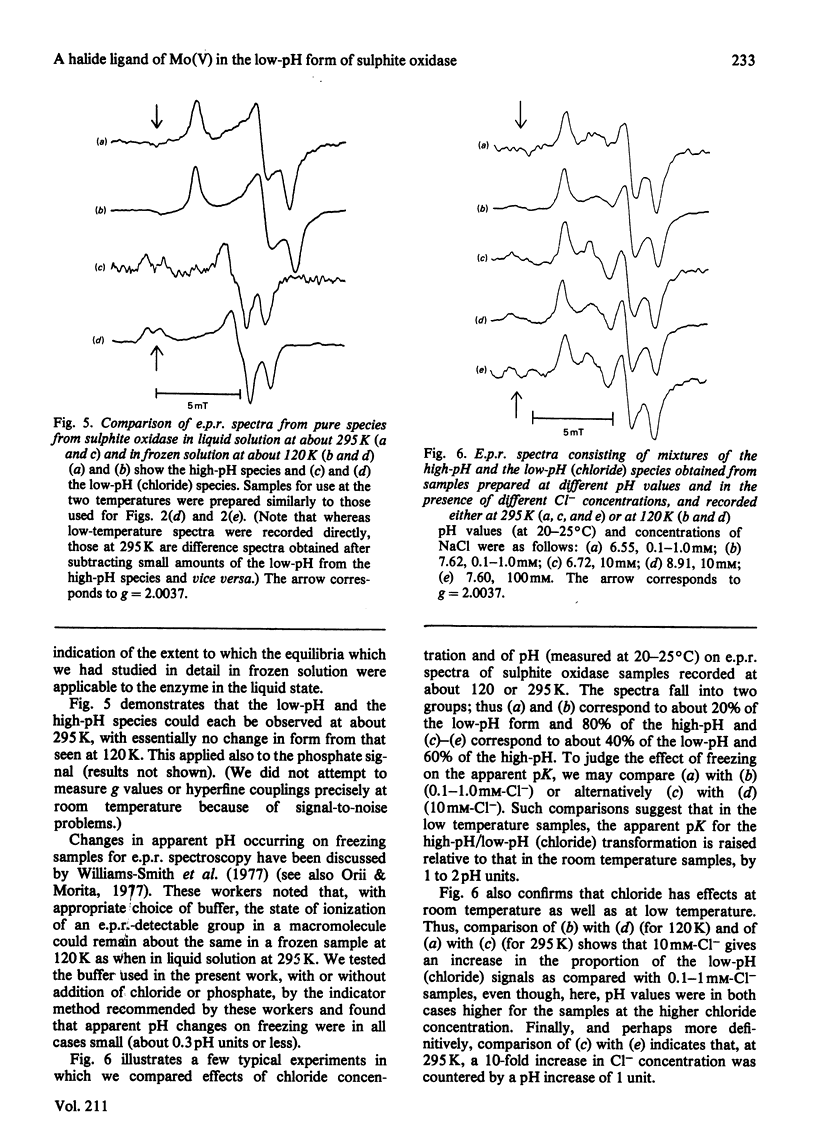
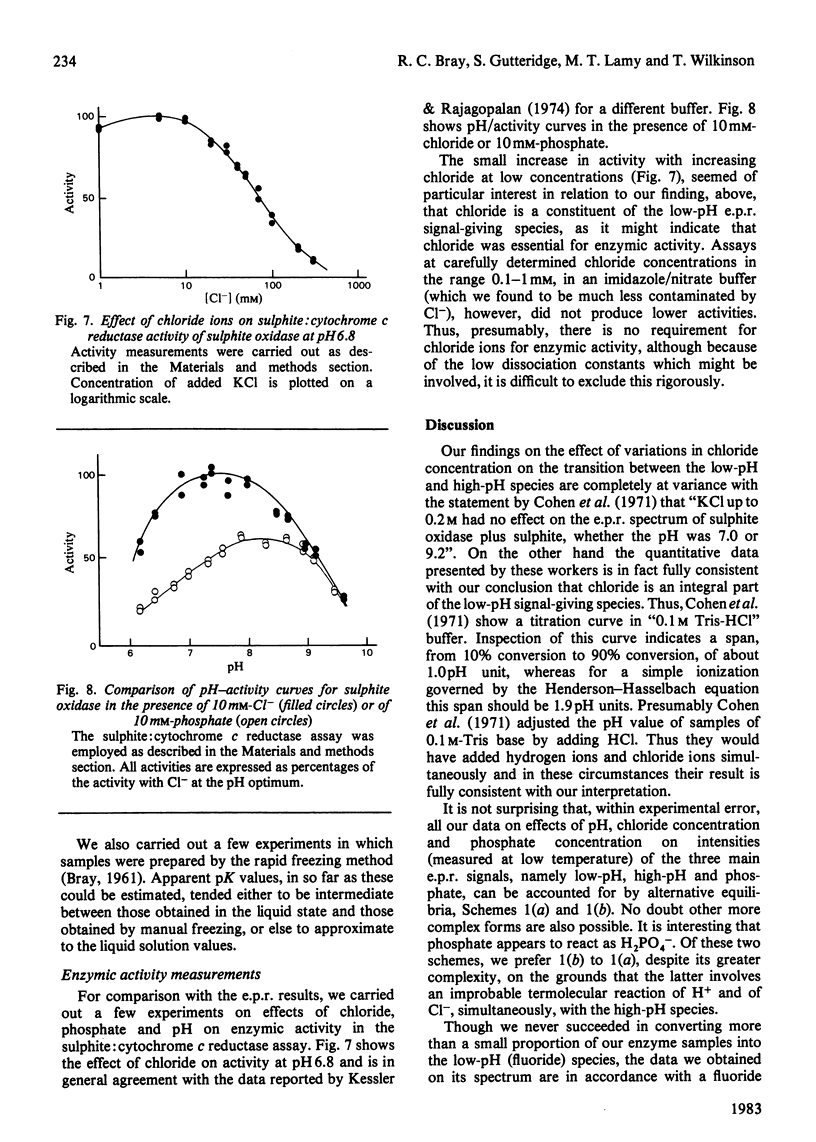
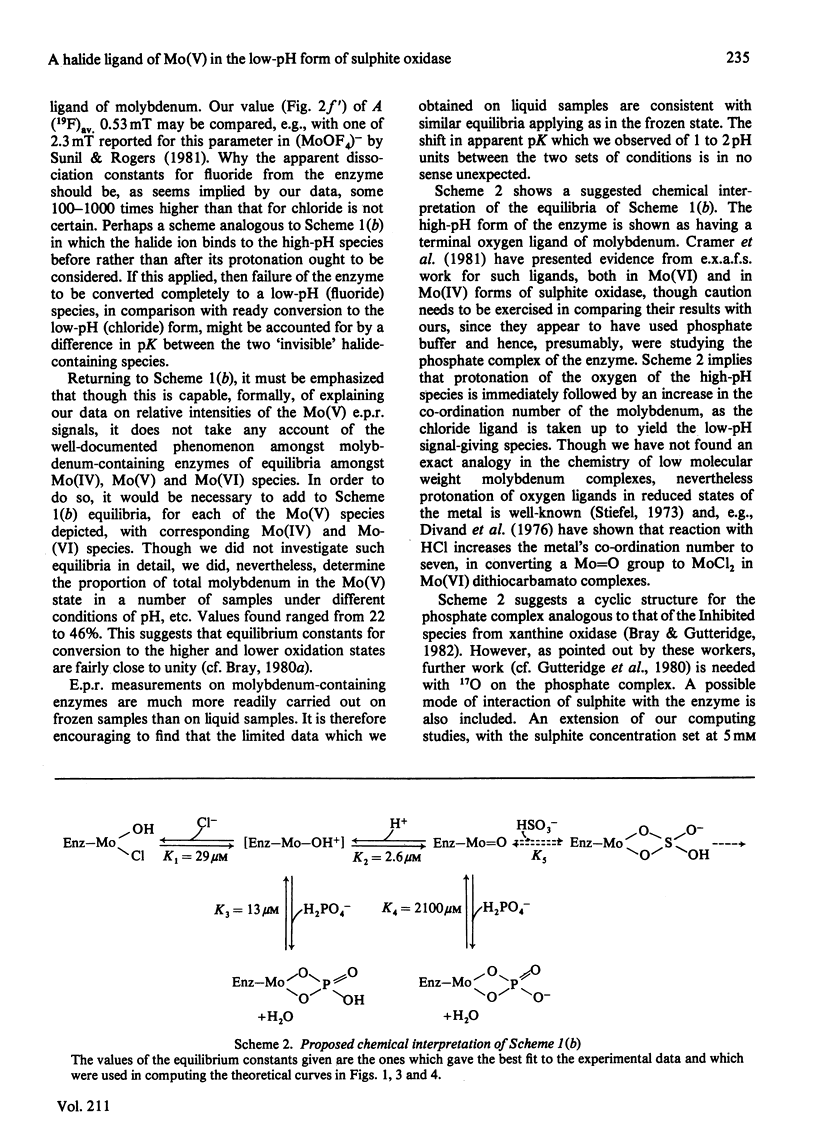
Abstract
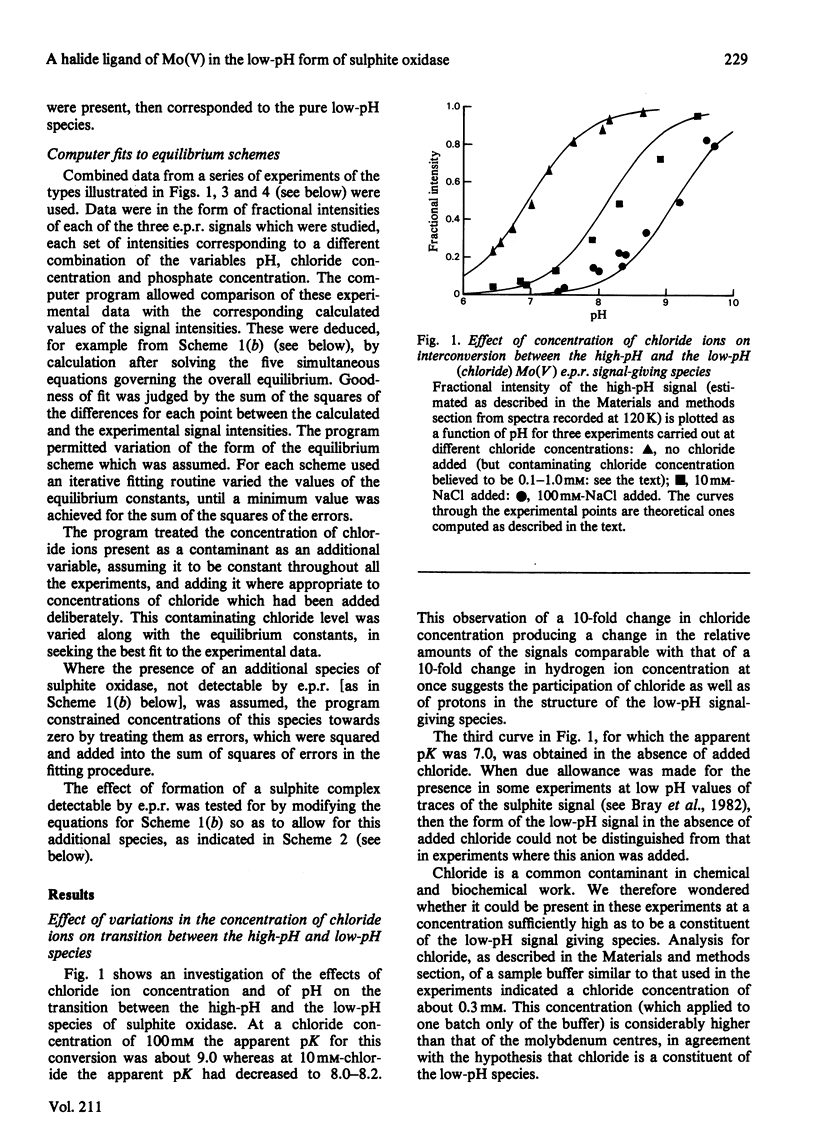
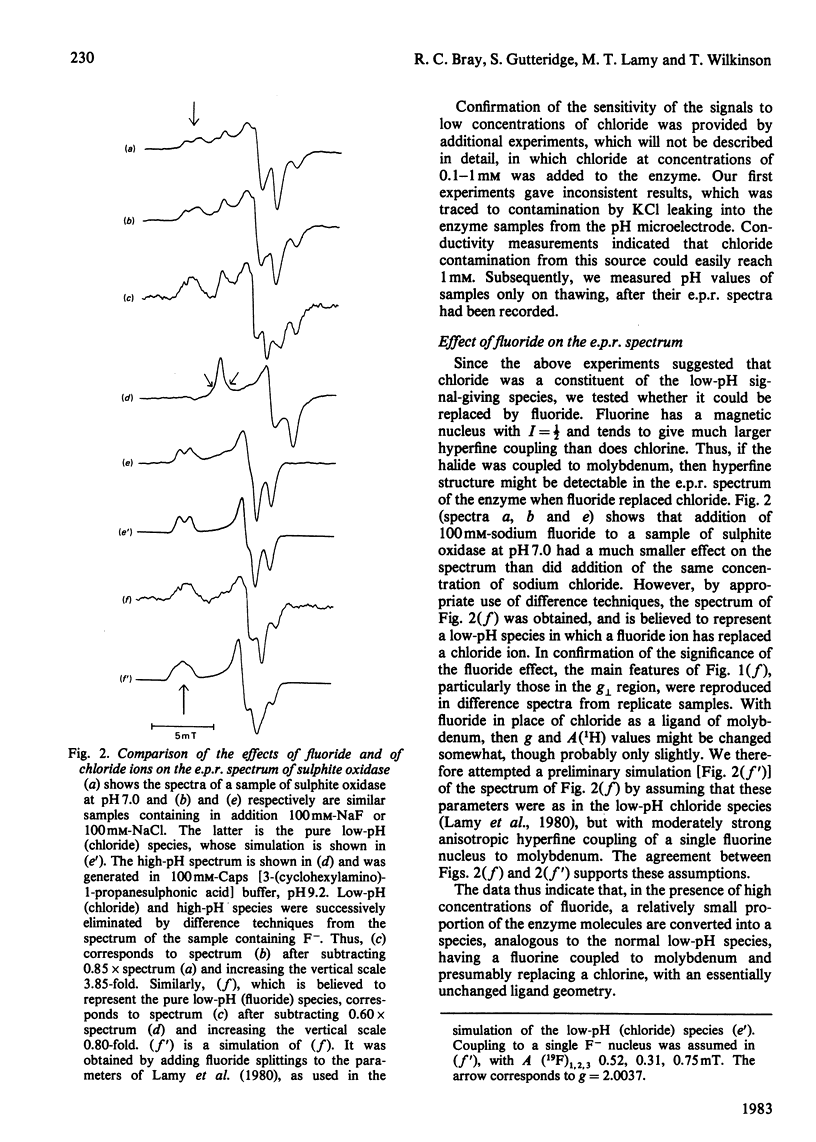
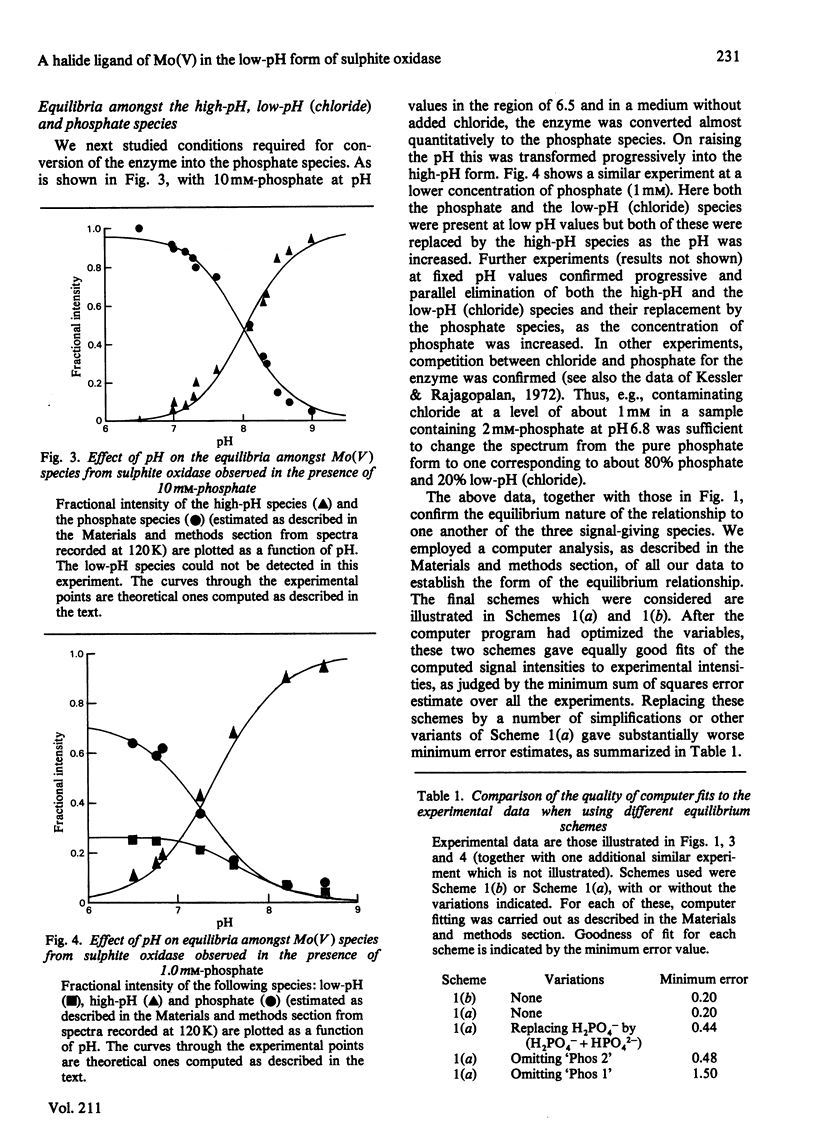
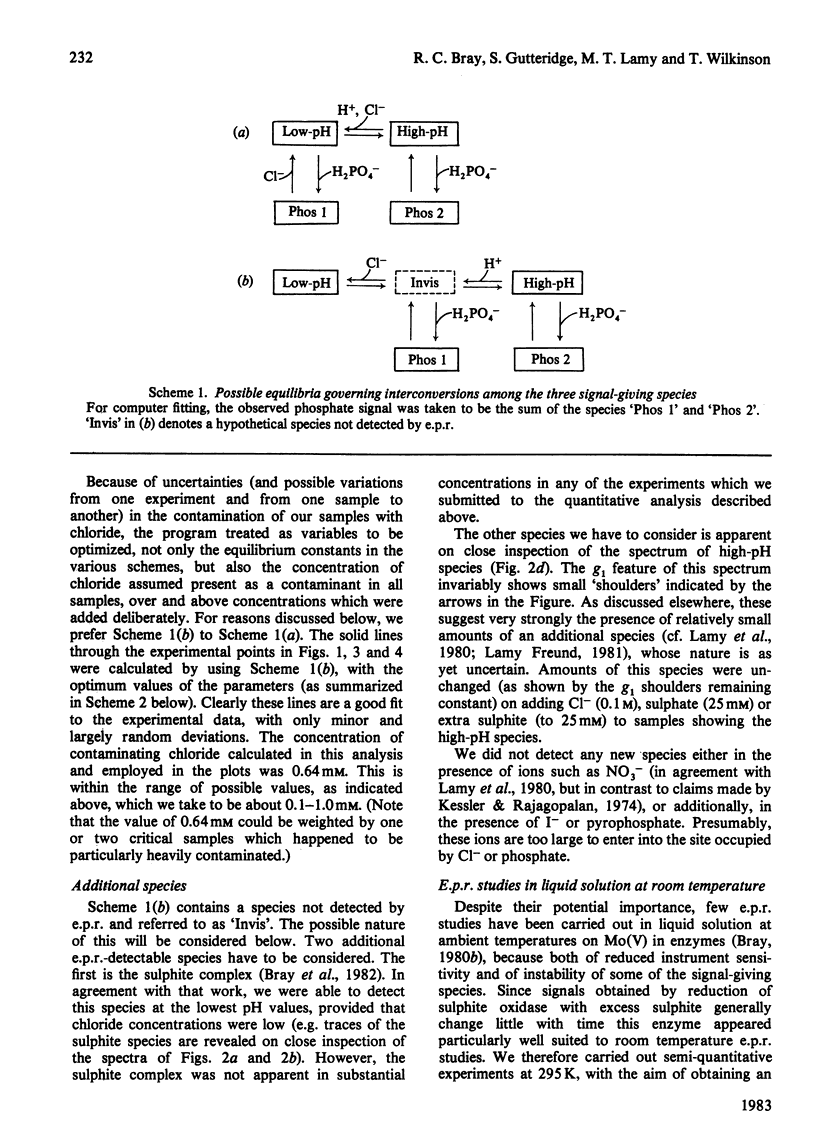
The interaction of chloride, fluoride and phosphate ions with the molybdenum centre of sulphite oxidase in the pH range 6.2 to 9.6 has been studied by e.p.r. of Mo(V) in the enzyme reduced by sulphite. Detailed studies were made from e.p.r. spectra recorded at about 120K and more limited studies from spectra of liquid samples at about 295K and also from enzyme activity measurements. Interconversion between low-pH and high-pH Mo(V) e.p.r. signal-giving species [described by Lamy, Gutteridge & Bray (1980) Biochem. J. 185, 397-403] is