Abstract
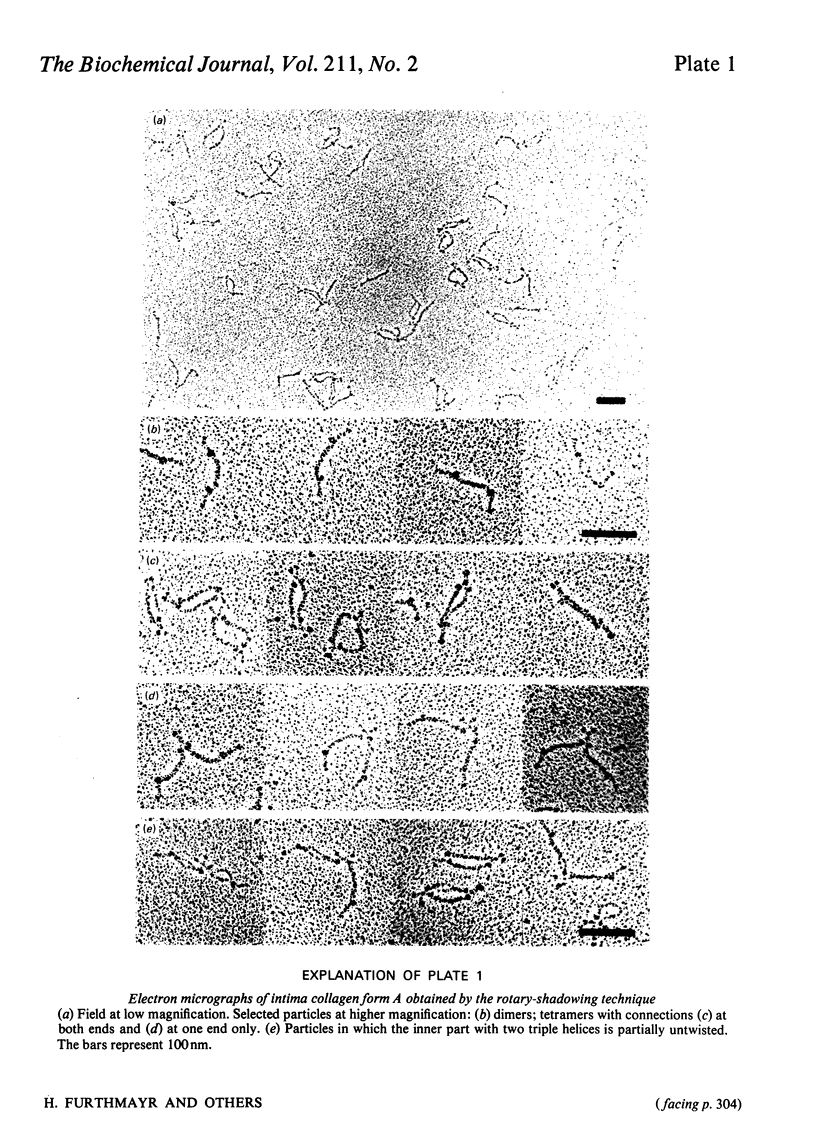
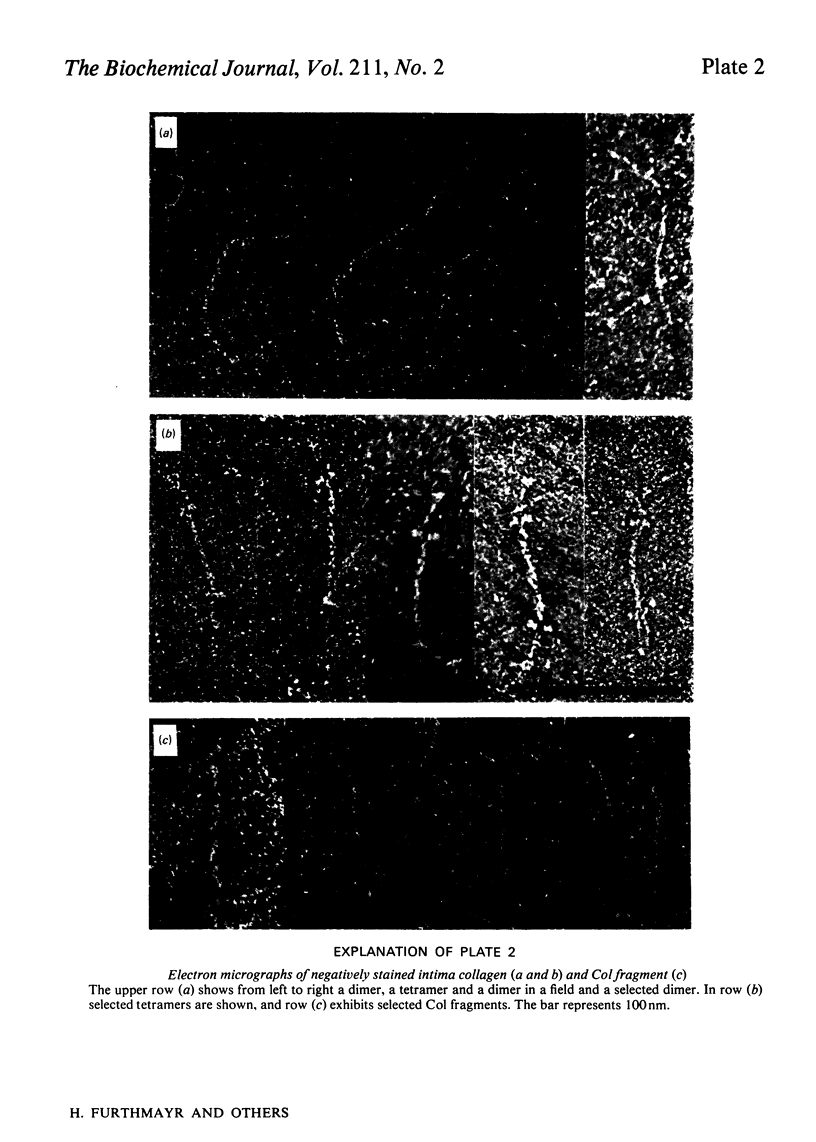
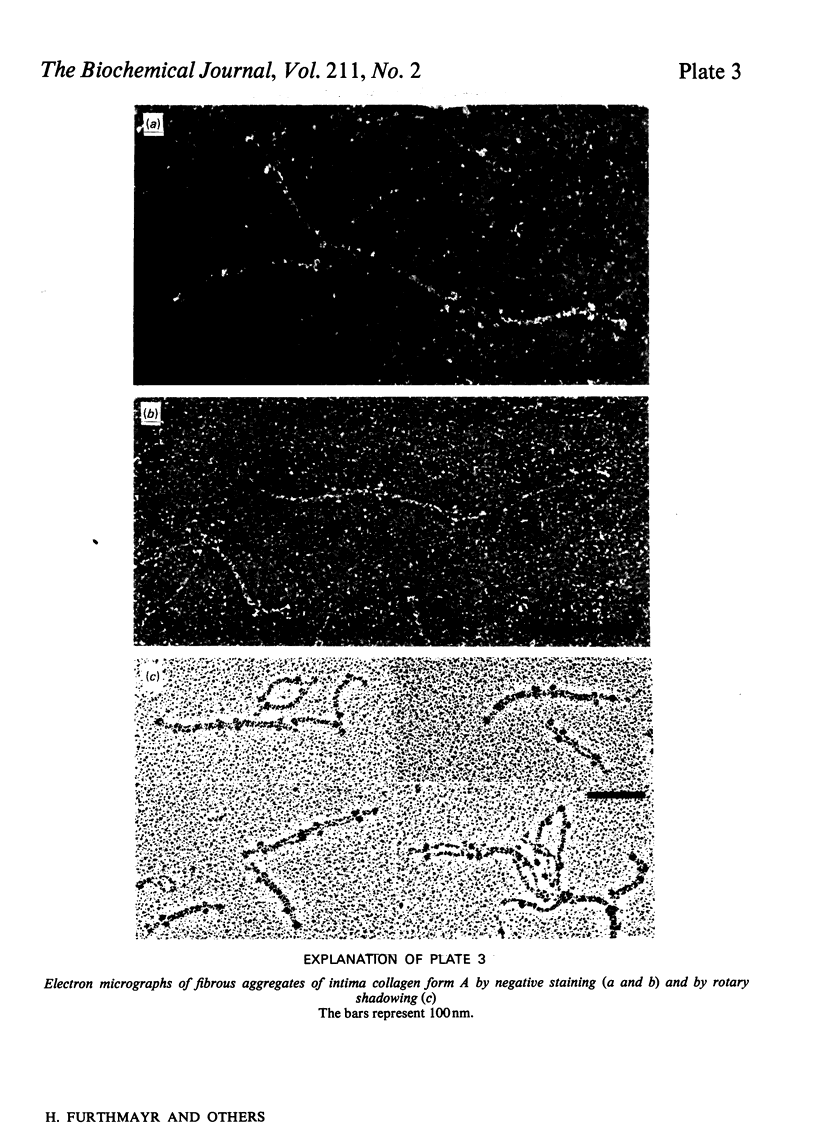
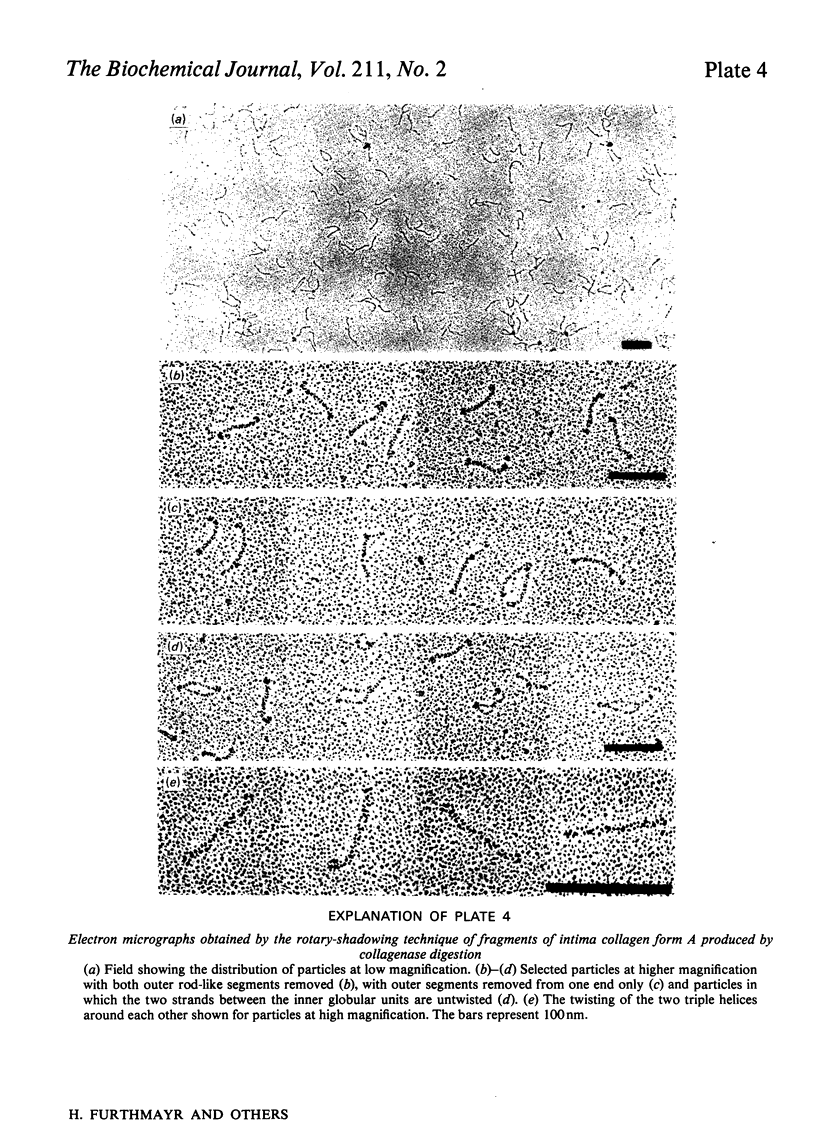
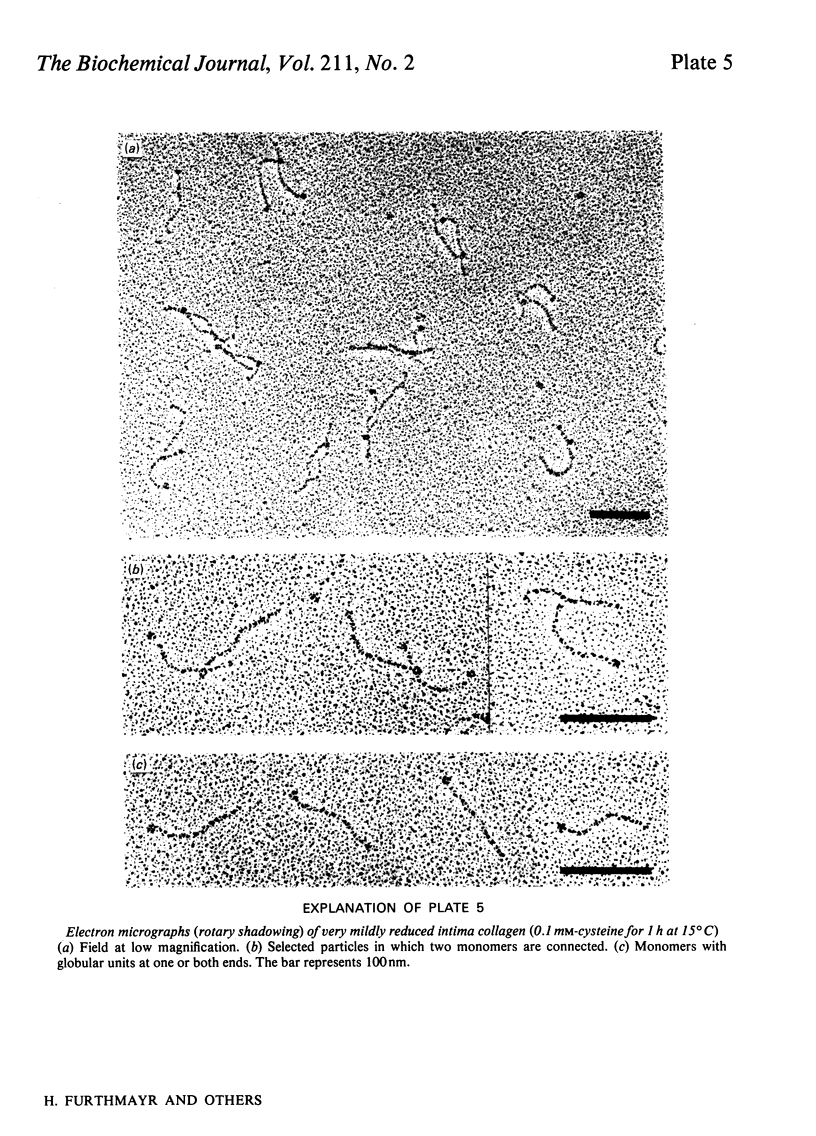
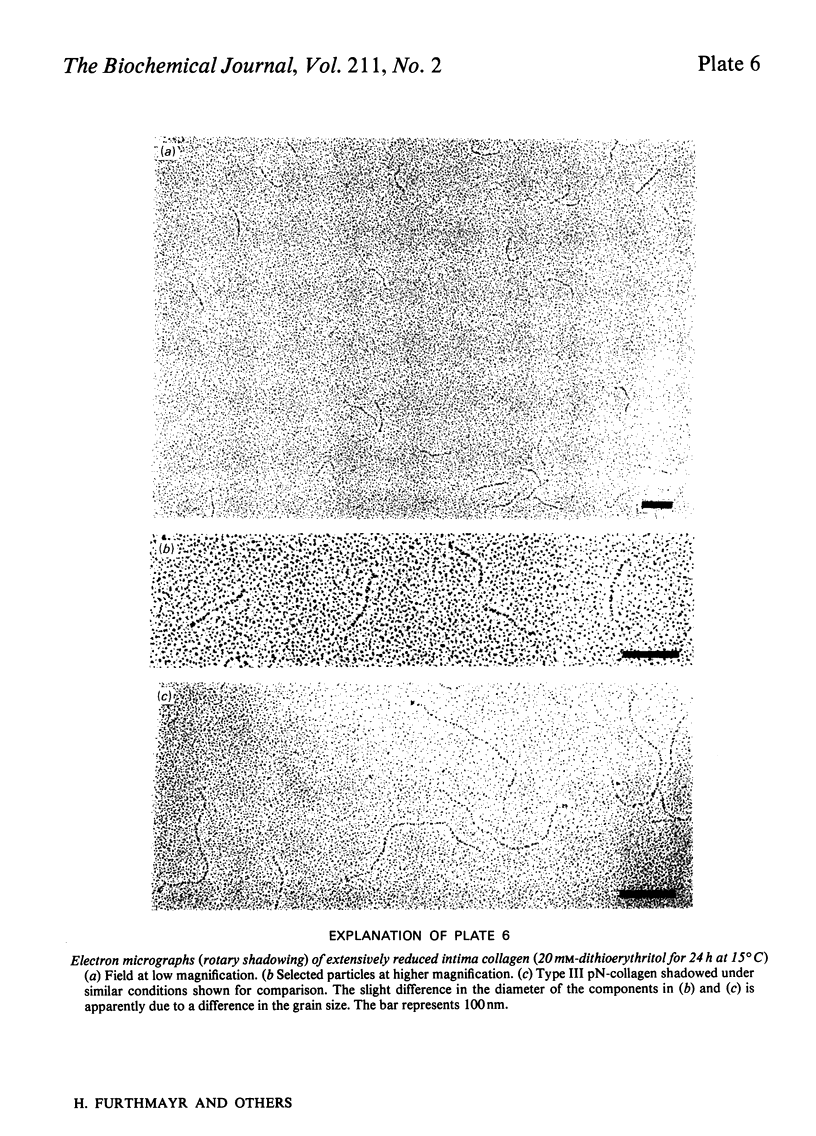
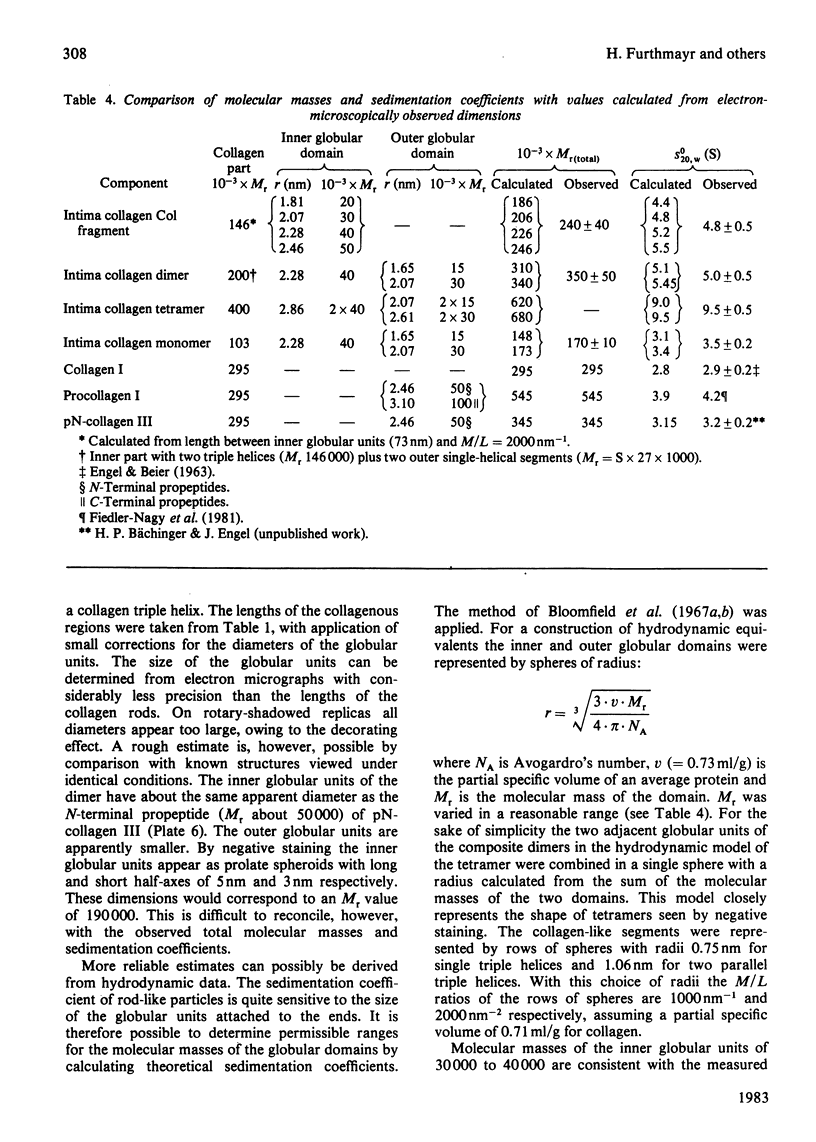
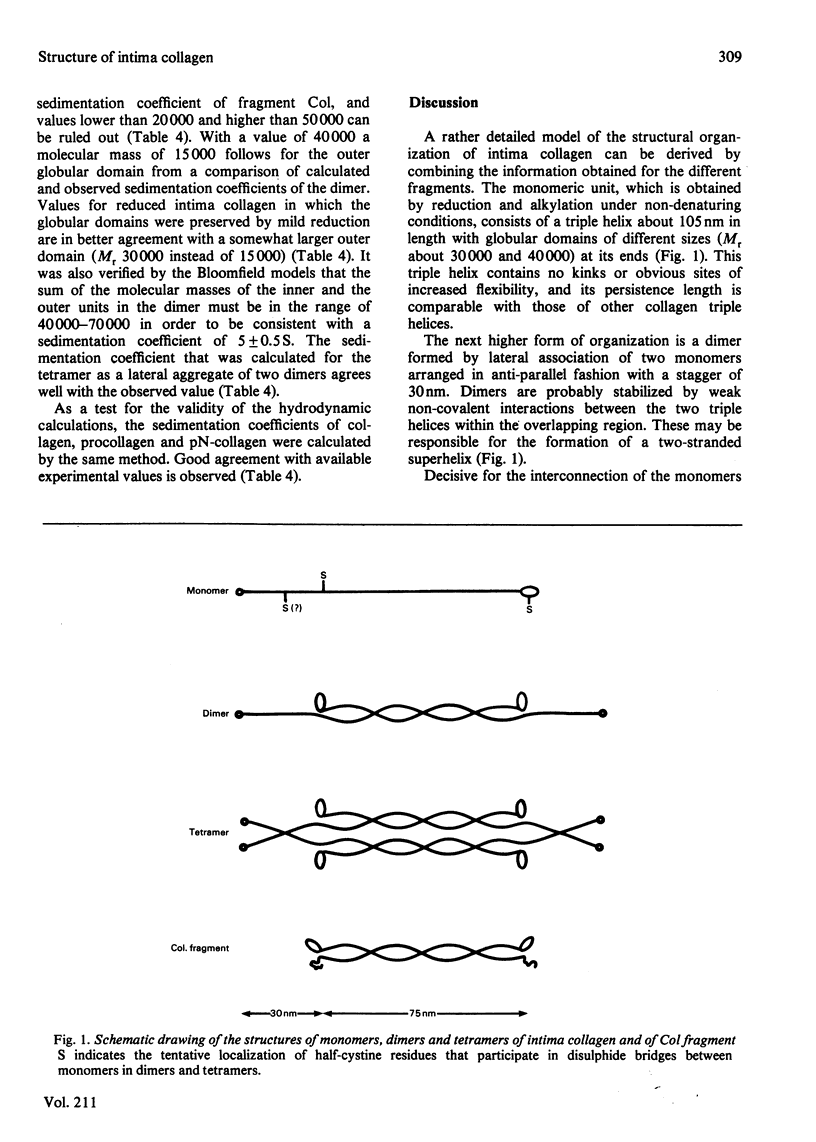
Intima collagen was studied by electron microscopy (rotary shadowing and negative staining) and by analytical ultracentrifugation. It was found that the monomeric unit (Mr 170 000) consists of a 105 nm-long triple helix terminated by a small globular domain (Mr about 30 000) at one end and a large globular domain (Mr about 40 000) at the other end. The monomer was produced by selective reduction of interchain disulphide bridges. Before reduction, dimers, tetramers and larger filamentous structures were found. Dimers are lateral staggered aggregates of two monomers aligned in an anti-parallel fashion. This gives rise