Abstract
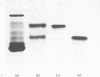
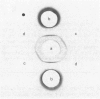
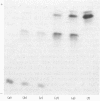
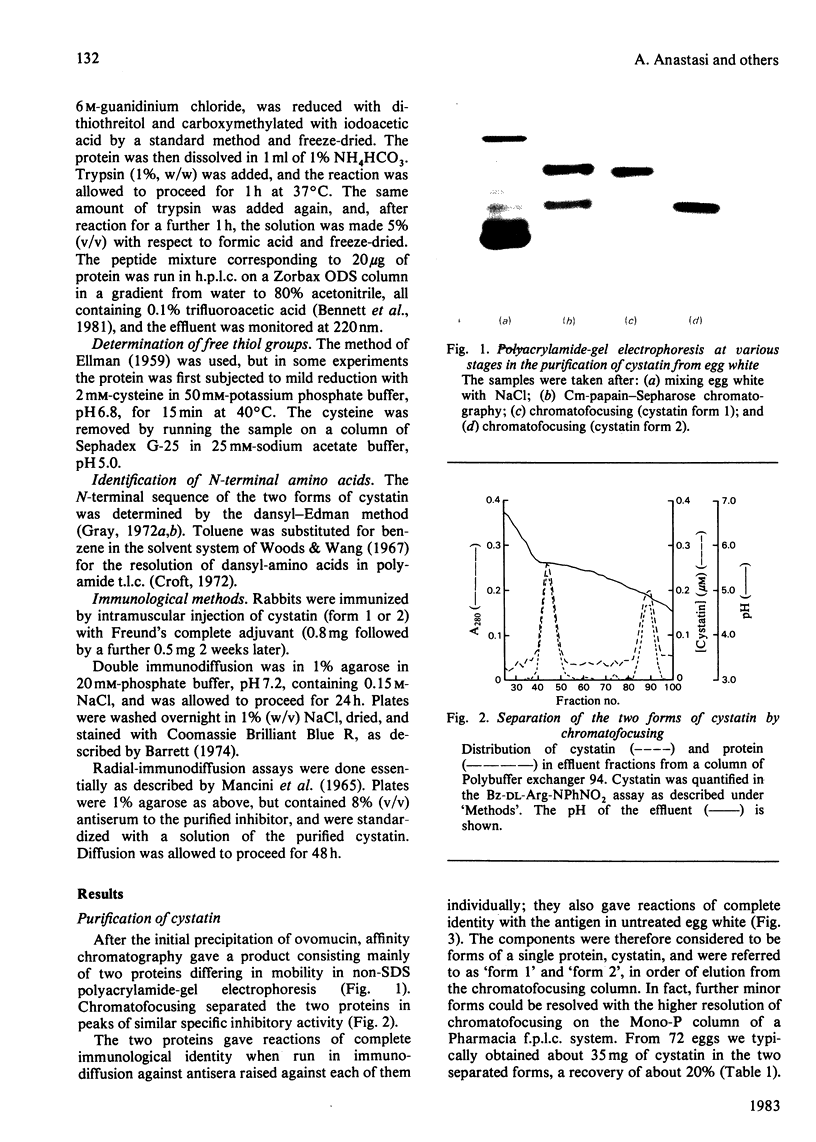
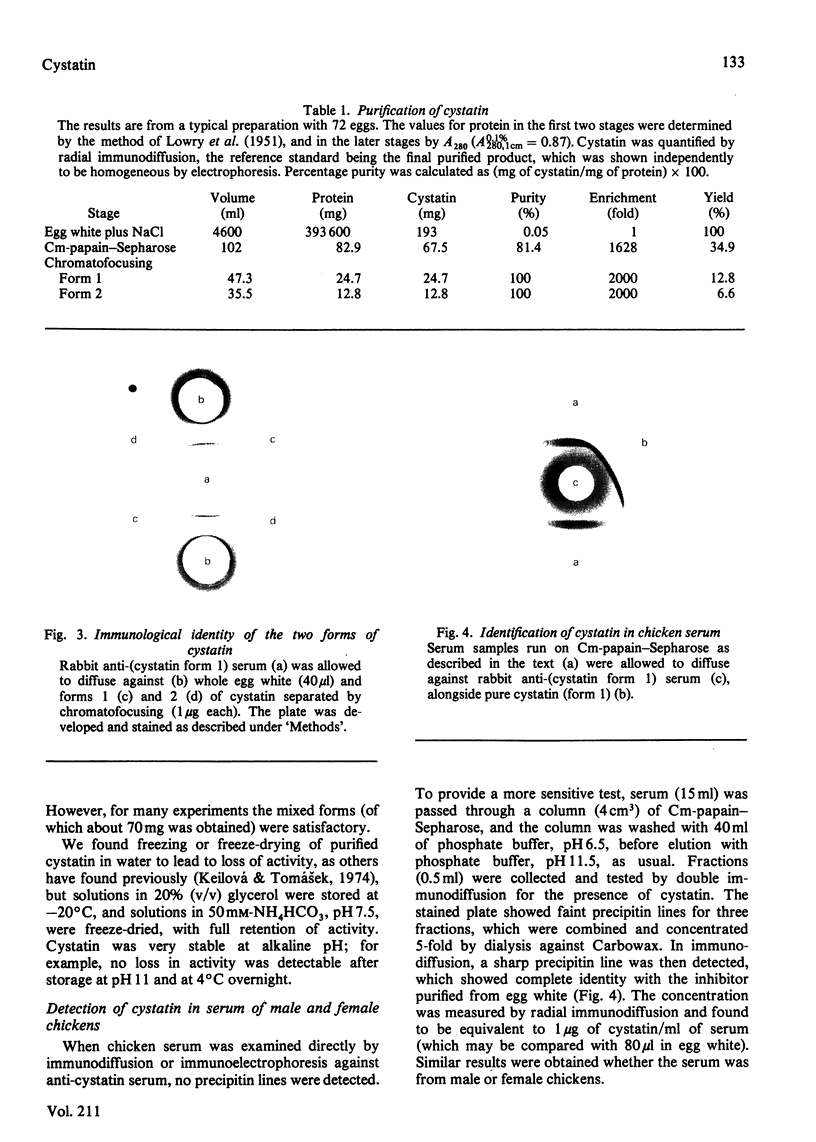
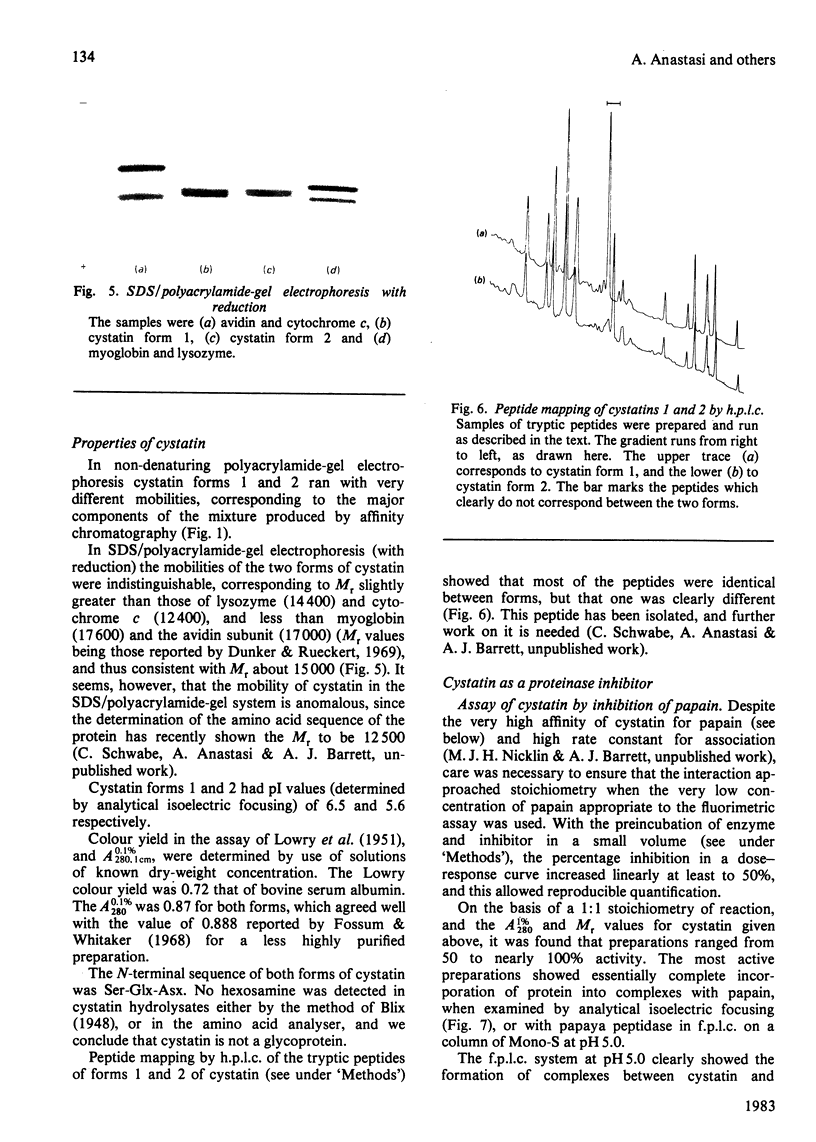
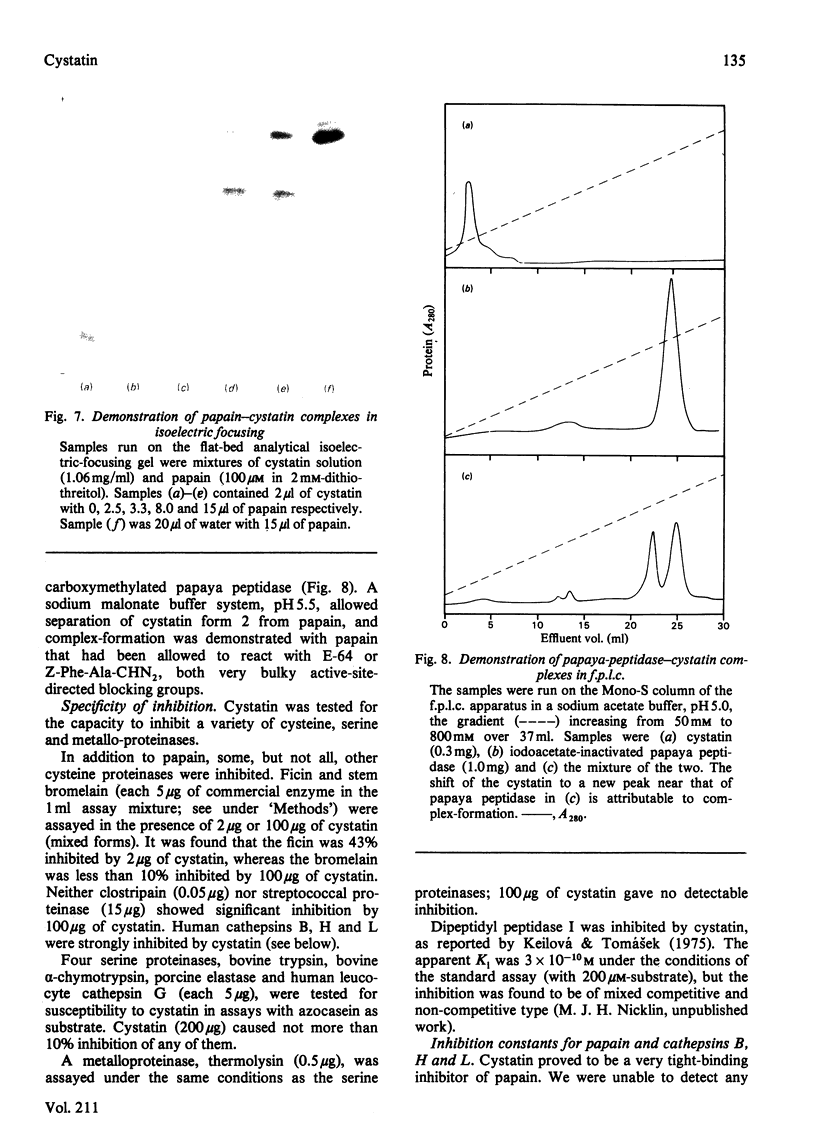
The protein from chicken egg white that inhibits cysteine proteinases, and has been named 'cystatin', was purified by ovomucin precipitation, affinity chromatography on carboxymethylpapain-Sepharose and chromatofocusing. The final purification step separated two major forms of the protein (pI 6.5 and 5.6), with a total recovery of about 20% from egg white. By use of affinity chromatography and immunodiffusion it was shown that the inhibitor is also present at low concentrations in the serum of male and female chickens. Tryptic peptide maps of the separated forms 1 and 2 of egg-white cystatin were closely similar, and each form had the N-terminal sequence Ser-Glx-Asx. The two forms showed complete immunological identity, and neither contained carbohydrate. Ki values for the inhibition of cysteine proteinases were as follows: papain (less than 1 X 10(-11)M), cathepsin B (8 X 10(-10)M), cathepsin H (about 2 X 10(-8)M) and cathepsin L (about 3 X 10(-12)M). Some other cysteine proteinases, and several non-cysteine proteinases, were found not to be significantly inhibited by cystatin. The inhibition of the exopeptidase dipeptidyl peptidase I by cystatin was confirmed and the Ki found to be 2 X 10(-10)M. Inhibitor complexes with active cysteine proteinases and the inactive