Abstract
Complications that occur during cancer therapy have emerged as a major contributor to the poor quality of life experienced by cancer patients as they live longer due to improved treatments. Many studies have investigated chemotherapy-induced peripheral neuropathy, but few have investigated the autonomic nervous system. Cardiovascular autonomic dysfunction (CAD) contributes to the distressing symptoms experienced by cancer patients, and it is also related to poor treatment outcomes. CAD has a multifactorial etiology in patients with cancer: it can be caused by the cancer itself, chemotherapy or radiation therapy, or other comorbidities. Its symptoms are nonspecific, and they include orthostatic hypotension, resting tachycardia, dizziness, chest tightness, and exertional dyspnea. It is important to suspect CAD and perform therapeutic interventions in a clinical context, because a patient who is more frail is less like to endure the treatment process. The quality of life of patients receiving active cancer treatments can be improved by evaluating the risk of CAD before and after chemotherapy, and combining both nonpharmacological and pharmacological management. Here we review the prevalence, pathogenesis, diagnosis, and treatment of CAD, which is the most common and a sometimes serious symptom in cancer patients.
Keywords: dysautonomia, cancer, chemotherapy, paraneoplastic syndromes
Graphical Abstract
INTRODUCTION
Chemotherapy-induced peripheral neuropathy (CIPN) is the most common neurological complication of chemotherapy, and its impact on the quality of life of cancer patients has received considerable attention. In contrast, cardiovascular autonomic dysfunction (CAD) has not received much attention despite its high prevalence in cancer patients.1 Studying the effects of CAD on patients with cancer is just as important as studying the effects of CIPN, because CAD can directly affect mortality.2 The increasing lifespans of cancer patients is making it more important to identify, prevent, and treat complications of CAD early.3
The mechanisms underlying CAD are more complicated than those of CIPN, since both cancer itself and cancer treatment can affect the cardiovascular system and autonomic neurons of the sympathetic and parasympathetic nervous systems.3,4,5 Cardiovascular changes caused by cancer and preexisting cardiovascular abnormalities may all contribute to CAD.3 Also, tumors secrete inflammatory cytokines that damage microvascular endothelia and mitochondria, leading to the accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that in turn can cause neuronal damage in the cardiovascular system.6 In addition to these direct mechanisms, depression, psychological stress, anxiety, and physical inactivity can exacerbate CAD.
Because CAD has been reported to cause syncope and other symptoms and be detrimental to patient survival,7 suspecting and diagnosing CAD can improve the quality of life and reduce morbidity and mortality. CAD can be diagnosed through history-taking and autonomic function tests.8 Prompt appropriate management should be implemented as soon as a diagnosis is made. Patient education and lifestyle modifications as well as pharmacological management are key in the clinical setting for preventing syncope and falls, which can have serious consequences in CAD.
This review summarizes the frequency, risk factors, clinical significance, diagnosis, and management of CAD, which represents a unique disease group of cancer patients.
PREVALENCE OF CAD
There has been insufficient research into the prevalence of CAD in cancer patients, with most studies focused on advanced cancer or specific types of chemotherapeutic agents.
Prevalence of CAD in advanced cancer
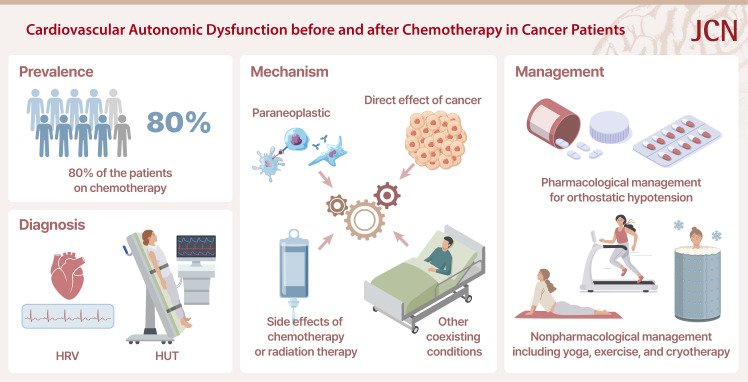
A prospective cohort study using the Ewing classification found that the prevalence of CAD in patients with any type of advanced cancer is reportedly 70%–80%.9 The Ewing battery of cardiovascular autonomic tests is a well-established diagnostic tool for CAD that includes assessments of parasympathetic function (heart-rate variability [HRV] during deep breathing, lying to standing, and the Valsalva maneuver) and sympathetic function (analyses of blood pressure changes upon standing and during sustained hand grip).10 That study found that the median survival time was approximately 100 days in patients with CAD, and that the 1-year survival rate in patients with CAD was half that in those without CAD (15% vs. 30%). Although it was not possible to determine whether the deaths were due to cancer progression, factors associated with CAD, or CAD itself, one clear finding was that the mortality rate was higher in patients with CAD. However, that study primarily involved patients with end-stage cancer, and so further research is needed that involves patients undergoing active chemotherapy or cancer survivors who are expected to survive for a long time period.
Another retrospective study found that only 20% of 47 patients who received chemotherapy did not have CAD, which was determined based on a Ewing score of less than 2.11 The total frequency of moderate CAD (defined as a Ewing score of 2.5–3.0) was 40%, while the frequency of severe CAD (Ewing score of ≥3.5) was 40%. Those authors concluded that CAD is associated with increased morbidity and mortality, and deserves special attention because its frequency is as high as 80% in cancer patients.
Prevalence of CAD according to chemotherapeutic agents
The chemotherapeutic agents taxanes, anthracyclines, platinum, vinca alkaloids, and bortezomib have been reported to cause CAD. Observing the specific effects of individual chemotherapeutic agents can be challenging since they are frequently administered in combination with other drugs or substituted with different regimens when the initial treatment is ineffective.
Approximately 20% of patients with ovarian cancer treated with paclitaxel and carboplatin exhibited a decrease in HRV when moving from the supine to the erect position (known as the 30/15 ratio) or orthostatic hypotension after 3–4 months, but most of the symptoms resolved by around 6 months after the end of treatment.12 That study used a combination of carboplatin- and taxane-based chemotherapy, and so the effects of taxanes alone on CAD could not be evaluated. In addition, the recovery observed after the end of treatment may have been due to most of the included ovarian cancer patients being young and the cancer being completely controlled in many cases by the chemotherapy. Another study found that HRV detected using power spectral analysis was impaired in all 14 paclitaxel-treated ovarian cancer patients.13 However, in a further study, CAD did not develop in patients with metastatic breast cancer who received docetaxel after anthracycline therapy.14
Doxorubicin induces CAD in addition to its direct toxicity to myocytes. A study of 20 high-dose anthracyclines found that CAD occurred in 85% of patients, even in those with a normal left ventricular ejection fraction.15 Doxorubicin treatment induces significant changes in the cardiovascular autonomic nervous system in survivors of childhood acute lymphoblastic leukemia (ALL).16 That study suggested that cumulative doses of doxorubicin can have a significant negative impact on the cardiovascular autonomic nervous system. Administering relatively low doses of epirubicin to 40 patients with breast cancer did not produce persistent alterations in HRV using 24-hour electrocardiogram.17 This also suggests that there is a dose–response relationship for anthracycline-induced CAD.
