Abstract
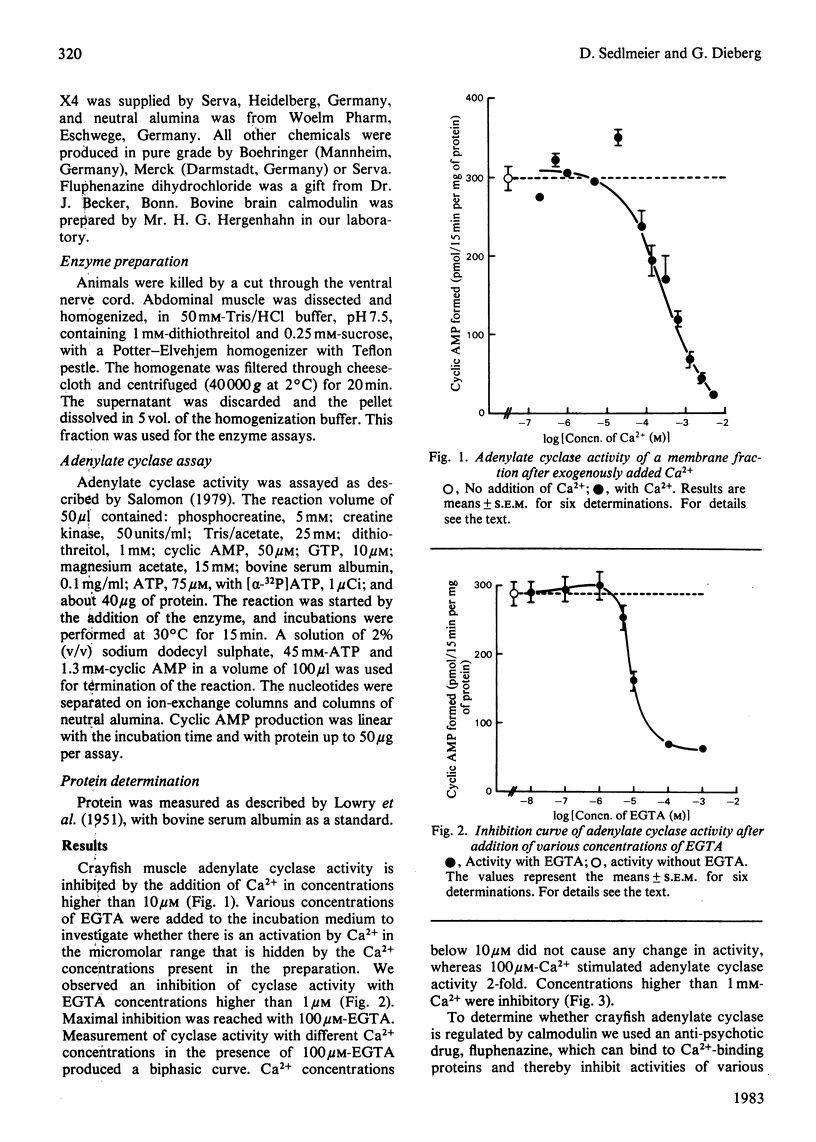
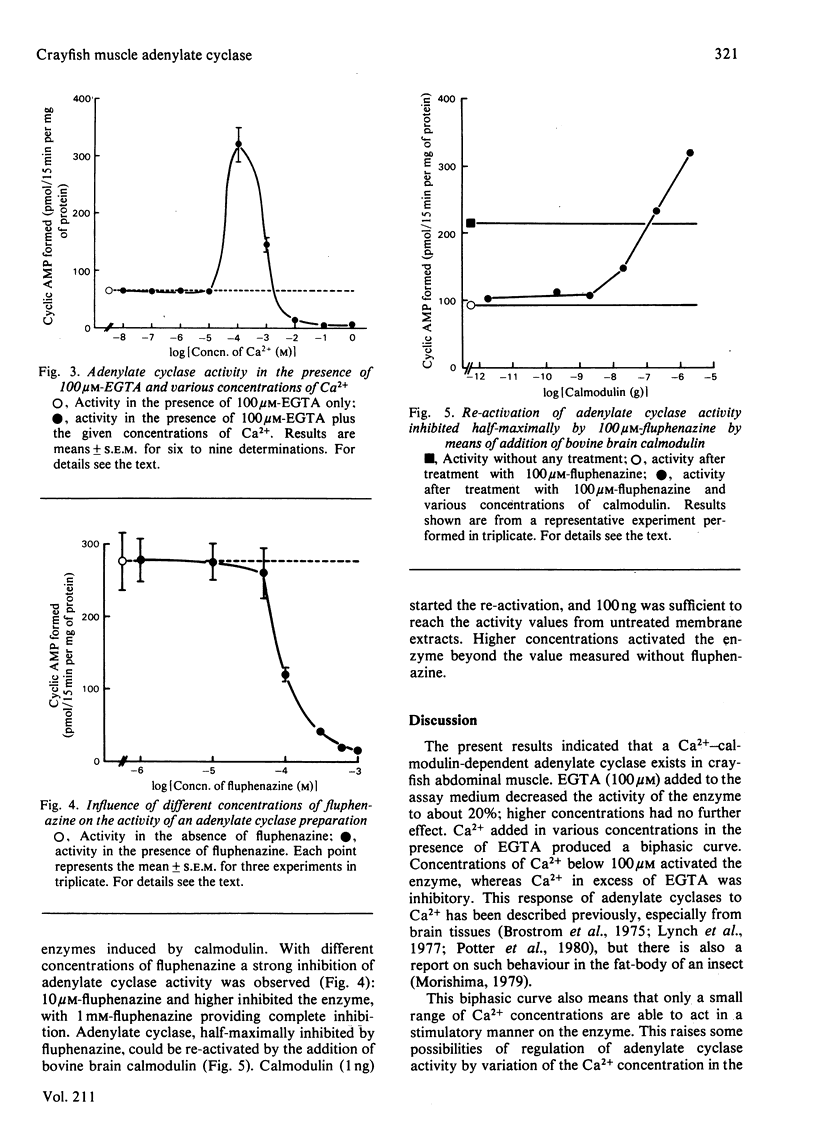
A plasma-membrane preparation of crayfish muscle showed an adenylate cyclase activity which is inhibited to about 80% of its original activity by 100 microM-EGTA. Measurements of the enzyme activity in the presence of 100 microM-EGTA and various concentrations of Ca2+ revealed an increase in enzyme activity of about 400%, indicating an adenylate cyclase which is dependent on Ca2+ for activity. Fluphenazine (1 mM), a blocker of the Ca2+-binding protein calmodulin, decreased enzyme activity to zero. The enzyme can be re-activated by the addition of certain concentrations of calmodulin to the assay medium. This suggests that crayfish muscle adenylate cyclase is dependent on Ca2+ and calmodulin for activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiranoff B., Laburthe M., Rouyer-Fessard C., Demaille J. G., Rosselin G. Régulation par la calmoduline de l'activité adenylate cyclasique de l'épithélium intestinal. C R Seances Acad Sci III. 1982 Mar 15;294(11):487–489. [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y., Bradham L. S., Lynch T. J., Lin Y. M., Tallant E. A. Protein activator of cyclic 3':5'-nucleotide phosphodiesterase of bovine or rat brain also activates its adenylate cyclase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1055–1062. doi: 10.1016/0006-291x(75)90747-0. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin and the adenylate cyclase-phosphodiesterase system. Cell Calcium. 1981 Aug;2(4):263–280. doi: 10.1016/0143-4160(81)90020-8. