Abstract
Background and Purpose
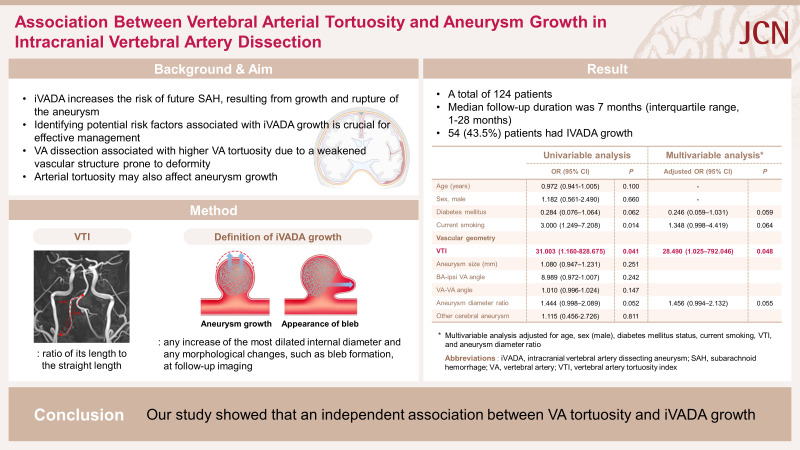
An intracranial vertebral artery dissecting aneurysm (iVADA) increases the risk of future subarachnoid hemorrhage, which is a severe complication with high rebleeding rates and poor outcomes. Identifying potential risk factors associated with iVADA growth is crucial for their effective management.
Methods
This observational study was carried out at a single center and included patients who had been diagnosed with iVADA based on neuroimaging findings. We divided the patients into two groups: with and without iVADA growth. Growth was defined as any enlargement of a dilated region or a morphological change in follow-up imaging. We measured the vertebral artery tortuosity index (VTI) in the contralateral vertebral artery (VA), defined as its actual length divided by its straight length. We investigated the factors associated with iVADA growth.
Results
This study included 124 patients. The median follow-up period was 7 months. We observed iVADA growth in 54 patients (43.5%), who were more likely to be current smokers (33.3% vs. 14.3%, p=0.012) and have a higher VTI (1.14±0.11 [mean±standard deviation] vs. 1.06±0.12, p=0.035) compared with those without iVADA growth. A multivariate analysis revealed that the VTI (adjusted odds ratio=28.490, 95% confidence interval=1.025–792.046, p=0.048) was independently associated with iVADA growth.
Conclusions
This study has identified an independent association between VA tortuosity and iVADA growth.
Keywords: dissecting vertebral artery aneurysm, arterial tortuosity, aneurysm growth
Graphical Abstract
INTRODUCTION
An intracranial vertebral artery dissecting aneurysm (iVADA) can manifest in various ways, ranging from incidental findings to symptoms such as headaches, signs of brainstem compression, and ischemic strokes.1 iVADA generally has a favorable prognosis.2 However, the devastating complication of subarachnoid hemorrhage (SAH) can result from the growth and rupture of iVADA.3,4
iVADA is becoming easier to detect using various imaging modalities, which is facilitating interventions before rupture occurs.5 Factors such as the size, neck/height ratio, and coexistence of arterial stenosis adjacent to an iVADA have been linked to the risk of rupture.6 We previously demonstrated an association between higher vertebral artery (VA) tortuosity and the occurrence of dissection or intracranial aneurysms, which can be attributed to a weakened vascular structure that can lead to increased tortuosity and dissection.7
Arterial tortuosity can also affect the growth of an aneurysm.8 Although dissecting aneurysms have different pathophysiological factors, the growth rates of dissecting and intracranial aneurysms may be influenced by similar factors, since they can share a common condition of fragmentation or a lack of internal elastic lamina.9,10 This study aimed to identify the factors associated with iVADA growth, including consideration of the VA tortuosity.