Decades-old platinum-based agents are also classically neurotoxic, with cisplatin, carboplatin, and oxaliplatin still widely used in ovarian, breast, gastric, colorectal, lung, and head and neck cancers. These agents are commonly known to cause CIPN, but there are conflicting findings regarding whether they cause CAD. The baroreflex sensitivity was maintained better in 44 patients with testicular cancer who received cisplatin-containing chemotherapy than in those who received orchiectomy alone.18 However, CAD was observed in 11 patients with ovarian cancer, as measured by the Valsalva ratio.19 In addition, in 10 of 28 patients with germ-cell tumors, the administration of cisplatin, vincristine, and bleomycin resulted in HRV impairment during the Valsalva maneuver, when standing up, and during deep breathing.20 In colorectal cancer patients treated with oxaliplatin, parasympathetic cardiac innervation was reduced at 3–4 months and 6–8 months after the initiation of oxaliplatin-based chemotherapy relative to baseline.21
Vincristine is well known to induce peripheral neuropathy, but it has also been shown to damage the autonomic nervous system in approximately one-third of patients.22 Decreased heart-rate and blood-pressure responses have been observed during hand grip, standing, deep breathing, and tilt tests in children with ALL.23,24 HRV during deep breathing decreased markedly after vincristine use and tended to recover after the completion of treatment,25 but the recovery period varied from several months to several years.26
Bortezomib is also known to cause CAD, but the prevalence of this effect remains unclear.27,28 Immune checkpoint inhibitors have revolutionized cancer therapy and are widely used for treating many cancers. In addition to acute inflammatory neuropathies such as Guillain-Barre syndrome, acute sensorimotor and autonomic neuropathies have been reported.29
While chemotherapeutic agents can cause CAD via various mechanisms and at varying frequencies, their extents and reversibilities are not well understood. These are likely to be closely related to the cancer severity, whether the types of chemotherapeutic agents used can act synergistically, the frailty of the patient, comorbidities, and the cumulative dose of chemotherapy.
MECHANISMS OF CAD IN CANCER
While the pathogenesis of CAD is not fully understood, several mechanisms have been proposed, including inflammatory, infectious, metabolic, neurodegenerative, and toxic processes, as well as cancer treatments.30 However, establishing a causal link between cancer itself or cancer therapy and CAD is difficult due to the presence of numerous confounding variables. CAD may be caused by preexisting neuropathy, paraneoplastic effects, tumor invasion, or mechanical compression of the autonomic nervous system. There is no single explanation for CAD since its causes overlap, and there is a variable time lag between the onset of an etiological process and the development of CAD. However, categorizing and understanding the mechanisms are important for subsequent treatment and therapeutic trials.
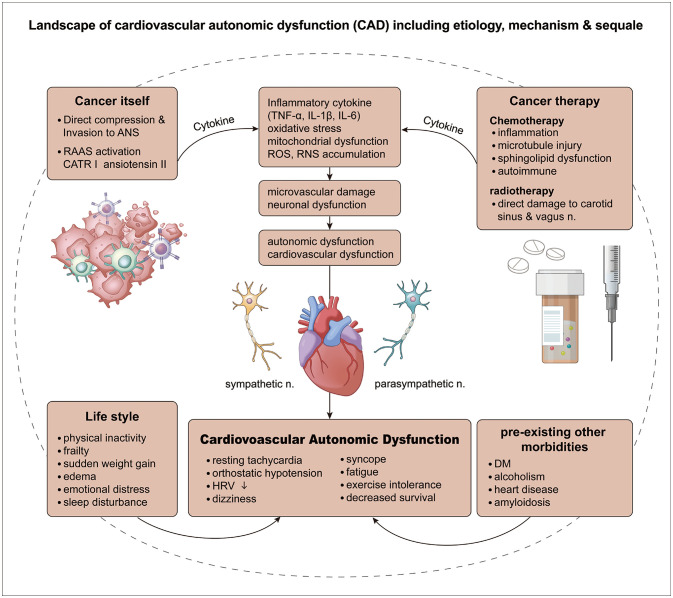
The potential complex mechanisms underlying CAD in cancer patients can be divided into three categories: 1) CAD caused by the cancer itself, 2) CAD caused by the side effects of chemotherapy or radiation therapy,3 and 3) CAD caused by a coexisting condition (Table 1 and Fig. 1).
Table 1. Potential mechanisms underlying CAD in cancer patients.
| Etiology | Mechanisms | Results |
|---|---|---|
| Cancer itself | Inflammatory cascade involving TNF-α, IL-1β, and IL-6 | Microvascular endothelial dysfunction |
| Oxidative stress | Neuron/axonal damage Demyelination | |
| ROS or RNS accumulation Mitochondrial dysfunction | Apoptosis | |
| RAAS activation: ATR1 and angiotensin 2; Sympathetic nerve activation: β-adrenergic receptor | Cardiac remodeling, cardiac hypertrophy, fibrosis, cardiac dysfunction | |
| Direct invasion, compression of autonomic nervous system | Cardiovascular autonomic neuropathy | |
| Chemotherapy | Microtubule injury, mitochondrial dysfunction, ion-channel dysfunction, inflammatory cascade, ROS accumulation, autoimmunity | Neuronal apoptosis, axonal dysregulation (similar to CIPN) attributed to thinly myelinated or unmyelinated nerve fibers |
| Radiation therapy | Direct injury to vagus nerve, carotid sinus, baroreflex | Cardiovascular autonomic neuropathy |
| Lifestyle factors | Decreased physical activity, sleep disturbances, anxiety, emotional stress, weight gain, weakness | CAD |
| Coexisting disease | Diabetes mellitus, amyloidosis, heart disease | CAD |
ATR1, angiotensin 1 receptor; CAD, cardiovascular autonomic dysfunction; CIPN, chemotherapy-induced peripheral neuropathy; IL, interleukin; RAAS, renin-angiotensin-aldosterone system; RNS, reactive nitrogen species; ROS, reactive oxygen species; TNF, tumor necrosis factor.
Fig. 1. CAD in cancer patients is characterized by dysfunction of the autonomic nervous system and the cardiovascular system due to various causes, leading to resting tachycardia, orthostatic hypotension, and decreased HRV and survival. CAD has four etiologies: 1) cancer itself, 2) cancer treatments, 3) lifestyle changes due to cancer, and 4) preexisting comorbidities. Although CAD caused by cancer itself and CAD caused by cancer treatment have distinct underlying mechanisms, the main common mechanisms are inflammatory cytokine-mediated oxidative stress, mitochondrial dysfunction, microvascular damage, and neuronal dysfunction. Cancer-induced lifestyle changes such as decreased physical activity, weakness, sudden weight gain, edema, emotional stress, and insomnia all contribute to CAD, and so these factors need to be managed. Other comorbidities besides cancer can also cause and worsen CAD, and also need to be treated. ANS, autonomic nervous system; CAD, cardiovascular autonomic dysfunction; CATR, CATR tumerogenic conversion 1 protein; DM, diabetes mellitus; HRV, heart-rate variability; IL, interleukin; RAAS, renin-angiotensin-aldosterone system; RNS, reactive nitrogen species; ROS, reactive oxygen species; TNF, tumor necrosis factor.