to an inner 75 nm-long region of two slightly intertwisted triple helices flanked by the large globular domains. The outer triple-helical segments (length 30 nm) with the small globular domains at their ends emerge at both sides of this structure. Interchain disulphide bridges are probably located in the vicinity of the large domains. Only the outer segments could be degraded by bacterial collagenase. In tetramers the outer segments of two dimers are covalently linked, forming a scissors-like structure. In the fibrous forms several tetramers are assembled end-to-end with an overlap between the outer segments. The molecular masses and sedimentation coefficients were calculated for these various forms from the electron-microscopically observed dimensions and agreed with results obtained by ultracentrifugation. The unique structure of intima collagen suggests that it originates from a microfibrillar component and that it can be considered a unique collagenous protein, for which we propose the designation type VI collagen.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomfield V., Dalton W. O., Van Holde K. E. Frictional coefficients of multisubunit structures. I. Theory. Biopolymers. 1967 Feb;5(2):135–148. doi: 10.1002/bip.1967.360050202. [DOI] [PubMed] [Google Scholar]
- Bloomfield V., Van Holde K. E., Dalton W. O. Frictional coefficients of multisubunit structures. II. Application to proteins and viruses. Biopolymers. 1967 Feb;5(2):149–159. doi: 10.1002/bip.1967.360050203. [DOI] [PubMed] [Google Scholar]
- Desgranges C., Razaka G., Larrue J., Bricaud H. Etude de la trame macromoléculaire extracellulaire élaborée par des cellules aortiques de rat en culture secondaire. C R Seances Soc Biol Fil. 1976;170(4):760–764. [PubMed] [Google Scholar]
- ENGEL J., BEIER G. VERGLEICH DER MOLEKULAREN DATEN VON TROPOKOLLAGEN VERSCHIEDENER KALBSHAEUTE IM NATIVEN UND DENATURIERTEN ZUSTAND. Hoppe Seylers Z Physiol Chem. 1963;334:201–214. doi: 10.1515/bchm2.1963.334.1.201. [DOI] [PubMed] [Google Scholar]