METHODS
Participants
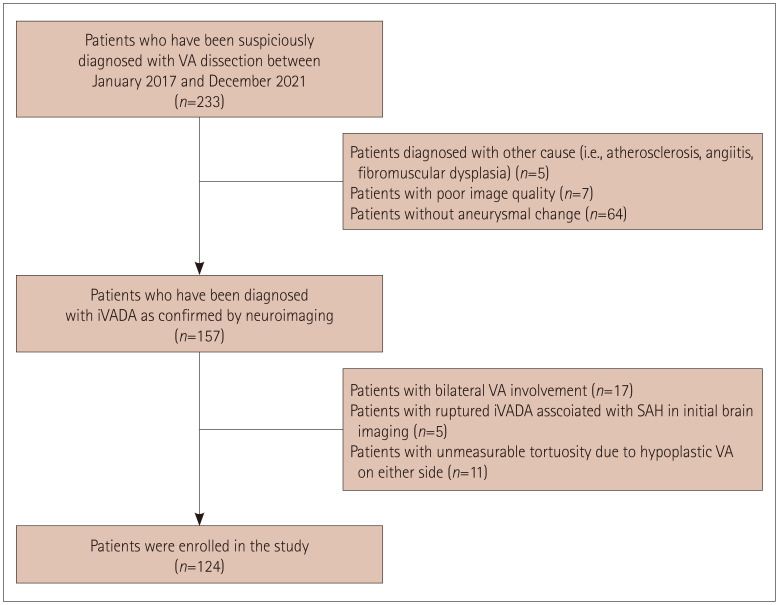
This observational study was conducted at Asan Medical Center and involved patients diagnosed with iVADA between January 2017 and December 2021. iVADA was diagnosed based on neurovascular imaging findings, including from digital-subtraction angiography (DSA), magnetic resonance angiography (MRA), computed tomography angiography (CTA), and/or high-resolution magnetic resonance imaging (HR-MRI). These imaging modalities revealed 1) fusiform or saccular aneurysmal dilatation at the distal VA, and 2) intramural hematoma, intimal flap, double lumen sign, pearl-and-string sign, or their combination.
We excluded patients with 1) poor image quality, 2) bilateral VA involvement, 3) ruptured iVADA associated with SAH as diagnosed in initial brain imaging, 4) any hypoplastic VA on either side for which tortuosity was unmeasurable, or 5) laboratory or radiographic findings indicating angiitis, fibromuscular dysplasia, or any other causes (e.g., atherosclerosis) (Fig. 1).
Fig. 1. Study flowchart. iVADA, intracranial vertebral artery dissecting aneurysm; SAH, subarachnoid hemorrhage; VA, vertebral artery.
We obtained demographic data and risk factors by reviewing the patients’ medical records and a stroke registry database. We assessed the presence of vascular risk factors, comorbidities, family history of stroke, and medication use at the time of the iVADA diagnosis. The study was approved by the Institutional Review Board of Asan Medical Center (IRB number: 2021-1782). The need to obtained informed consents from patients was waived due to the retrospective design of the study.
Measuring geometric parameters
MRI and MRA were performed using a 3.0-T Philips scanner (Philips Healthcare, Eindhoven, The Netherlands), while CTA and DSA were performed using a Siemens scanner (Siemens Healthneers, Erlangen, Germany). All morphological parameters were evaluated on two-dimensional (2D) angiographic images obtained from reconstructed coronal sections perpendicular to the anterior-posterior commissure plane.11
We determined the size of each dissecting aneurysm by measuring its most-dilated internal diameter.12,13 The diameter of the basilar artery (BA) was measured 1 mm above the vertebrobasilar junction. We also measured the angle between the BA and the ipsilateral VA as well as the angle between the contralateral VA and VAs.14 The aneurysm diameter ratio was calculated by dividing the most-dilated diameter by the vessel diameter at a point proximal to the dilatation where its diameter was normal. We considered a VA to be dominant if it had a larger diameter (a side-to-side diameter difference of ≥0.3 mm) or directly connected to the BA when the two VAs had comparable diameters.15
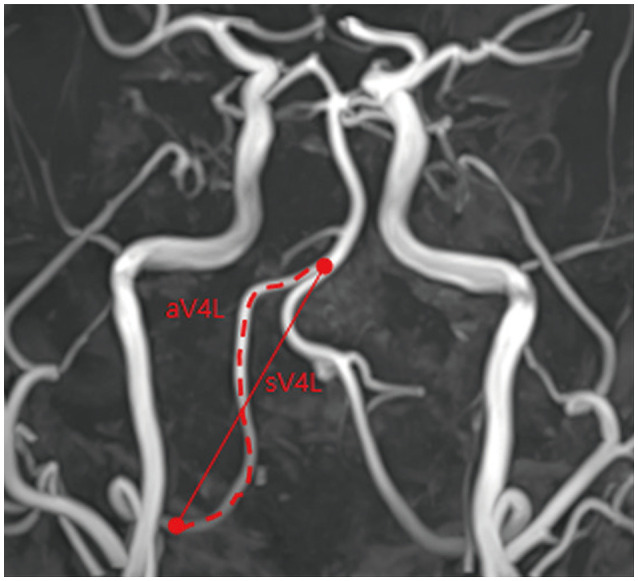
We determined the vertebral artery tortuosity index (VTI) by calculating the ratio of its actual length to its straight length. The VA actual length was determined by tracing the vessel’s course from the union of both VAs to the dura mater, while it straight length was determined by measuring the linear distance from the origin to the end of its V4 portion (Fig. 2).14,16 We measured the VTI from the contralateral VA, since the ipsilateral VA might have been deformed after the dissection, and in several patients the ipsilateral VA was not traceable.