influenced by chloride concentration, a 10-fold increase in concentration (in the range of about 1 mM to 100 mM) causing an increase of about 1 pH unit in the apparent pK for the conversion. This suggests that chloride is a constituent of the low-pH species. In support of this, high concentrations of fluoride modified the e.p.r. spectrum. Partial conversion to a Mo(V) species, in which F- has presumably replaced Cl- and showing hyperfine coupling of A(19F)av. 0.5mT, is indicated. It is proposed that interconversion between high-pH and low-pH species is of the form: (formula; see text) No evidence that Cl- is essential for enzymic activity was found. Data relating to equilibria amongst low-pH, high-pH and also the phosphate species are presented. Depending on pH and on concentrations of Cl- and H2PO4-, one, two, or all three species may be present. Qualitatively, under appropriate conditions, the phosphate species tends to replace some or all of the low-pH species. Quantitative analysis by a computer procedure permitted an appropriate scheme to be deduced and equilibrium constants to be evaluated. Studies on the e.p.r. signals at 295K indicated that similar equilibria applied in liquid solution, but with some changes in the values of the constants. The structure of the molybdenum centre in its various states and the nature of the enzymic reaction are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray R. C., Barber M. J., Lowe D. J. Electron-paramagnetic-resonance spectroscopy of complexes of xanthine oxidase with xanthine and uric acid. Biochem J. 1978 Jun 1;171(3):653–658. doi: 