derivatives formed by treatment with iodoacetate, E-64 [L-trans-epoxysuccinylleucylamido(4-guanidino)butane] and benzyloxycarbonylphenylalanylalanyldiazomethane were demonstrated by isoelectric focusing and cation-exchange chromatography. The complexes dissociated in sodium dodecyl sulphate/polyacrylamide-gel electrophoresis (with or without reduction) with no sign of fragmentation of the inhibitor. Cystatin was found not to contain a free thiol group, and there was no indication that disulphide exchange plays any part in the mechanism of inhibition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Ernback S. Chemical fixation of enzymes to cyanogen halide activated polysaccharide carriers. Eur J Biochem. 1971 Feb 1;18(3):351–360. doi: 10.1111/j.1432-1033.1971.tb01250.x. [DOI] [PubMed] [Google Scholar]
- Baines B. S., Brocklehurst K. A necessary modification to the preparation of papain from any high-quality latex of Carica papaya and evidence for the structural integrity of the enzyme produced by traditional methods. Biochem J. 1979 Feb 1;177(2):541–548. doi: 10.1042/bj1770541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Chicken alpha2-proteinase inhibitor: a serum protein homologous with ovoinhibitor of egg white. Biochim Biophys Acta. 1974 Nov 5;371(1):52–62. doi: 10.1016/0005-2795(74)90154-8. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Bennett H. P., Browne C. A., Solomon S. Purification of the two major forms of rat pituitary corticotropin using only reversed-phase liquid chromatography. Biochemistry. 1981 Aug 4;20(16):4530–4538. doi: 10.1021/bi00519a004. [DOI] [PubMed] [Google Scholar]