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Sutherland E. W. Detergent-dispersed adenylate cyclase from rat brain. Effects of fluoride, cations, and chelators. J Biol Chem. 1973 Jul 25;248(14):5114–5121. [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Yamazaki R., Kambayashi J., Sakon M., Kosaki G. Lack of tissue specificity of calmodulin: a rapid and high-yield purification method. FEBS Lett. 1981 Apr 20;126(2):203–207. doi: 10.1016/0014-5793(81)80242-6. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Specificity of the binding of trifluoperazine to the calcium-dependent activator of phosphodiesterase and to a series of other calcium-binding proteins. Biochim Biophys Acta. 1978 May 3;540(2):197–204. doi: 10.1016/0304-4165(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Lynch T. J., Tallant E. A., Cheung W. Y. Rat brain adenylate cyclase. Further studies on its stimulation by a Ca2+-binding protein. Arch Biochem Biophys. 1977 Jul;182(1):124–133. doi: 10.1016/0003-9861(77)90290-9. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Calmodulin: a protein for all seasons. Science. 1980 Apr 18;208(4441):274–276. doi: 10.1126/science.6102798. [DOI] [PubMed] [Google Scholar]
- Means A. R. Calmodulin: properties, intracellular localization, and multiple roles in cell regulation. Recent Prog Horm Res. 1981;37:333–367. doi: 10.1016/b978-0-12-571137-1.50011-6. [DOI] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Ofulue A. F., Nijjar M. S. Calmodulin activation of rat lung adenylate cyclase is independent of the cytoplasmic factors modulating the enzyme. Biochem J. 1981 Dec 15;200(3):475–480. doi: 10.1042/bj2000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piascik M. T., Piascik M. F., Hitzemann R. J., Potter J. D. Ca2+-dependent regulation of rat caudate nucleus adenylate cyclase and effects on the response to dopamine. Mol Pharmacol. 1981 Sep;20(2):319–325. [PubMed] [Google Scholar]