Direct effects of cancer
The mechanisms by which cancer causes cardiovascular damage include systemic inflammation, oxidative stress, autonomic dysfunction, and metabolic abnormalities, of which inflammation and oxidative stress are the most common.6 Most cancers create a protumorigenic microenvironment by secreting proinflammatory cytokines or acute-phase reactants.31,32 The involved inflammatory cytokines include TNF-α (tumor necrosis factor-alpha), interleukin (IL)-1β and IL-6.33 This inflammatory response is both a precursor to cancer and a trigger for cardiovascular damage.34,35 Therefore, the pathogenesis of cancer and cardiovascular damage may involve similar mechanisms and may occur simultaneously prior to cancer treatment.36
The inflammatory response not only causes microvascular endothelial damage but also adversely affects cardiac remodeling, leading to decreased cardiac contractile function.35 Oxidative stress-induced ROS and RNS by tumors also causes cardiac dysfunction. Mitochondrial dysfunction is a prominent feature in cardiac dysfunction that affects calcium homeostasis and reduces cardiac contractility (Fig. 1).34,37
Overactivation of the renin-angiotensin-aldosterone system, which plays an important pathogenic role in heart failure by causing the remodeling, hypertrophy, and fibrosis of cardiac muscle, has been identified in various cancers.38,39 In addition, angiotensin 1 receptor and angiotensin 2 are involved in angiogenesis, proliferation, invasion, and metastasis in cancer.40 Persistent sympathetic hyperactivity has also been reported in cancer patients and is associated with cancer development, progression, and angiogenesis.35
CAD caused by chemotherapy
Chemotherapy has significantly increased the survival time of cancer patients,41,42 but many patients experience side effects from this treatment modality.5 CIPN is a common condition, and its pathogenesis has received considerable attention.43 Sensory fibers are more susceptible than motor fibers to damage because they are either thinly myelinated or unmyelinated. Similarly, postganglionic autonomic nerve fibers are not myelinated and preganglionic fibers are thinly myelinated, and so autonomic nerve fibers may be susceptible to damage caused by the same mechanisms as CIPN damage.44,45
Various anticancer drugs cause neuron damage via inflammatory cascades and oxidative stress (Table 2).3 Platinum, taxanes, proteasome inhibitors, and thalidomide induce the secretion of various inflammatory cytokines via mitochondrial damage, which increases oxidative stress and leads to the accumulation of ROS.43 Another mechanism is microtubular injury caused by taxanes, vinca alkaloids, and epothilones (e.g., ixabepilone), which directly damages neurons (including their axons) of the autonomic nervous system.46 Both taxanes and platinum disrupt intracellular signaling by altering calcium homeostasis, eventually leading to autonomic nerve damage. These agents also cause peripheral neuropathy via voltage-gated-ion-channel dysfunction. Thalidomide causes neuron death via peripheral vascular ischemia.43,47 Proteasome inhibitors such as bortezomib cause nerve damage by sphingolipid dysregulation, activation of neuroinflammation, and mitochondrial damage.43,48
Table 2. Chemotherapeutic agents and their mechanisms contributing to autonomic neuropathy.
| Anticancer drugs | Possible mechanisms underlying autonomic neuropathy |
|---|---|
| Taxanes (docetaxel, paclitaxel) | Microtubule injury, mitochondrial damage, inflammatory cytokines, oxidative stress, direct axonal damage, voltage-gated-ion-channel dysfunction |
| Vinca alkaloids (vinblastine, vincristine) | Microtubule injury |
| Platinum-based agents (cisplatin, carboplatin, oxaliplatin) | Mitochondrial damage, inflammatory cytokines, oxidative stress, voltage-gated-ion-channel dysfunction, parasympathetic dysfunction |
| Proteasome inhibitors (bortezomib) | Sphingolipid dysregulation, neuroinflammatory signaling pathway activation, mitochondrial damage, inflammatory cytokines, oxidative stress |
| Immune checkpoint inhibitors (ipilimumab, nivolumab, pembrolizumab) | Possible autoimmunity |
A prospective study from 2021 found that approximately 70% of patients with gastrointestinal cancer who received taxane- or platinum-based palliative anticancer therapy for 12 weeks developed CIPN of grades I to III, and that a decrease in the resting-state HRV was a predictor of CAD.1 In addition, a significant association between decreased resting-state HRV and the development of CIPN has been reported, suggesting that monitoring and managing CAD in patients with clinically developed CIPN can help in the management of both conditions simultaneously and have positive impacts on the quality of life, morbidity, and mortality.49,50
Radiation-therapy-associated CAD
CAD has been reported after applying radiation therapy to head and neck cancer as well as mediastinal tumors when autonomic nerves were within the radiation field.30 Direct irradiation of the carotid and vagus nerves has been shown to lead to inflammation, fibrosis, and eventual damage to the vagal nerve and attenuation of the baroreflex.51,52,53 Vagal nerve damage during irradiation is also related to long-term mortality. A cohort study found a link between mediastinal radiation and CAD in survivors of Hodgkin’s lymphoma.54 Those authors found that the exercise capacity during treadmill testing was worse in 263 survivors of Hodgkin’s lymphoma treated with radiation therapy and that their all-cause mortality rate was higher at a median interval of 19 years after the therapy.
Other coexisting conditions
CAD is associated with both cancer-related and non-cancer-related comorbidities. Non-cancer-related comorbidities that cause CAD include diabetes mellitus, amyloidosis, and underlying heart disease. In addition to medical diseases, many lifestyle factors are also associated with the symptoms of CAD. Anxiety, depression, emotional stress, sleep disturbances, and sudden weight gain can lead to autonomic imbalance via chronic stimulation of the hypothalamus-pituitary axis and subsequent upregulation of the sympathetic-adrenal-medullary system.55 Physical inactivity is one of the common causes of orthostatic intolerance in patients with cancer. Disuse atrophy of skeletal muscles due to extended bed rest worsens the symptoms of orthostatic hypotension by reducing venous return due to the pumping function being compromised. It is therefore important to recognize these factors and treat them through lifestyle modifications.
PARANEOPLASTIC CAD
Paraneoplastic neurological syndrome (PNS) is a rare neurological disorder that affects less than 1% of cancer patients.56 It can affect any part of the nervous system and is associated with specific malignancies and neuronal antibodies, which influence the clinical manifestations.
Few studies have investigated the prevalence of paraneoplastic autonomic dysfunction in PNS, with one reason being the patient or physician neglecting this condition if it occurs in a localized or limited form, such as isolated constipation, dry mouth, or focal anhidrosis. Also, due to its frequent association with the symptoms and signs of central nervous system or somatic nerve involvement, dysautonomia may go undiagnosed if its symptoms are not readily apparent or are overshadowed by other significant symptoms.
PNS typically manifests before cancer is detected, and very few cases begin during cancer treatment. It can be challenging to determine whether dysautonomia falls within the spectrum of PNS or is a side effect of chemotherapy. It is therefore crucial for clinicians to acquaint themselves with the distinctive symptoms of PNS and be able to distinguish between the two situations in order to ascertain the appropriate course of treatment.
Lambert-Eaton myasthenic syndrome (LEMS) is a high-risk neurological phenotype of PNS, previously referred to as a classical PNS.57 LEMS is characterized by proximal muscle weakness, loss of tendon reflexes, and autonomic dysfunction. The most common autonomic symptoms are impotence (in 60% of male patients) and dry mouth (in 80% of all patients). Although orthostatic hypotension is an infrequent symptom, autonomic function tests show abnormalities in almost all patients.58 LEMS is associated with the presence of antibodies to P/Q-type voltage-gated calcium channels in the presynaptic neuromuscular junction in around 90% of patients. Additionally, anti-SOX-1 (Sry-like high-mobility group box-1) antibodies have been observed in some cases.59 Both of these antibodies are commonly associated with small-cell lung cancer and are often detected before the diagnosis of malignancy.
An intermediate-risk phenotype of PNS showing dysautonomia is Morvan syndrome, which is characterized by peripheral nerve hyperexcitability, hallucinations, insomnia, and excessive sweating. Symptoms of CAD include extrasystole associated with tachycardia in patients with anti-voltage-gated-potassium-channel antibodies,60 and postural tachycardia syndrome in patients with CASPR2 (contactin-associated protein-like 2) antibodies.61 Autonomic function tests applied to two patients with Morvan syndrome revealed a combination of peripheral autonomic neuropathy and autonomic hyperactivity, indicating that CAD affects both the central and peripheral nervous systems.62 CAD is linked to malignant thymoma in approximately 50% of cases.