- Croft L. R. The amino acid sequence of -crystallin (fraction II) from calf lens. Biochem J. 1972 Jul;128(4):961–970. doi: 10.1042/bj1280961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fossum K., Whitaker J. R. Ficin and papain inhibitor from chicken egg white. Arch Biochem Biophys. 1968 Apr;125(1):367–375. doi: 10.1016/0003-9861(68)90672-3. [DOI] [PubMed] [Google Scholar]
- Henderson P. J. A linear equation that describes the steady-state kinetics of enzymes and subcellular particles interacting with tightly bound inhibitors. Biochem J. 1972 Apr;127(2):321–333. doi: 10.1042/bj1270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen M. Purification and properties of two protease inhibitors from rat skin inhibiting papain and other SH-proteases. Acta Chem Scand B. 1976;30(10):933–940. doi: 10.3891/acta.chem.scand.30b-0933. [DOI] [PubMed] [Google Scholar]
- Järvinen M. Purification and some characteristics of the human epidermal SH-protease inhibitor. J Invest Dermatol. 1978 Aug;71(2):114–118. doi: 10.1111/1523-1747.ep12546165. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Riemann S., Wiederanders B., Ansorge S., Bohley P. Lysosomal cysteine proteinases. Ciba Found Symp. 1979;(75):15–35. doi: 10.1002/9780470720585.ch2. [DOI] [PubMed] [Google Scholar]
- Kominami E., Wakamatsu N., Katunuma N. Endogenous thiol protease inhibitor from rat liver. Biochem Biophys Res Commun. 1981 Mar 31;99(2):568–575. doi: 10.1016/0006-291x(81)91783-6. [DOI] [PubMed] [Google Scholar]
