google Scholar]
- 21. Kiela P, Zabielski R, Podgurniak P. et al. Cholecystokinin-8 and vasoactive intestinal polypeptide stimulate exocrine pancreatic secretion via duodenally mediated mechanisms in the conscious pig. Exp Physiol 1996;81:375–84. 10.1113/expphysiol.1996.sp003942 [DOI] [PubMed] [Google Scholar]
- 22. Choi J, Sabikhi L, Hassan A. et al. Bioactive peptides in dairy products. Int J of Dairy Tech 2012;65:1–12. 10.1111/j.1471-0307.2011.00725.x [DOI] [Google Scholar]
- 23. Pellegrini A, Hulsmeier AJ, Hunziker P. et al. Proteolytic fragments of ovalbumin display antimicrobial activity. Biochim Biophys Acta 2004;1672:76–85. 10.1016/j.bbagen.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 24. Gilbert ER, Wong EA, Webb KE. Jr,. Board-invited review: peptide absorption and utilization: implications for animal nutrition and health. J Anim Sci 2008;86:2135–55. 10.2527/jas.2007-0826 [DOI] [PubMed] [Google Scholar]
- 25. Ozorio L, Mellinger-Silva C, Cabral LMC. et al. The influence of peptidases in intestinal brush border membranes on the absorption of oligopeptides from whey protein hydrolysate: an ex vivo study using an ussing chamber. Foods 2020;9:1415. 10.3390/foods9101415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adibi SA, Gray SJ, Menden E.. The kinetics of amino acid absorption and alteration of plasma composition of free amino acids after intestinal perfusion of amino acid mixtures. Am J Clin Nutr 1967;20:24–33. 10.1093/ajcn/20.1.24 [DOI] [PubMed] [Google Scholar]
- 27. Frame EG. The levels of individual free amino acids in the plasma of normal man at various intervals after a high-protein meal. J Clin Invest 1958;37:1710–23. 10.1172/JCI103763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hartmann S, Rydzewska G, Domínguez-Muñoz JE.. Kreon® (Creon®) vs. Lipancrea®: in vitro comparison of two encapsulated pancreatin preparations. Pharmaceuticals (Basel) 2022;15:1570. 10.3390/ph15121570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmed N, Corey M, Forstner G. et al. Molecular consequences of cystic fibrosis transmembrane regulator (CFTR) gene mutations in the exocrine pancreas. Gut 2003;52:1159–64. 10.1136/gut.52.8.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keller J, Layer P.. Human pancreatic exocrine response to nutrients in health and disease. Gut 2005;54:vi1–28. 10.1136/gut.2005.065946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seracu DI. The study of UV and VIS absorption spectra of the complexes of amino acids with ninhydrin. Analytical Letters 1987;20:1417–28. 10.1080/00032718708066323 [DOI] [Google Scholar]
- 32. Kurz A, Seifert J.. Factors influencing proteolysis and protein utilization in the intestine of pigs: a review. Animals (Basel) 2021;11:3551. 10.3390/ani11123551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kambhampati S, Li J, Evans BS. et al. Accurate and efficient amino acid analysis for protein quantification using hydrophilic interaction chromatography coupled tandem mass spectrometry. Plant Methods 2019;15:46. 10.1186/s13007-019-0430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bernard L, Chauveau B, Rémond D.. Effect of the methodology on peptide amino acid concentrations in blood and plasma of sheep. Arch Tierernahr 2001;54:281–96. 10.1080/17450390109381985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in supplementary data.