Fig. 2. Measurement of the VTI. If the actual length of the VA is aV4L and its straight length is sV4L, then VTI is calculated as aV4L/sV4L. VA, vertebral artery; VTI, vertebral artery tortuosity index.
Neuroimaging follow-up and aneurysm growth
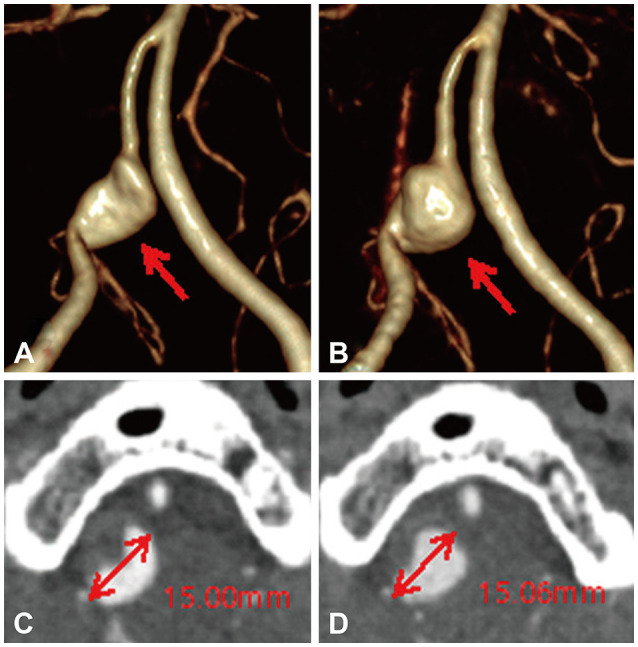
Follow-up evaluations of iVADA growth were conducted in each patient using the same modalities (DSA, CTA, and/or MRA) utilized in the initial examination. The follow-up period was determined by the physician. iVADA growth was defined as any increase in the most-dilated internal diameter and any morphological changes such as bleb formation detected in the follow-up imaging (Fig. 3).17,18 Two experienced researchers who were blinded to the patients’ clinical information independently interpreted the images and reached consensus decisions.
Fig. 3. Image of growth in the right iVADA. A: At the initial presentation, three-dimensional CTA demonstrated the pearl-and-string sign with dilatation (arrow) of the right VA where it branched from the posterior inferior cerebellar artery. B: 1-Year follow-up CTA showed an enlarged aneurysm sac and changes in the morphology of the right iVADA. C and D: Images showing the diameter of the dissecting aneurysm at the initial presentation and at the 1-year follow-up in axial CTA. CTA, computed tomography angiography; iVADA, intracranial vertebral artery dissecting aneurysm; VA, vertebral artery.
Statistical analyses
We compared the characteristics of patients between those with and without iVADA growth, using chi-squared or Fisher’s exact tests for categorical variables and Student’s t-tests or Mann–Whitney U-tests for continuous variables. Univariate and multivariate logistic regression models were analyzed to identify the factors associated with iVADA growth. In the multivariable logistic regression we included age, sex, and variables with p values of <0.1 in the univariate analyses. The optimal predictor cut points for the VTI were determined by constructing receiver operating characteristic (ROC) curves. We used SPSS software (version 21.0, IBM Corp., Armonk, NY, USA) for the statistical analyses, and a p value of <0.05 was considered indicative of statistical significance.