- Kopitar M., Brzin J., Locnikar P., Turk V. Inhibitory mechanism of sericystatin, an intracellular proteinase inhibitor, reacting with cysteine proteinases. Hoppe Seylers Z Physiol Chem. 1981 Oct;362(10):1411–1414. [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- MATSUSHIMA I. An undescribed trypsin inhibitor in egg white. Science. 1958 May 16;127(3307):1178–1179. doi: 10.1126/science.127.3307.1178. [DOI] [PubMed] [Google Scholar]
- Macartney H. W., Tschesche H. Lantent collagenase from human polymorphonuclear leucocytes and activation to collagenase by removal of a inhibitor. FEBS Lett. 1980 Oct 6;119(2):327–332. doi: 10.1016/0014-5793(80)80282-1. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McDonald J. K., Zeitman B. B., Reilly T. J., Ellis S. New observations on the substrate specificity of cathepsin C (dipeptidyl aminopeptidase I). Including the degradation of beta-corticotropin and other peptide hormones. J Biol Chem. 1969 May 25;244(10):2693–2709. [PubMed] [Google Scholar]
- Robinson G. W. Isolation and characterization of papaya peptidase A from commercial chymopapain. Biochemistry. 1975 Aug 12;14(16):3695–3700. doi: 10.1021/bi00687a028. [DOI] [PubMed] [Google Scholar]
- Schwartz W. N., Barrett A. J. Human cathepsin H. Biochem J. 1980 Nov 1;191(2):487–497. doi: 10.1042/bj1910487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen L. C., Whitaker J. R. Some properties of a ficin-papain inhibitor from avian egg white. Arch Biochem Biophys. 1973 Oct;158(2):623–632. doi: 10.1016/0003-9861(73)90554-7. [DOI] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]