10.1042/bj1710653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Gutteridge S. Numbers and exchangeability with water of oxygen-17 atoms coupled to molybdenum (V) in different reduced forms of xanthine oxidase. Biochemistry. 1982 Nov 9;21(23):5992–5999. doi: 10.1021/bi00266a041. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Lamy M. T., Gutteridge S., Wilkinson T. Evidence from electron-paramagnetic-resonance spectroscopy for a complex of sulphite ions with the molybdenum centre of sulphite oxidase. Biochem J. 1982 Jan 1;201(1):241–243. doi: 10.1042/bj2010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C. The reactions and the structures of molybdenum centers in enzymes. Adv Enzymol Relat Areas Mol Biol. 1980;51:107–165. doi: 10.1002/9780470122969.ch3. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Fridovich I., Rajagopalan K. V. Hepatic sulfite oxidase. A functional role for molybdenum. J Biol Chem. 1971 Jan 25;246(2):374–382. [PubMed] [Google Scholar]
- Gutteridge S., Lamy M. T., Bray R. C. The nature of the phosphate inhibitor complex of sulphite oxidase from electron-paramagnetic-resonance studies using oxygen-17. Biochem J. 1980 Oct 1;191(1):285–288. doi: 10.1042/bj1910285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S., Tanner S. J., Bray R. C. The molybdenum centre of native xanthine oxidase. Evidence for proton transfer from substrates to the centre and for existence of an anion-binding site. Biochem J. 1978 Dec 1;175(3):869–878. doi: 10.1042/bj1750869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem J. 1970 Mar;116(5):851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. L., Rajagopalan K. V. Hepatic sulfite oxidase. Effect