- Piascik M. T., Wisler P. L., Johnson C. L., Potter J. D. Ca2+-dependent regulation of guinea pig brain adenylate cyclase. J Biol Chem. 1980 May 10;255(9):4176–4181. [PubMed] [Google Scholar]
- Potter J. D., Piascik M. T., Wisler P. L., Robertson S. P., Johnson C. L. Calcium dependent regulation of brain and cardiac muscle adenylate cyclase. Ann N Y Acad Sci. 1980;356:220–231. doi: 10.1111/j.1749-6632.1980.tb29613.x. [DOI] [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Sedlmeier D., Keller R. The mode of action of the crustacean neurosecretory hyperglycemic hormone. I. Involvement of cyclic nucleotides. Gen Comp Endocrinol. 1981 Sep;45(1):82–90. doi: 10.1016/0016-6480(81)90172-6. [DOI] [PubMed] [Google Scholar]
- Sedlmeier D. The mode of action of the crustacean neurosecretory hyperglycemic hormone (CHH). II. Involvement of glycogen synthase. Gen Comp Endocrinol. 1982 Aug;47(4):426–432. doi: 10.1016/0016-6480(82)90120-4. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Wiedenkeller D. E., Kaelin D., Siegel E. G., Wollheim C. B. Stimulation of adenylate cyclase by Ca2+ and calmodulin in rat islets of langerhans: explanation for the glucose-induced increase in cyclic AMP levels. Diabetes. 1980 Jan;29(1):74–77. doi: 10.2337/diab.29.1.74. [DOI] [PubMed] [Google Scholar]
- Valverde I., Vandermeers A., Anjaneyulu R., Malaisse W. J. Calmodulin activation of adenylate cyclase in pancreatic islets. Science. 1979 Oct 12;206(4415):225–227. doi: 10.1126/science.225798. [DOI] [PubMed] [Google Scholar]
- Waisman D., Stevens F. C., Wang J. H. The distribution of the Ca++-dependent protein activator of cyclic nucleotide phosphodiesterase in invertebrates. Biochem Biophys Res Commun. 1975 Aug 4;65(3):975–982. doi: 10.1016/s0006-291x(75)80481-5. [DOI] [PubMed] [Google Scholar]


