Abstract
1. The action of dilute H2O2 on a series of ovarian-cyst glycoproteins and glycopolypeptides was investigated. 2. Both native glycoproteins and the glycopolypeptides were carbohydrate-rich, of relatively low molecular weight and of simple structure. 3. At pH 5.6 and 37 degrees C, exposure to H2O2 for a limited time brought about a partial degradation, the molecular weight being decreased by 2-4-fold. 4. Carbohydrate analysis showed very little change in the oligosaccharide moiety, apart from a small decrease in sialic acid in some samples. 5. Amino acid analysis showed minor changes in serine, threonine and proline contents, but almost total loss of histidine. Concomitantly, there was a small gain in aspartic acid. 6. Myosin, examined at both pH 5.7 and 6.7, exhibited generally similar behaviour, there being losses of other amino acid residues as well as histidine: the viscosity was decreased to a low value, and a range of peptides of widely varying size was produced. 7. It is suggested that attack on the histidine residue, with partial conversion into aspartic acid, is accompanied by scission of the histidyl peptide bond.
Full text
PDF



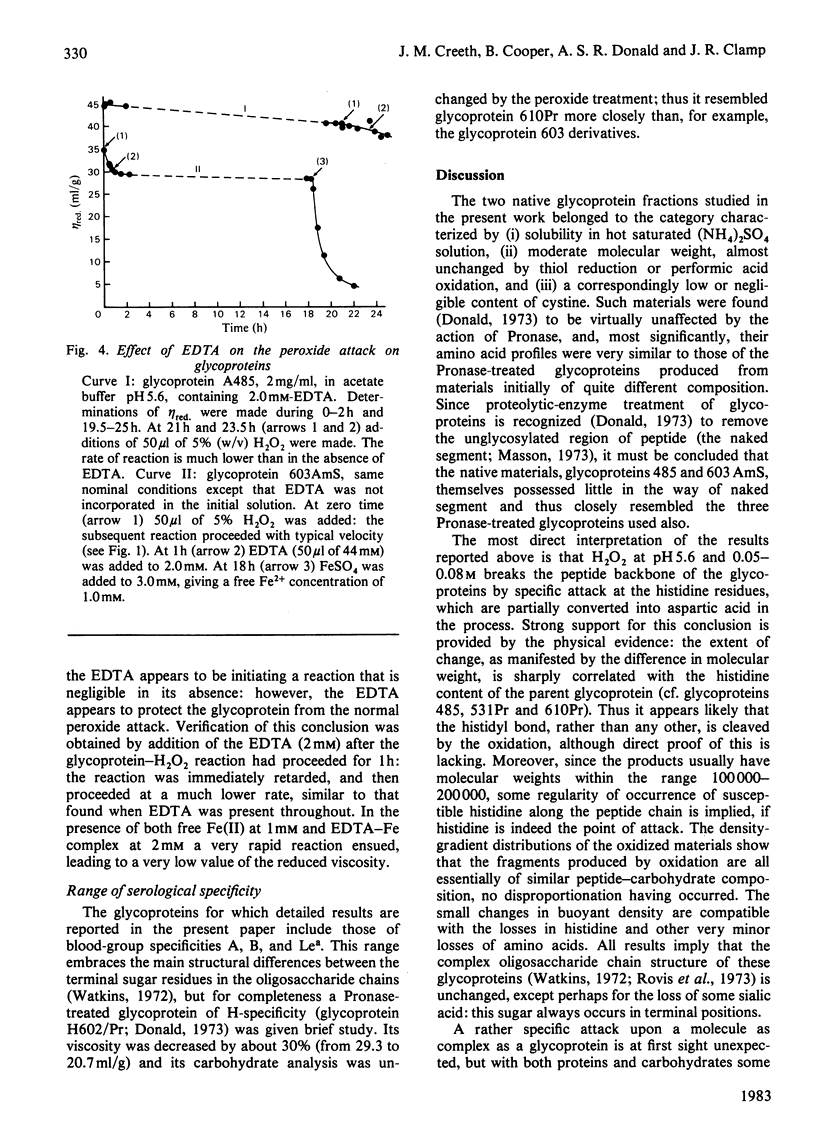


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhaskar K. R., Creeth J. M. The macromolecular properties of blood-group-specific glycoproteins. Characterization of a series of fractions obtained by density-gradient ultracentrifugation. Biochem J. 1974 Dec;143(3):669–679. doi: 10.1042/bj1430669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar K. R., Donald A. S., Morgan W. T., Creeth J. C. The macromolecular properties of blood-group-specific glycoproteins. Characterization of a series of fractions obtained by solvent fractionation. Biochem J. 1974 Oct;143(1):159–170. doi: 10.1042/bj1430159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Treby D. A., Halliwell B., Gutteridge J. M. Protection against superoxide and hydrogen peroxide in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1981 Oct;61(4):483–486. doi: 10.1042/cs0610483. [DOI] [PubMed] [Google Scholar]
- Clamp J. R. A gas-liquid chromatographic approach to the analysis of carbohydrates. Biochem Soc Trans. 1977;5(6):1693–1695. doi: 10.1042/bst0051693. [DOI] [PubMed] [Google Scholar]