of anions on interaction with cytochrome c. Biochim Biophys Acta. 1974 Dec 29;370(2):389–398. doi: 10.1016/0005-2744(74)90100-4. [DOI] [PubMed] [Google Scholar]
- Kessler D. L., Rajagopalan K. V. Purification and properties of sulfite oxidase from chicken liver. Presence of molybdenum in sulfite oxidase from diverse sources. J Biol Chem. 1972 Oct 25;247(20):6566–6573. [PubMed] [Google Scholar]
- Lamy M. T., Gutteridge S., Bary R. C. Electron-paramagnetic-resonance parameters of molybdenum(V) in sulphite oxidase from chicken liver. Biochem J. 1980 Feb 1;185(2):397–403. doi: 10.1042/bj1850397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J. Electron paramagnetic resonance in biochemistry. Computer simulation of spectra from frozen aqueous samples. Biochem J. 1978 Jun 1;171(3):649–651. doi: 10.1042/bj1710649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orii Y., Morita M. Measurement of the pH of frozen buffer solutions by using pH indicators. J Biochem. 1977 Jan;81(1):163–168. doi: 10.1093/oxfordjournals.jbchem.a131431. [DOI] [PubMed] [Google Scholar]
- Stiefel E. I. Proposed molecular mechanism for the action of molybedenum in enzymes: coupled proton and electron transfer. Proc Natl Acad Sci U S A. 1973 Apr;70(4):988–992. doi: 10.1073/pnas.70.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Smith D. L., Bray R. C., Barber M. J., Tsopanakis A. D., Vincent S. P. Changes in apparent pH on freezing aqueous buffer solutions and their relevance to biochemical electron-paramagnetic-resonance spectroscopy. Biochem J. 1977 Dec 1;167(3):593–600. doi: 10.1042/bj1670593. [DOI] [PMC free article] [PubMed] [Google Scholar]