RESULTS
This study included 124 patients aged 54±12 years (mean±standard deviation), of whom 64.2% were male. The median follow-up period was 7 months (interquartile range= 1–28 months).
iVADA growth occurred in 54 (43.5%) patients, with 2 showing morphological changes and 52 having an enlarged dilated region. There were no significant differences between those with and without iVADA growth in vascular risk factors or symptoms, except for the prevalence of current smoking being higher in patients with iVADA growth (33.3% vs. 14.3%, p=0.012) (Table 1). The only significant intergroup difference in geometric parameters was for VTI, which was higher in those with iVADA growth (1.14±0.11 vs. 1.06±0.12, p=0.035) (Table 2).
Table 1. Baseline characteristics of patients with and without iVADA growth.
| Characteristics | Growth (n=54) | No growth (n=70) | p | |
|---|---|---|---|---|
| Age (yr) | 54±11 | 57±11 | 0.097 | |
| Sex, male | 36 (66.7) | 44 (62.9) | 0.660 | |
| Follow-up period (months) | 2 [0–8] | 13 [3–36] | 0.709 | |
| Risk factors | ||||
| Hypertension | 31 (57.4) | 43 (61.4) | 0.651 | |
| Diabetes mellitus | 3 (5.6) | 12 (17.1) | 0.050 | |
| Hyperlipidemia | 14 (25.9) | 16 (22.9) | 0.692 | |
| Coronary artery disease | 2 (3.7) | 6 (8.6) | 0.464 | |
| Current smoking | 18 (33.3) | 10 (14.3) | 0.012 | |
| Initial blood pressure | ||||
| SBP (mm Hg) | 140.30±17.01 | 139.98±21.39 | 0.924 | |
| DBP (mm Hg) | 86.15±10.24 | 86.79±16.62 | 0.804 | |
| Family history | 14 (25.9) | 11 (15.7) | 0.160 | |
| Previous stroke history | 4 (7.4) | 1 (1.4) | 0.166 | |
| Medication | ||||
| Antiplatelets | 16 (29.6) | 13 (18.6) | 0.149 | |
| Anticoagulants | 1 (1.9) | 2 (2.9) | 0.999 | |
| Antihypertensives | 16 (29.6) | 28 (40.0) | 0.231 | |
| Antidiabetics | 3 (5.6) | 7 (10.0) | 0.511 | |
| Antihyperlipidemics | 16 (29.6) | 16 (22.9) | 0.393 | |
| Ischemic stroke | 7 (12.7) | 4 (5.6) | 0.208 | |
| TIA | 1 (1.8) | 1 (1.4) | 0.999 | |
| Symptom | 0.564 | |||
| Headache | 31 (58.5) | 40 (57.1) | ||
| Dizziness | 9 (16.6) | 6 (8.6) | ||
| Others | 4 (7.5) | 9 (12.9) | ||
| Asymptomatic | 10 (18.9) | 15 (21.4) | ||
Data are n (%), mean±standard-deviation, or median [interquartile range] values.
DBP, diastolic blood pressure; iVADA, intracranial vertebral artery dissecting aneurysm; SBP, systolic blood pressure; TIA, transient ischemic attack.
Table 2. Vascular geometry in patients with and without iVADA growth.
| Geometric characteristic | Growth (n=54) | No growth (n=70) | p |
|---|---|---|---|
| VTI | 1.14±0.11 | 1.06±0.12 | 0.035 |
| Size of aneurysm (mm) | 6.88±3.00 | 6.24±2.79 | 0.248 |
| BA–ipsiVA angle (degrees) | 145.56±22.56 | 150.18±19.54 | 0.243 |
| VA–VA angle (degrees) | 57.03±28.02 | 49.62±26.08 | 0.144 |
| Aneurysm ratio | 2.83±1.32 | 2.42±0.83 | 0.054 |
| BA diameter (mm) | 3.10±0.91 | 3.16±0.65 | 0.677 |
| VA diameter ratio | 1.48±0.39 | 1.54±0.58 | 0.533 |
| Ipsilateral dominant | 24 (44.4) | 33 (47.1) | 0.765 |
| Other cerebral aneurysm | 11 (20.0) | 13 (18.3) | 0.397 |
Data are n (%) or mean±standard-deviation values. The VA diameter ratio was calculated by dividing the diameter at the initiation of the affected V4 segment by the diameter at the termination of the affected V4 segment. Other cerebral aneurysm refers to an aneurysm located in any intracranial and/or extracranial artery other than the ipsilateral or contralateral distal VA.