Autoimmune autonomic ganglionopathy may occur when the autonomic nervous system is selectively involved. This condition is characterized by subacute panautonomic failure and is caused by antibodies against the ganglionic nicotinic acetylcholine (ACh) receptor.63 Approximately 70% of patients experience orthostatic hypotension and constipation, which are often accompanied by symptoms associated with cholinergic impairment such as dry mouth, dry eyes, and urinary retention. Although most cases have an autoimmune etiology, there are instances where they manifest as part of PNS. Reported underlying malignancies are lung cancer or thymoma, and ganglionic nicotinic ACh receptor antibodies are detected in some patients.64
Paraneoplastic autonomic neuropathy can occur alone or in combination with subacute sensory neuronopathy, and is associated with anti-Hu and anti-CV2/CRMP5 (collapsing response-mediator protein 5) antibodies.65
The prognosis is reportedly poor when any form of dysautonomia manifests as PNS.65 A recent retrospective study identified autonomic dysfunction in 26% of 477 anti-Hu patients, with gastrointestinal and cardiovascular systems being the most commonly affected.66 While dysautonomia itself did not worsen overall survival, central hypoventilation and CAD were associated with a higher risk of death within 1 year from clinical onset.
The PNS criteria proposed in 2021 suggested applying the PNS-Care Score for diagnostic certainty. Definite, probable, and possible PNS can be categorized according to the clinical level, laboratory level, and the existence of cancer consistent with the phenotype.57 This requires the presence of high- or intermediate-risk antibodies in the serum or cerebrospinal fluid for a diagnosis of definite PNS.57 However, regardless of the presence of neural antibodies, a diagnostic workup for finding underlying malignancy should be performed according to the clinical phenotype when PNS is suspected clinically.67 If malignancy is not identified or is not consistent with the phenotype during the initial investigation, follow-up testing should be repeated every 4–6 months for a period of 2 years. Screening the chest for tumors must be included because paraneoplastic CAD is usually associated with small-cell lung cancer and thymoma (Table 3).
Table 3. Antibodies and cancers associated with paraneoplastic autonomic Dysfunction.
| Antibody | Clinical phenotype | Associated cancers | |
|---|---|---|---|
| High-risk antibodies | |||
| SOX-1 | LEMS | SCLC, NSCLC | |
| CV2/CRMP5 | Autonomic neuropathy | SCLC, thymoma, NSCLC | |
| Hu | Pandysautonomia | SCLC | |
| Intermediate-risk antibodies | |||
| P/Q-type VGCCs | LEMS | SCLC | |
| CASPR2 | Morvan syndrome | Thymoma | |
CASPR2, contactin-associated protein-like 2; CRMP5, collapsing response-mediator protein 5; LEMS, Lambert-Eaton myasthenic syndrome; NSCLC, non-SCLC; SCLC, small-cell lung cancer; SOX-1, Sry-like high-mobility group box-1; VGCCs, voltage-gated calcium channels.
The possibility of permanent neuronal damage in onconeural antibody-associated PNS make it crucial to initiate therapy as soon as possible, including before all results of any additional investigations are available.68 PNS is treated by the removal or treatment of the underlying cancer and the implementation of immunotherapy including intravenous methylprednisolone, intravenous immunoglobulin, and plasma exchange.68 Rituximab and/or cyclophosphamide should be considered as a second-line therapy in patients who do not respond.
DIAGNOSIS OF CAD IN CANCER PATIENTS
Clinical features of CAD
Autonomic dysfunction has heterogeneous manifestations that depend on the specific autonomic nerves or organs involved.69 CAD is particularly notable within the spectrum of autonomic dysfunction because of its association with increased mortality. Clinicians therefore need to be aware of the signs and symptoms of CAD, which include resting tachycardia, reduced exercise tolerance, orthostatic dizziness, and syncope. Orthostatic hypotension leads to a secondary reduction in cerebral perfusion, resulting in a constellation of symptoms attributable to widespread cortical hypofunction, including dizziness, lightheadedness, fatigue, brain fog, blurring of vision, tremor, and anxiety. In addition, reduced perfusion to the shoulder musculature may cause bilateral shoulder and nuchal pain after prolonged orthostasis or ambulation, which is sometimes referred to as “coat hanger headache” because the distribution of the discomfort resembles the shape of a coat hanger.70
Orthostatic hypotension must be distinguished from cardiac syncope owing to structural or rhythmic abnormalities of the heart, including arrhythmias, carotid sinus syndrome, cardiomyopathy, and arterial dissection.
Autonomic function tests for CAD
Conventional autonomic function testing can be a reliable noninvasive tool for objective clinical evaluations of CAD. The most common and easiest test to perform is measuring HRV when deep breathing. This is a reliable test for assessing the function of the cardiac vagal nerve.71 During deep inhalation, cardiac output decreases due to reduced venous return, causing the heart rate to increase; conversely, the heart rate becomes slower during deep exhalation. HRV during deep breathing is calculated as the ratio of the longest heart-rate interval to the shortest heart-rate interval, or as the ratio of the maximum heart rate to the minimum heart rate (E:I ratio).
Heart-rate and blood-pressure changes during the Valsalva maneuver reflect both sympathetic and parasympathetic functions.72 The Valsalva ratio is the peak pulse rate induced by the Valsalva maneuver divided by the lowest subsequent reflex pulse rate. This test is useful for evaluating baroreflex function, but is not commonly performed because it requires specialized equipment for measuring the continuous beat-to-beat blood pressure.
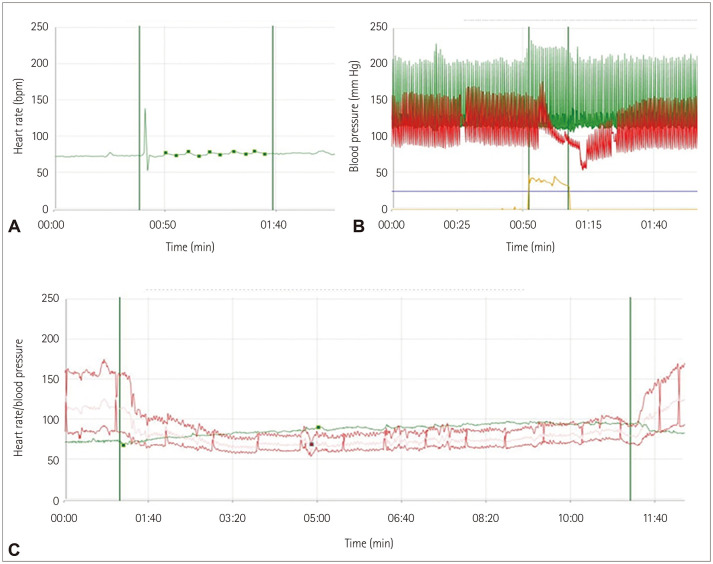
The head-up tilt test can be used to distinguish the underlying cause of orthostatic intolerance such as orthostatic tachycardia, orthostatic hypotension, or syncope. The current diagnostic criteria for orthostatic hypotension are a decrease in systolic blood pressure of more than 20 mm Hg and a decrease in diastolic blood pressure of 10 mm Hg within 3 minutes of applying the simulus.73 Neurogenic orthostatic hypotension is characterized by a continuous decrease in blood pressure without sufficient compensating tachycardia.74 Fig. 2 depicts typical abnormalities observed in autonomic function tests in a patient with breast cancer and CAD.