- Elliott A., Offer G. Shape and flexibility of the myosin molecule. J Mol Biol. 1978 Aug 25;123(4):505–519. doi: 10.1016/0022-2836(78)90204-8. [DOI] [PubMed] [Google Scholar]
- Engel J., Odermatt E., Engel A., Madri J. A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981 Jul 25;150(1):97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Carrell N., McDonagh J. Fibronectin molecule visualized in electron microscopy: a long, thin, flexible strand. J Cell Biol. 1981 Dec;91(3 Pt 1):673–678. doi: 10.1083/jcb.91.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Timpl R., Tuderman L., Raisher L., Wiestner M., Perlish J. S., Graves P. N. Ultrastructural identification of extension aminopropeptides of type I and III collagens in human skin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7360–7364. doi: 10.1073/pnas.78.12.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foidart J. M., Tryggvason K., Robey P. G., Liotta L. A., Martin G. R. Biosynthesis of type IV and V (alpha A-alpha B) collagens by human placenta. Coll Relat Res. 1981 Feb;1(2):137–150. doi: 10.1016/s0174-173x(81)80016-7. [DOI] [PubMed] [Google Scholar]
- Frontali C., Dore E., Ferrauto A., Gratton E., Bettini A., Pozzan M. R., Valdevit E. An absolute method for the determination of the persistence length of native DNA from electron micrographs. Biopolymers. 1979 Jun;18(6):1353–1373. doi: 10.1002/bip.1979.360180604. [DOI] [PubMed] [Google Scholar]
- Furuto D. K., Miller E. J. Isolation of a unique collagenous fraction from limited pepsin digests of human placental tissue. Characterization of one of the constituent polypeptide chains. J Biol Chem. 1980 Jan 10;255(1):290–295. [PubMed] [Google Scholar]
- Gibson M. A., Cleary E. G. A collagen-like glycoprotein from elastin-rich tissues. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1288–1295. doi: 10.1016/0006-291x(82)90926-3. [DOI] [PubMed] [Google Scholar]