BA, basilar artery; ipsiVA, ipsilateral VA; iVADA, intracranial vertebral artery dissecting aneurysm; VA, vertebral artery; VTI, vertebral artery tortuosity index.
Factors associated with iVADA growth
Current smoking (odds ratio [OR]=3.000, 95% confidence interval [CI]=1.249–7.208, p=0.014) and the VTI (OR=31.003, 95% CI=1.160–828.675, p=0.041) were associated with iVADA growth. In the multivariate analysis, VTI remained significantly associated with growth after adjusting for age, sex, diabetes mellitus, and current smoking (adjusted OR=28.490, 95% CI=1.025–792.046, p=0.048) (Table 3). ROC curve analysis showed that VTI had predictive value for iVADA growth, with an area under the ROC curve of 0.633 (95% CI=0.529–0.738, p=0.015). The optimal cutoff value for VTI was 1.065, which yielded sensitivity and specificity values for predicting iVADA growth of 63.0% and 61.8%, respectively (Supplementary Table 1 and Supplementary Fig. 1 in the online-only Data Supplement).
Table 3. Factors associated with iVADA growth.
| Univariate analysis | Multivariate analysis* | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p | Adjusted OR (95% CI) | p | ||
| Age | 0.972 (0.941–1.005) | 0.100 | - | ||
| Sex, male | 1.182 (0.561–2.490) | 0.660 | - | ||
| Risk factors | |||||
| Hypertension | 0.674 (0.328–1.385) | 0.283 | |||
| Diabetes mellitus | 0.284 (0.076–1.064) | 0.062 | 0.246 (0.059–1.031) | 0.059 | |
| Current smoking | 3.000 (1.249–7.208) | 0.014 | 1.348 (0.998–4.419) | 0.064 | |
| Family history | 1.877 (0.774–4.553) | 0.163 | |||
| Previous stroke history | 5.520 (0.599–50.892) | 0.132 | |||
| Medication | |||||
| Antiplatelets | 1.846 (0.798–4.272) | 0.152 | |||
| Anticoagulants | 0.639 (0.056–7.233) | 0.717 | |||
| Antihypertensives | 0.687 (0.326–1.446) | 0.323 | |||
| Antidiabetics | 0.527 (0.130–2.141) | 0.371 | |||
| Antihyperlipidemics | 1.410 (0.630–3.155) | 0.403 | |||
| Vascular geometry | |||||
| VTI | 31.003 (1.160–828.675) | 0.041 | 28.490 (1.025–792.046) | 0.048 | |
| Size of aneurysm | 1.080 (0.947–1.231) | 0.251 | |||
| BA–ipsiVA angle | 8.989 (0.972–1.007) | 0.242 | |||
| VA–VA angle | 1.010 (0.996–1.024) | 0.147 | |||
| Aneurysm diameter ratio | 1.444 (0.998–2.089) | 0.052 | 1.456 (0.994–2.132) | 0.055 | |
| Other cerebral aneurysm | 1.115 (0.456–2.726) | 0.811 | |||
*Multivariate analysis with adjustment for age, sex, diabetes mellitus, current smoking, VTI, and aneurysm diameter ratio.
BA, basilar artery; CI, confidence interval; ipsiVA, ipsilateral VA; iVADA, intracranial vertebral artery dissecting aneurysm; OR, odds ratio; VA, vertebral artery; VTI, vertebral artery tortuosity index.
DISCUSSION
iVADA growth was present in 43.5% of the patients in this study. These patients were more likely to be current smokers and had a higher VTI compared with those without iVADA growth. VTI was independently associated with iVADA growth.