Fig. 2. Results of autonomic function tests in a cancer patient who complained of orthostatic dizziness and recurrent syncope. A low E:I ratio implied cardiovagal impairment (A), an abnormal Valsalva phase and ratio suggested an inadequate baroreflex and vasoconstriction (B), and a head-up tilt test showed marked orthostatic hypotension (C). E:I ratio, expiration:inspiration ratio.
MANAGEMENT OF CAD IN CANCER PATIENTS
The underlying cause should be corrected once it has been identified. Because many medications can cause orthostatic hypotension, offending drugs should be discontinued. If severe autonomic neuropathy occurs due to chemotherapy or as a component within the spectrum of CIPN, it may be advisable to temporarily discontinue chemotherapeutic agents. Symptomatic management can be achieved through nonpharmacological or pharmacological approaches, as described below.
Nonpharmacological treatments
It is essential to provide education for promoting a moderate increase in physical activity since this can improve autonomic function by enhancing cardiopulmonary function and oxygen consumption in the skeletal muscles. These positive effects have been consistently found in both healthy individuals and patients with autonomic neuropathy.75,76,77 Furthermore, studies have shown that structured aerobic exercise during or after chemotherapy helps to reverse autonomic neuropathy in patients with breast and testicular cancer.78,79 Recommended aerobic exercises include stationary biking and swimming, which not only exert direct positive effects on the autonomic nervous system but also indirectly improve sleep quality. A systematic review and meta-analysis investigating the effects of exercise on autonomic dysfunction in cancer patients and survivors also found that both resistance and endurance training can improve HRV.80 While the positive effects of exercise on CAD in cancer patients are clear, further research is necessary to determine the optimal exercise type and intensity for maximizing these benefits, since the few previous studies have included small samples and have applied diverse methods to measure autonomic modulation.
Mental stress, anxiety, and depression in patients with cancer can stimulate the sympathetic nervous system and reduce parasympathetic activity, leading to instability in autonomic function.81 Moreover, a systematic review of 12 epidemiological studies demonstrated that high vagal nerve activity predicted a better cancer outcome, suggesting that vagal activation improves the prognosis of cancer.82 Yoga activates the parasympathetic nervous system, increases HRV, and reduces stress and anxiety. In patients undergoing chemotherapy for breast cancer, the addition of meditation and yoga five times a week for 40 minutes produced significant improvements in HRV at 18 weeks after the end of chemotherapy relative to controls.83 This finding suggests that yoga can prevent chemotherapy-induced CAD.
Recent studies have indicated that cryotherapy exerts prophylactic effects against CIPN. Cryotherapy is known to prevent axonal degeneration and mitochondrial dysfunction by inducing vasoconstriction and reducing toxicity in the applied region. A systemic review and meta-analysis found that applying Elasto-Gel frozen gloves and socks 15–30 minutes before chemotherapy infusion and removing them 15–30 minutes after the end of the infusion (with their temperature being maintained) significantly reduced the incidence of CIPN and improved the quality of life during chemotherapy.84 However, most studies of the effects of cryotherapy have focused on patient-reported sensory symptoms or the severity of the motor and sensory components of CIPN. One randomized controlled trial demonstrated a possible benefit of cryotherapy on autonomic function in a patient receiving paclitaxel, although a preventive effect on sensory neuropathy was not evident.85 More well-designed studies with objective measures of autonomic function are required to establish a therapeutic or preventive effect of cryotherapy on CAD.
Recommendations for the symptomatic management of orthostatic hypotension include consuming 2.0–2.5 liters of water daily and an adequate amount of salt. Wearing an abdominal binder or compression stockings while performing daily activities can help prevent excessive venous pooling. Compression stockings should exert a pressure of at least 15–20 mm Hg, and waist-high compression stockings are more effective than other types of stocking. The use of an adjustable bed in which the upper body can be elevated by 30–45 degrees during sleep has been shown to promote the secretion of antidiuretic hormones, alleviate orthostatic hypotension, and prevent supine hypertension, which occurs in approximately half of these patients.86
Pharmacological treatments
CAD frequently presents in patients with cancer as an imbalance between the sympathetic and parasympathetic nervous systems, resulting in cardiovascular problems including arrhythmia and hypertension.35 Antihypertensive drugs can be effective against CAD in cancer patients by modulating the autonomic nervous system or by reducing the cardiotoxicity of chemotherapeutic agents.
Beta blockers are notable for their ability to decrease the risk of anthracycline-induced cardiomyopathy and reduce the occurrences of heart failure in patients with breast cancer by counteracting enhanced sympathetic activity,55 although more research is needed to clarify their role in other forms of chemotherapy-related CAD. Angiotensin-converting enzyme inhibitors and angiotensin 2 receptor blockers have also shown cardioprotective effects in cancer patients treated with high-dose chemotherapy and epirubicin, respectively.87,88 However, these studies measured the left ventricular ejection fraction or cardiac enzymes to assess cardiac function, and so their relevance to CAD remains unclear. Large-scale randomized controlled trials are needed to confirm the direct clinical relevance of these therapies to CAD.
Aggressive pharmacological management may be necessary to treat orthostatic hypotension since this is associated with syncope and falls. The alpha-1 adrenoreceptor agonist midodrine increases the peripheral vascular resistance and the blood pressure. It is typically administered at a dose of 2.5 mg or 5 mg three times daily. Since supine hypertension is often a problem, midodrine should not be taken 3–4 hours before bedtime.89 Droxidopa is converted to norepinephrine by aromatic L-amino acid decarboxylase after ingestion, which activates the sympathetic nervous system. Droxidopa is approved by the Food and Drug Administration for neurogenic orthostatic hypotension associated with Parkinson’s disease and multiple-system atrophy. The recommended dose is 100–600 mg three times daily, although the number of doses can be adjusted depending on the patient’s condition. The potential side effects of droxidopa include supine hypertension, nausea, and headache.90 The mineralocorticoid fludrocortisone increases the blood pressure by promoting sodium and water reabsorption in the kidneys.91 The recommended dose is 0.05–0.20 mg daily, but it is important to monitor for supine hypertension, hypokalemia, and rare renal toxicity. However, long-term use can increase the risk of heart and renal failure.
Patients with cancer often have anemia of chronic disease, which can worsen the symptoms of orthostatic hypotension. It is essential to maintain the hematocrit level within the normal range through active treatment. Erythropoietin can be a useful treatment, and this can be combined with iron supplementation.92
CONCLUSIONS AND FUTURE PERSPECTIVES
The increasing survival time of cancer patients is resulting in a concomitant increase in the number of cancer survivors. The various etiologies of CAD include the cancer itself, chemotherapy with certain anticancer drugs, radiation therapy, and confounding factors. Because CAD tends to persist for years or even decades after the end of chemotherapy, it reduces both the quality of life and the survival rate of patients. In addition, the impact of CAD on patient mortality varies depending on whether the cancer is early or metastatic, and the patient’s age, chemotherapy regimen, chemotherapy dose, and underlying disease. Therefore, it is essential to always suspect CAD so that it can be diagnosed as early as possible.
The pathogenesis of CAD has been described as the effect of cancer itself, a toxic effect of chemotherapeutics or irradiation, and other coexisting diseases, and in many cases a combination of these factors. CAD as a component within the spectrum of PNS is an important issue since CAD can occur before a cancer diagnosis, and the prognosis is grave when CAD manifests as a paraneoplastic syndrome. It is therefore important to first establish clinical suspicion, and this can be achieved by considering the patient’s symptoms. Autonomic function tests measuring HRV and the orthostatic blood pressure can provide a confirmative diagnosis and be used to determine the severity of CAD.