- Jander R., Rauterberg J., Voss B., von Bassewitz D. B. A cysteine-rich collagenous protein from bovine placenta. Isolation of its constituent polypeptide chains and some properties of the non-denatured protein. Eur J Biochem. 1981;114(1):17–25. [PubMed] [Google Scholar]
- Jones C. J., Sear C. H., Grant M. E. An ultrastructural study of fibroblasts derived from bovine ligamentum nuchae and their capacity for elastogenesis in culture. J Pathol. 1980 May;131(1):35–53. doi: 10.1002/path.1711310104. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A., Fessler J. H. Biosynthesis of A,B procollagen. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6434–6438. doi: 10.1073/pnas.77.11.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Fessler J. H. Propeptides of procollagen V (A,B) in chick embryo crop. J Biol Chem. 1981 Jul 10;256(13):7053–7058. [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt E., Risteli J., van Delden V., Timpl R. Structural diversity and domain composition of a unique collagenous fragment (intima collagen) obtained from human placenta. Biochem J. 1983 May 1;211(2):295–302. doi: 10.1042/bj2110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage H., Pritzl P., Bornstein P. A unique, pepsin-sensitive collagen synthesized by aortic endothelial cells in culture. Biochemistry. 1980 Dec 9;19(25):5747–5755. doi: 10.1021/bi00566a013. [DOI] [PubMed] [Google Scholar]
- Sear C. H., Grant M. E., Jackson D. S. The nature of the microfibrillar glycoproteins of elastic fibres. A biosynthetic study. Biochem J. 1981 Feb 15;194(2):587–598. doi: 10.1042/bj1940587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini A., Field J. M. The ultrastructure and mechanics of elastic ligaments. Adv Exp Med Biol. 1977;79:97–103. doi: 10.1007/978-1-4684-9093-0_9. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Takebayashi T., Morita Y., Oosawa F. Electronmicroscopic investigation of the flexibility of F-actin. Biochim Biophys Acta. 1977 Jun 24;492(2):357–363. doi: 10.1016/0005-2795(77)90086-1. [DOI] [PubMed] [Google Scholar]
- Timpl R., Glanville R. W., Nowack H., Wiedemann H., Fietzek P. P., Kühn K. Isolation, chemical and electron microscopical characterization of neutral-salt-soluble type III collagen and procollagen from fetal bovine skin. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1783–1792. doi: 10.1515/bchm2.1975.356.2.1783. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Anderson J. M., Branton D. Structural comparison of several actin-binding macromolecules. J Cell Biol. 1980 May;85(2):489–495. doi: 10.1083/jcb.85.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Reinhardt B. N., Branton D. Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J Biol Chem. 1980 Jul 25;255(14):7034–7039. [PubMed] [Google Scholar]