iVADA growth has been attributed to repeated hemodynamic injuries to the aneurysm wall. Elastin is a protein that is important for the maintenance of mechanical tension, and is functionally impaired in arterial walls with aneurysm. These walls progressively weaken due to turbulent blood flow in the aneurysm sac, which makes the wall less able to resist pulsatile stresses, resulting in aneurysm growth.19 A recent study demonstrated that preserved endothelial function, as measured by flow-mediated dilatation, is associated with a reduction in the iVADA size, whereas aneurysmal enlargement is more common in individuals with a lower arterial stiffness.20
Weakening of the arterial connective tissue manifests as an increased vascular tortuosity of the cerebral arteries. For example, a mutation in the transforming growth factor β receptor activates signaling cascades in the vessel wall, resulting in selective deterioration of the extracellular matrix. This ultimately leads to aneurysm formation and high vascular tortuosity.21,22,23 We previously found VA dissection to be associated with higher VA tortuosity due to a weakened vascular structure being prone to deformity.7 Another study showed that high tortuosity of the coronary artery disrupts laminar blood flow, increasing the artery wall shear stress (WSS) and weakening the artery. This may lead to spontaneous coronary artery dissection.24
Arterial tortuosity can also affect the growth of an aneurysm. A previous study showed that the high vessel tortuosity can change the local hemodynamics, such as the WSS and flow angle, which impacts aneurysm growth.8 However, we found that iVADA growth was associated with high VA tortuosity on the contralateral side. The ipsilateral VA might have shown morphological changes following dissection. Most previous studies have found temporal changes in unruptured VA dissection in follow-up imaging.25 Nevertheless, contralateral tortuosity can be represented by uncharacterized underlying systemic factors that weaken the arterial wall structure. Also, a previous study found that the contralateral tortuosity was similar to the ipsilateral tortuosity, which indicates that the contralateral tortuosity is particularly useful since ipsilateral measurements are often rendered infeasible due to various causes.26
The present study had several limitations. Firstly, it was conducted at a single center with relatively few patients. Secondly, the vascular geometry data relied on 2D images derived from reconstructed three-dimensional angiographic images. However, measuring tortuosity in the lateral dimensions seems highly feasible. Thirdly, although we suggest that an impaired hemodynamic status is associated with iVADA growth, this could not be assessed in the present retrospective study. Also, we could not make adjustments for all possible confounding factors such as the initial and follow-up systolic and diastolic blood pressures due to only a small number of patients being evaluated. Fourthly, the prevalence of iVADA in this study (43.5%) was higher than in previous studies, which was probably due to many patients being referred to our tertiary hospital for a second consultation.27,28,29,30 It is known that iVADA aggravation often progresses within 1 month. In our study, most patients were followed up regardless of symptoms. Finally, although we investigated the family history, we did not perform genetic testing, and so the influence of genetics on the present results cannot be excluded.
Notwithstanding these limitations, VA tortuosity was found to be independently associated with iVADA growth, which is in turn associated with severe complications such as high rebleeding rates and poor outcomes. Therefore, patients exhibiting iVADA with high VA tortuosity should be carefully managed in order to prevent further growth.
Footnotes
- Conceptualization: Sang Hee Ha, Bum Joon Kim, Jae Young Park.
- Data curation: Sang Hee Ha, Jae Young Park.
- Formal analysis: Sang Hee Ha, Jae Young Park.
- Funding acquisition: Bum Joon Kim.
- Investigation: Bum Joon Kim, Soo Jeong, Jun Young Chang, Dong-Wha Kang, Sun U. Kwon.
- Methodology: Sang Hee Ha, Jae Young Park, Soo Jeong, Jun Young Chang, Dong-Wha Kang, Sun U. Kwon.
- Supervision: Bum Joon Kim.
- Writing—original draft: Sang Hee Ha, Jae Young Park.
- Writing—review & editing: Bum Joon Kim.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: This research was supported by the Brain Convergence Research Program of the National Research Foundation (NRF), funded by the Korean government (MSIT) (No. 2020M3E5D2A01084576), and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1A2C2100077).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2024.0139.
Cutoff value of vascular geometric factors influencing iVADA growth
ROC curve for prediction of growth of iVADA vascular geometric factors. ROC curve for prediction of growth of iVADA by VTI (blue), aneurysm diameter (green), BA-ipsiVA angle (ivoy), VA-VA angle (purple) and aneurysm ratio (yellow). Results are presented as an odds ratio and 95% CIs. BA, basilar artery; CI, confidence interval; ipsiVA, ipsilateral VA; iVADA, intracranial vertebral artery dissecting aneurysm; ROC, receiver operating characteristic; VA, vertebral artery; VTI, vertebral artery tortuosity index.