Due to the variety of possible underlying mechanisms, CAD is treated using both pharmacological and nonpharmacological approaches. Managing CAD by patients and specialists making a shared tailored decisions is critical for increasing the survival time and quality of life of cancer patients.
Dysautonomia in cancer patients has recently been receiving increasing interest. The incidence of CAD in different cancer types, stages, and chemotherapy regimens and the impacts of these factors on the quality of life should be investigated in future research.
Footnotes
- Conceptualization: So Young Yoon.
- Data curation: So Young Yoon, Jeeyoung Oh.
- Formal analysis: So Young Yoon, Jeeyoung Oh.
- Funding acquisition: Jeeyoung Oh.
- Investigation: So Young Yoon, Jeeyoung Oh.
- Methodology: So Young Yoon, Jeeyoung Oh.
- Visualization: So Young Yoon, Jeeyoung Oh.
- Writing—original draft: So Young Yoon, Jeeyoung Oh.
- Writing—review & editing: So Young Yoon, Jeeyoung Oh.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: This work was supported by Konkuk University Medical Center Research Grant 2021.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article.
References
- 1.Jang A, Seol YM. Heart rate variability as a non-invasive objective parameter for predicting the occurrence of chemotherapy-induced peripheral neuropathy in patients with gastrointestinal cancer. Anticancer Res. 2021;41:2637–2645. doi: 10.21873/anticanres.15044. [DOI] [PubMed] [Google Scholar]
- 2.Fadul N, Strasser F, Palmer JL, Yusuf SW, Guo Y, Li Z, et al. The association between autonomic dysfunction and survival in male patients with advanced cancer: a preliminary report. J Pain Symptom Manage. 2010;39:283–290. doi: 10.1016/j.jpainsymman.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Teng AE, Noor B, Ajijola OA, Yang EH. Chemotherapy and radiation-associated cardiac autonomic dysfunction. Curr Oncol Rep. 2021;23:14. doi: 10.1007/s11912-020-01013-7. [DOI] [PubMed] [Google Scholar]
- 4.Perrino C, Schiattarella GG, Magliulo F, Ilardi F, Carotenuto G, Gargiulo G, et al. Cardiac side effects of chemotherapy: state of art and strategies for a correct management. Curr Vasc Pharmacol. 2014;12:106–116. doi: 10.2174/157016111201140327163302. [DOI] [PubMed] [Google Scholar]
- 5.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 6.Fabiani I, Panichella G, Aimo A, Grigoratos C, Vergaro G, Pugliese NR, et al. Subclinical cardiac damage in cancer patients before chemotherapy. Heart Fail Rev. 2022;27:1091–1104. doi: 10.1007/s10741-021-10151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J. 2019;43:3–30. doi: 10.4093/dmj.2018.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai YR, Huang CC, Chang HW, Chiu WC, Tsai NW, Cheng BC, et al. Severity of cardiovascular autonomic neuropathy is a predictor associated with major adverse cardiovascular events in adults with type 2 diabetes mellitus: a 6-year follow-up study. Can J Diabetes. 2021;45:155–161. doi: 10.1016/j.jcjd.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Stone CA, Kenny RA, Nolan B, Lawlor PG. Autonomic dysfunction in patients with advanced cancer; prevalence, clinical correlates and challenges in assessment. BMC Palliat Care. 2012;11:3. doi: 10.1186/1472-684X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewing DJ, Borsey DQ, Bellavere F, Clarke BF. Cardiac autonomic neuropathy in diabetes: comparison of measures of R-R interval variation. Diabetologia. 1981;21:18–24. doi: 10.1007/BF03216217. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Palmer JL, Strasser F, Yusuf SW, Bruera E. Heart rate variability as a measure of autonomic dysfunction in men with advanced cancer. Eur J Cancer Care (Engl) 2013;22:612–616. doi: 10.1111/ecc.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dermitzakis EV, Kimiskidis VK, Lazaridis G, Alexopoulou Z, Timotheadou E, Papanikolaou A, et al. The impact of paclitaxel and carboplatin chemotherapy on the autonomous nervous system of patients with ovarian cancer. BMC Neurol. 2016;16:190. doi: 10.1186/s12883-016-0710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekholm EM, Salminen EK, Huikuri HV, Jalonen J, Antila KJ, Salmi TA, et al. Impairment of heart rate variability during paclitaxel therapy. Cancer. 2000;88:2149–2153. doi: 10.1002/(sici)1097-0142(20000501)88:9<2149::aid-cncr22>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Ekholm E, Rantanen V, Bergman M, Vesalainen R, Antila K, Salminen E. Docetaxel and autonomic cardiovascular control in anthracycline treated breast cancer patients. Anticancer Res. 2000;20:2045–2048. [PubMed] [Google Scholar]
- 15.Tjeerdsma G, Meinardi MT, van Der Graaf WT, van Den Berg MP, Mulder NH, Crijns HJ, et al. Early detection of anthracycline induced cardiotoxicity in asymptomatic patients with normal left ventricular systolic function: autonomic versus echocardiographic variables. Heart. 1999;81:419–423. doi: 10.1136/hrt.81.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caru M, Corbin D, Périé D, Lemay V, Delfrate J, Drouin S, et al. Doxorubicin treatments induce significant changes on the cardiac autonomic nervous system in childhood acute lymphoblastic leukemia long-term survivors. Clin Res Cardiol. 2019;108:1000–1008. doi: 10.1007/s00392-019-01427-9. [DOI] [PubMed] [Google Scholar]
- 17.Meinardi MT, van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, van den Berg MP, et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol. 2001;19:2746–2753. doi: 10.1200/JCO.2001.19.10.2746. [DOI] [PubMed] [Google Scholar]
- 18.Nuver J, Smit AJ, van der Meer J, van den Berg MP, van der Graaf WT, Meinardi MT, et al. Acute chemotherapy-induced cardiovascular changes in patients with testicular cancer. J Clin Oncol. 2005;23:9130–9137. doi: 10.1200/JCO.2005.01.4092. [DOI] [PubMed] [Google Scholar]
- 19.Boogerd W, ten Bokkel Huinink WW, Dalesio O, Hoppenbrouwers WJ, van der Sande JJ. Cisplatin induced neuropathy: central, peripheral and autonomic nerve involvement. J Neurooncol. 1990;9:255–263. doi: 10.1007/BF02341156. [DOI] [PubMed] [Google Scholar]
- 20.Hansen SW. Autonomic neuropathy after treatment with cisplatin, vinblastine, and bleomycin for germ cell cancer. BMJ. 1990;300:511–512. doi: 10.1136/bmj.300.6723.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dermitzakis EV, Kimiskidis VK, Eleftheraki A, Lazaridis G, Konstantis A, Basdanis G, et al. The impact of oxaliplatin-based chemotherapy for colorectal cancer on the autonomous nervous system. Eur J Neurol. 2014;21:1471–1477. doi: 10.1111/ene.12514. [DOI] [PubMed] [Google Scholar]
- 22.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 23.Nazir HF, AlFutaisi A, Zacharia M, Elshinawy M, Mevada ST, Alrawas A, et al. Vincristine-induced neuropathy in pediatric patients with acute lymphoblastic leukemia in Oman: Frequent autonomic and more severe cranial nerve involvement. Pediatr Blood Cancer. 2017;64:e26677. doi: 10.1002/pbc.26677. [DOI] [PubMed] [Google Scholar]
- 24.Roca E, Bruera E, Politi PM, Barugel M, Cedaro L, Carraro S, et al. Vinca alkaloid-induced cardiovascular autonomic neuropathy. Cancer Treat Rep. 1985;69:149–151. [PubMed] [Google Scholar]
- 25.Hirvonen HE, Salmi TT, Heinonen E, Antila KJ, Välimäki IA. Vincristine treatment of acute lymphoblastic leukemia induces transient autonomic cardioneuropathy. Cancer. 1989;64:801–805. doi: 10.1002/1097-0142(19890815)64:4<801::aid-cncr2820640406>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63:419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 27.Giannoccaro MP, Donadio V, Gomis Pèrez C, Borsini W, Di Stasi V, Liguori R. Somatic and autonomic small fiber neuropathy induced by bortezomib therapy: an immunofluorescence study. Neurol Sci. 2011;32:361–363. doi: 10.1007/s10072-010-0475-2. [DOI] [PubMed] [Google Scholar]
- 28.Stratogianni A, Tosch M, Schlemmer H, Weis J, Katona I, Isenmann S, et al. Bortezomib-induced severe autonomic neuropathy. Clin Auton Res. 2012;22:199–202. doi: 10.1007/s10286-012-0164-8. [DOI] [PubMed] [Google Scholar]
- 29.Gu Y, Menzies AM, Long GV, Fernando SL, Herkes G. Immune mediated neuropathy following checkpoint immunotherapy. J Clin Neurosci. 2017;45:14–17. doi: 10.1016/j.jocn.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Coumbe BGT, Groarke JD. Cardiovascular autonomic dysfunction in patients with cancer. Curr Cardiol Rep. 2018;20:69. doi: 10.1007/s11886-018-1010-y. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Yang X, Wang L, Zhang C. Interplay between inflammatory tumor microenvironment and cancer stem cells. Oncol Lett. 2018;16:679–686. doi: 10.3892/ol.2018.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 34.Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, et al. Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27:494–510. doi: 10.1177/2047487319870344. [DOI] [PubMed] [Google Scholar]
- 35.Bertero E, Canepa M, Maack C, Ameri P. Linking heart failure to cancer: background evidence and research perspectives. Circulation. 2018;138:735–742. doi: 10.1161/CIRCULATIONAHA.118.033603. [DOI] [PubMed] [Google Scholar]
- 36.de Boer RA, Meijers WC, van der Meer P, van Veldhuisen DJ. Cancer and heart disease: associations and relations. Eur J Heart Fail. 2019;21:1515–1525. doi: 10.1002/ejhf.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 38.Ager EI, Neo J, Christophi C. The renin-angiotensin system and malignancy. Carcinogenesis. 2008;29:1675–1684. doi: 10.1093/carcin/bgn171. [DOI] [PubMed] [Google Scholar]
- 39.Hanif K, Bid HK, Konwar R. Reinventing the ACE inhibitors: some old and new implications of ACE inhibition. Hypertens Res. 2010;33:11–21. doi: 10.1038/hr.2009.184. [DOI] [PubMed] [Google Scholar]
- 40.Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab. 2005;16:293–299. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 42.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975-2015 [Internet] Bethesda: National Cancer Institute; [cited 2018 Sep 10]. Available from: https://seer.cancer.gov/csr/1975_2015/ [Google Scholar]
- 43.Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. 2019;20:1451. doi: 10.3390/ijms20061451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34:57–61. doi: 10.1002/mus.20551. [DOI] [PubMed] [Google Scholar]
- 45.Hoitsma E, Reulen JP, de Baets M, Drent M, Spaans F, Faber CG. Small fiber neuropathy: a common and important clinical disorder. J Neurol Sci. 2004;227:119–130. doi: 10.1016/j.jns.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 46.LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology. 2013;37:231–239. doi: 10.1016/j.neuro.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neurosci Lett. 2015;596:90–107. doi: 10.1016/j.neulet.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Wani TH, Chakrabarty A, Shibata N, Yamazaki H, Guengerich FP, Chowdhury G. The dihydroxy metabolite of the teratogen thalidomide causes oxidative DNA damage. Chem Res Toxicol. 2017;30:1622–1628. doi: 10.1021/acs.chemrestox.7b00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marstrand SD, Buch-Larsen K, Andersson M, Jensen LT, Schwarz P. Vibration perception threshold and heart rate variability as methods to assess chemotherapy-induced neuropathy in women with breast cancer - a pilot study. Cancer Treat Res Commun. 2021;28:100426. doi: 10.1016/j.ctarc.2021.100426. [DOI] [PubMed] [Google Scholar]
- 50.Marstrand SD, Buch-Larsen K, Andersson M, Jensen LT, Schwarz P. Heart rate variability and vibration perception threshold to assess chemotherapy-induced neuropathy in women with breast cancer - a systematic review. Cancer Treat Res Commun. 2021;26:100295. doi: 10.1016/j.ctarc.2020.100295. [DOI] [PubMed] [Google Scholar]
- 51.Sharabi Y, Dendi R, Holmes C, Goldstein DS. Baroreflex failure as a late sequela of neck irradiation. Hypertension. 2003;42:110–116. doi: 10.1161/01.HYP.0000077441.45309.08. [DOI] [PubMed] [Google Scholar]
- 52.Kong L, Lu JJ, Liss AL, Hu C, Guo X, Wu Y, et al. Radiation-induced cranial nerve palsy: a cross-sectional study of nasopharyngeal cancer patients after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1421–1427. doi: 10.1016/j.ijrobp.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Goodman BP, Schrader SL. Radiation-induced cranial neuropathies manifesting as baroreflex failure and progressive bulbar impairment. Neurologist. 2009;15:102–104. doi: 10.1097/NRL.0b013e31817ba3a6. [DOI] [PubMed] [Google Scholar]
- 54.Groarke JD, Tanguturi VK, Hainer J, Klein J, Moslehi JJ, Ng A, et al. Abnormal exercise response in long-term survivors of hodgkin lymphoma treated with thoracic irradiation: evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol. 2015;65:573–583. doi: 10.1016/j.jacc.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 55.Geraldes V, Caldeira E, Afonso A, Machado F, Amaro-Leal Â, Laranjo S, et al. Cardiovascular dysautonomia in patients with breast cancer. Open Cardiovasc Med J. 2022;16:e187419242206271 [Google Scholar]
- 56.Kadish R, Clardy SL. Epidemiology of paraneoplastic neurologic syndromes. Handb Clin Neurol. 2024;200:57–77. doi: 10.1016/B978-0-12-823912-4.00011-6. [DOI] [PubMed] [Google Scholar]
- 57.Graus F, Vogrig A, Muñiz-Castrillo S, Antoine JG, Desestret V, Dubey D, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1014. doi: 10.1212/NXI.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lipka AF, Verschuuren JJGM. Lambert-Eaton myasthenic syndrome. Handb Clin Neurol. 2024;200:307–325. doi: 10.1016/B978-0-12-823912-4.00012-8. [DOI] [PubMed] [Google Scholar]
- 59.Sun X, Tan J, Sun H, Liu Y, Guan W, Jia J, et al. Anti-SOX1 antibodies in paraneoplastic neurological syndrome. J Clin Neurol. 2020;16:530–546. doi: 10.3988/jcn.2020.16.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liguori R, Vincent A, Clover L, Avoni P, Plazzi G, Cortelli P, et al. Morvan’s syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain. 2001;124:2417–2426. doi: 10.1093/brain/124.12.2417. [DOI] [PubMed] [Google Scholar]
- 61.Swayang PS, Nalini A, Preethish-Kumar V, Udupa K, Yadav R, Vengalil S, et al. CASPR2-related morvan syndrome: autonomic, polysomnographic, and neuropsychological observations. Neurol Clin Pract. 2021;11:e267–e276. doi: 10.1212/CPJ.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Josephs KA, Silber MH, Fealey RD, Nippoldt TB, Auger RG, Vernino S. Neurophysiologic studies in Morvan syndrome. J Clin Neurophysiol. 2004;21:440–445. doi: 10.1097/00004691-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Golden EP, Vernino S. Autoimmune autonomic neuropathies and ganglionopathies: epidemiology, pathophysiology, and therapeutic advances. Clin Auton Res. 2019;29:277–288. doi: 10.1007/s10286-019-00611-1. [DOI] [PubMed] [Google Scholar]