References
- 1.Hosoya T, Adachi M, Yamaguchi K, Haku T, Kayama T, Kato T. Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke. 1999;30:1083–1090. doi: 10.1161/01.str.30.5.1083. [DOI] [PubMed] [Google Scholar]
- 2.Park KW, Park JS, Hwang SC, Im SB, Shin WH, Kim BT. Vertebral artery dissection: natural history, clinical features and therapeutic considerations. J Korean Neurosurg Soc. 2008;44:109–115. doi: 10.3340/jkns.2008.44.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo J, Liu F, Zhao L, Cheng B, Hu Y, Wang X. Endovascular treatment of intracranial vertebral artery dissecting aneurysm, a case series study with two years follow up on complications. Heliyon. 2023;9:e15568. doi: 10.1016/j.heliyon.2023.e15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali MS, Amenta PS, Starke RM, Jabbour PM, Gonzalez LF, Tjoumakaris SI, et al. Intracranial vertebral artery dissections: evolving perspectives. Interv Neuroradiol. 2012;18:469–483. doi: 10.1177/159101991201800414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Z, Tian X, Tian B, Meddings Z, Zhang X, Li J, et al. Identification of high risk clinical and imaging features for intracranial artery dissection using high-resolution cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23:74. doi: 10.1186/s12968-021-00766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park GJ, Cho JH, Kim KH. Angiographic characteristics of ruptured versus unruptured vertebral artery dissecting aneurysm. J Cerebrovasc Endovasc Neurosurg. 2022;24:10–15. doi: 10.7461/jcen.2021.E2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim BJ, Yang E, Kim NY, Kim MJ, Kang DW, Kwon SU, et al. Vascular tortuosity may be associated with cervical artery dissection. Stroke. 2016;47:2548–2552. doi: 10.1161/STROKEAHA.116.013736. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Song HN, Lee JE, Kim YC, Baek IY, Kim YS, et al. How cerebral vessel tortuosity affects development and recurrence of aneurysm: outer curvature versus bifurcation type. J Stroke. 2021;23:213–222. doi: 10.5853/jos.2020.04399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung KH. New pathophysiological considerations on cerebral aneurysms. Neurointervention. 2018;13:73–83. doi: 10.5469/neuroint.2018.01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32:1659–1676. doi: 10.1038/jcbfm.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha SH, Ryu JC, Bae JH, Koo S, Chang JY, Kang DW, et al. Factors associated with two different stroke mechanisms in perforator infarctions regarding the shape of arteries. Sci Rep. 2022;12:16752. doi: 10.1038/s41598-022-21329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SH, Kim SH, Jang JH, Kim YZ, Kim KH, Nam TM. Diffusion-weighted imaging-positive lesions following endovascular treatment for ruptured and unruptured aneurysms: its incidence according to antithrombotic drugs. J Cerebrovasc Endovasc Neurosurg. 2022;24:249–256. doi: 10.7461/jcen.2022.E2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho DY, Kim BS, Choi JH, Park YK, Shin YS. The fate of unruptured intracranial vertebrobasilar dissecting aneurysm with brain stem compression according to different treatment modalities. AJNR Am J Neuroradiol. 2019;40:1924–1931. doi: 10.3174/ajnr.A6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omotoso BR, Harrichandparsad R, Satyapal KS, Moodley IG, Lazarus L. Radiological anatomy of the intracranial vertebral artery in a select South African cohort of patients. Sci Rep. 2021;11:12138. doi: 10.1038/s41598-021-91744-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J, Chen J, Tong X, Han M, Peng F, Niu H, et al. Morphological characteristics associated with ruptured intracranial vertebral artery dissecting aneurysms. J Neurointerv Surg. 2023;15:321–324. doi: 10.1136/neurintsurg-2022-018744. [DOI] [PubMed] [Google Scholar]