- 64.Golden EP, Vernino S. Paraneoplastic autonomic neuropathies and GI dysmotility. Handb Clin Neurol. 2024;200:275–282. doi: 10.1016/B978-0-12-823912-4.00005-0. [DOI] [PubMed] [Google Scholar]
- 65.Villagrán-García M, Farina A, Campetella L, Arzalluz-Luque J, Honnorat J. Autonomic nervous system involvement in autoimmune encephalitis and paraneoplastic neurological syndromes. Rev Neurol (Paris) 2024;180:107–116. doi: 10.1016/j.neurol.2023.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Villagrán-García M, Farina A, Arzalluz-Luque J, Campetella L, Muñiz-Castrillo S, Benaiteau M, et al. Dysautonomia in anti-Hu paraneoplastic neurological syndromes. J Neurol. 2024;271:3359–3369. doi: 10.1007/s00415-024-12278-4. [DOI] [PubMed] [Google Scholar]
- 67.Graus F. Clinical approach to diagnosis of paraneoplastic neurologic syndromes. Handb Clin Neurol. 2024;200:79–96. doi: 10.1016/B978-0-12-823912-4.00007-4. [DOI] [PubMed] [Google Scholar]
- 68.Kerstens J, Titulaer MJ. Overview of treatment strategies in paraneoplastic neurological syndromes. Handb Clin Neurol. 2024;200:97–112. doi: 10.1016/B978-0-12-823912-4.00015-3. [DOI] [PubMed] [Google Scholar]
- 69.Cheshire WP, Jr, Goldstein DS. The physical examination as a window into autonomic disorders. Clin Auton Res. 2018;28:23–33. doi: 10.1007/s10286-017-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khurana RK. Coat-hanger ache in orthostatic hypotension. Cephalalgia. 2012;32:731–737. doi: 10.1177/0333102412449932. [DOI] [PubMed] [Google Scholar]
- 71.Low PA, Tomalia VA, Park KJ. Autonomic function tests: some clinical applications. J Clin Neurol. 2013;9:1–8. doi: 10.3988/jcn.2013.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quispe RC, Novak P. Auxiliary tests of autonomic functions. J Clin Neurophysiol. 2021;38:262–273. doi: 10.1097/WNP.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 73.Cheshire WP, Freeman R, Gibbons CH, Cortelli P, Wenning GK, Hilz MJ, et al. Electrodiagnostic assessment of the autonomic nervous system: a consensus statement endorsed by the American Autonomic Society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin Neurophysiol. 2021;132:666–682. doi: 10.1016/j.clinph.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 74.Cheshire WP, Jr, Goldstein DS. Autonomic uprising: the tilt table test in autonomic medicine. Clin Auton Res. 2019;29:215–230. doi: 10.1007/s10286-019-00598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goh JK, Koh L. Evaluating treatment options for cardiovascular autonomic neuropathy in patients with diabetes mellitus: a systematic review. Diabetol Int. 2023;14:224–242. doi: 10.1007/s13340-023-00629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamasaki H. The effect of exercise on cardiovascular autonomic nervous function in patients with diabetes: a systematic review. Healthcare (Basel) 2023;11:2668. doi: 10.3390/healthcare11192668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vágvölgyi A, Ábrahám JE, Máthéné Köteles É, Korom A, Barnai M, Szücs M, et al. A three-month physical training program improves cardiovascular autonomic function in patients with metabolic syndrome with and without diabetes - a pilot study. Front Endocrinol (Lausanne) 2023;14:1224353. doi: 10.3389/fendo.2023.1224353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adams SC, DeLorey DS, Davenport MH, Stickland MK, Fairey AS, North S, et al. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: a phase 2 randomized controlled trial. Cancer. 2017;123:4057–4065. doi: 10.1002/cncr.30859. [DOI] [PubMed] [Google Scholar]
- 79.Kirkham AA, Lloyd MG, Claydon VE, Gelmon KA, McKenzie DC, Campbell KL. A longitudinal study of the association of clinical indices of cardiovascular autonomic function with breast cancer treatment and exercise training. Oncologist. 2019;24:273–284. doi: 10.1634/theoncologist.2018-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lavín-Pérez AM, Collado-Mateo D, Mayo X, Liguori G, Humphreys L, Jiménez A. Can exercise reduce the autonomic dysfunction of patients with cancer and its survivors? A systematic review and meta-analysis. Front Psychol. 2021;12:712823. doi: 10.3389/fpsyg.2021.712823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldstein DS. Stress and the autonomic nervous system. Auton Neurosci. 2023;247:103096. doi: 10.1016/j.autneu.2023.103096. [DOI] [PubMed] [Google Scholar]
- 82.De Couck M, Caers R, Spiegel D, Gidron Y. The role of the vagus nerve in cancer prognosis: a systematic and a comprehensive review. J Oncol. 2018;2018:1236787. doi: 10.1155/2018/1236787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inbaraj G, Udupa K, Raghavendra RM, Ram A, Patil S, Rajeswaran J, et al. Effects of an 18-week integrated yoga program on cardiac autonomic function in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Integr Cancer Ther. 2023;22:15347354231168795. doi: 10.1177/15347354231168795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tai HY, Lin LY, Huang TW, Gautama MSN. Efficacy of cryotherapy in the prevention of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Support Care Cancer. 2024;32:482. doi: 10.1007/s00520-024-08680-3. [DOI] [PubMed] [Google Scholar]
- 85.Ng DQ, Tan CJ, Soh BC, Tan MML, Loh SY, Tan YE, et al. Impact of cryotherapy on sensory, motor, and autonomic neuropathy in breast cancer patients receiving paclitaxel: a randomized, controlled trial. Front Neurol. 2020;11:604688. doi: 10.3389/fneur.2020.604688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wieling W, Kaufmann H, Claydon VE, van Wijnen VK, Harms MPM, Juraschek SP, et al. Diagnosis and treatment of orthostatic hypotension. Lancet Neurol. 2022;21:735–746. doi: 10.1016/S1474-4422(22)00169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 88.Cadeddu C, Piras A, Mantovani G, Deidda M, Dessì M, Madeddu C, et al. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J. 2010;160:487.e1–487.e7. doi: 10.1016/j.ahj.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 89.Smith W, Wan H, Much D, Robinson AG, Martin P. Clinical benefit of midodrine hydrochloride in symptomatic orthostatic hypotension: a phase 4, double-blind, placebo-controlled, randomized, tilt-table study. Clin Auton Res. 2016;26:269–277. doi: 10.1007/s10286-016-0363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biaggioni I, Arthur Hewitt L, Rowse GJ, Kaufmann H. Integrated analysis of droxidopa trials for neurogenic orthostatic hypotension. BMC Neurol. 2017;17:90. doi: 10.1186/s12883-017-0867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park JW, Okamoto LE, Shibao CA, Biaggioni I. Pharmacologic treatment of orthostatic hypotension. Auton Neurosci. 2020;229:102721. doi: 10.1016/j.autneu.2020.102721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rao SV, Stamler JS. Erythropoietin, anemia, and orthostatic hypotension: the evidence mounts. Clin Auton Res. 2002;12:141–143. doi: 10.1007/s10286-002-0031-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the study are included in this published article.