- 16.Shang K, Chen X, Cheng C, Luo X, Xu S, Wang W, et al. Arterial tortuosity and its correlation with white matter hyperintensities in acute ischemic stroke. Neural Plast. 2022;2022:4280410. doi: 10.1155/2022/4280410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinjikji W, Zhu YQ, Lanzino G, Cloft HJ, Murad MH, Wang Z, et al. Risk factors for growth of intracranial aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2016;37:615–620. doi: 10.3174/ajnr.A4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng JC, Wang J, Li H, Jiao YM, Fu WL, Huo R, et al. Aspirin and growth of small unruptured intracranial aneurysm: results of a prospective cohort study. Stroke. 2020;51:3045–3054. doi: 10.1161/STROKEAHA.120.029967. [DOI] [PubMed] [Google Scholar]
- 19.Sforza DM, Putman CM, Cebral JR. Hemodynamics of cerebral aneurysms. Annu Rev Fluid Mech. 2009;41:91–107. doi: 10.1146/annurev.fluid.40.111406.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SJ, Lee JS, Kim M, Park SY, Park JH, Park B, et al. Influence of endothelial function and arterial stiffness on the behavior of cervicocephalic arterial dissections: an observational study. Front Neurol. 2022;13:968488. doi: 10.3389/fneur.2022.968488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebas H, Boutigny A, Maupu C, Salfati J, Orset C, Mazighi M, et al. Imaging cerebral arteries tortuosity and velocities by transcranial Doppler ultrasound is a reliable assessment of brain aneurysm in mouse models. Stroke Vasc Interv Neurol. 2023;3:e000476. doi: 10.1161/SVIN.122.000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debette S. Pathophysiology and risk factors of cervical artery dissection: what have we learnt from large hospital-based cohorts? Curr Opin Neurol. 2014;27:20–28. doi: 10.1097/WCO.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 23.Ciurică S, Lopez-Sublet M, Loeys BL, Radhouani I, Natarajan N, Vikkula M, et al. Arterial tortuosity. Hypertension. 2019;73:951–960. doi: 10.1161/HYPERTENSIONAHA.118.11647. [DOI] [PubMed] [Google Scholar]
- 24.Buradi A, Mahalingam A. Impact of coronary tortuosity on the artery hemodynamics. Biocybern Biomed Eng. 2020;40:126–147. [Google Scholar]
- 25.Kuwabara M, Sakamoto S, Okazaki T, Mitsuhara T, Ishii D, Shimonaga K, et al. Natural history of acute unruptured vertebral basilar artery dissection: temporal changes in imaging findings and contributory factors. Clin Neurol Neurosurg. 2022;222:107450. doi: 10.1016/j.clineuro.2022.107450. [DOI] [PubMed] [Google Scholar]
- 26.Hoshino T, Sato S, Kushi K, Tanaka Y, Mochizuki T, Ishikawa T, et al. Tortuosity of middle cerebral artery M1 segment and outcomes after mechanical thrombectomy. Interv Neuroradiol. 2024;30:154–162. doi: 10.1177/15910199221104922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kai Y, Nishi T, Watanabe M, Morioka M, Hirano T, Yano S, et al. Strategy for treating unruptured vertebral artery dissecting aneurysms. Neurosurgery. 2011;69:1085–1091. doi: 10.1227/NEU.0b013e3182262adf. discussion 1091-1092. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi N, Murayama Y, Yuki I, Ishibashi T, Ebara M, Arakawa H, et al. Natural course of dissecting vertebrobasilar artery aneurysms without stroke. AJNR Am J Neuroradiol. 2014;35:1371–1375. doi: 10.3174/ajnr.A3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn SS, Kim BM, Suh SH, Kim DJ, Kim DI, Shin YS, et al. Spontaneous symptomatic intracranial vertebrobasilar dissection: initial and follow-up imaging findings. Radiology. 2012;264:196–202. doi: 10.1148/radiol.12112331. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa K, Touho H, Morisako T, Osaka Y, Tatsuzawa K, Nakae H, et al. Long-term follow-up study of unruptured vertebral artery dissection: clinical outcomes and serial angiographic findings. J Neurosurg. 2000;93:19–25. doi: 10.3171/jns.2000.93.1.0019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cutoff value of vascular geometric factors influencing iVADA growth
ROC curve for prediction of growth of iVADA vascular geometric factors. ROC curve for prediction of growth of iVADA by VTI (blue), aneurysm diameter (green), BA-ipsiVA angle (ivoy), VA-VA angle (purple) and aneurysm ratio (yellow). Results are presented as an odds ratio and 95% CIs. BA, basilar artery; CI, confidence interval; ipsiVA, ipsilateral VA; iVADA, intracranial vertebral artery dissecting aneurysm; ROC, receiver operating characteristic; VA, vertebral artery; VTI, vertebral artery tortuosity index.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.