- Cleland R. L., Stoolmiller A. C., Rodén L., Laurent T. C. Partial characterization of reaction products formed by the degradation of hyaluronic acid with ascorbic acid. Biochim Biophys Acta. 1969 Dec 30;192(3):385–394. doi: 10.1016/0304-4165(69)90387-0. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Bhaskar K. R., Horton J. R., Das I., Lopez-Vidriero M. T., Reid L. The separation and characterization of bronchial glycoproteins by density-gradient methods. Biochem J. 1977 Dec 1;167(3):557–569. doi: 10.1042/bj1670557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M. Some physical properties of mucins and their molecular origin. Mod Probl Paediatr. 1976 Oct 24;19:34–45. [PubMed] [Google Scholar]
- Creeth M. J., Horton J. R. Macromolecular distribution near the limits of density-gradient columns. Some applications to the separation and fractionation of glycoproteins. Biochem J. 1977 Mar 1;161(3):449–463. doi: 10.1042/bj1610449a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstone J. R., Morgan W. T. Further observations on the glycoproteins in human ovarian cyst fluids. Biochim Biophys Acta. 1965 Nov 1;101(3):300–314. doi: 10.1016/0926-6534(65)90009-6. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Emes C. H., Rowe A. J. Hydrodynamic studies on the self association of vertebrate skeletal muscle myosin. Biochim Biophys Acta. 1978 Nov 20;537(1):110–124. doi: 10.1016/0005-2795(78)90607-4. [DOI] [PubMed] [Google Scholar]
- Fraser D., Clamp J. R. The glycoprotein content of meconium. Clin Chim Acta. 1975 Mar 24;59(3):301–307. doi: 10.1016/0009-8981(75)90005-4. [DOI] [PubMed] [Google Scholar]
- Gibbons R. A. Mucus of the mammalian genital tract. Br Med Bull. 1978 Jan;34(1):34–38. [PubMed] [Google Scholar]
- Goodwin S. D., Watkins W. M. The peptide moiety of blood-group-specific glycoproteins. Some amino-acid sequences in the regions carrying the carbohydrate chains. Eur J Biochem. 1974 Sep 1;47(2):371–382. doi: 10.1111/j.1432-1033.1974.tb03702.x. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- HACHIMORI Y., HORINISHI H., KURIHARA K., SHIBATA K. STATES OF AMINO ACID RESIDUES IN PROTEINS. V. DIFFERENT REACTIVITIES WITH H2O2 OF TRYPTOPHAN RESIDUES IN LYSOZYME, PROTEINASES AND ZYMOGENS. Biochim Biophys Acta. 1964 Nov 8;93:346–346. doi: 10.1016/0304-4165(64)90385-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett. 1978 Dec 15;96(2):238–242. doi: 10.1016/0014-5793(78)80409-8. [DOI] [PubMed] [Google Scholar]
- Harris M. J., Herp A., Pigman W. Metal catalysis in the depolymerization of hyaluronic acid by autoxidants. J Am Chem Soc. 1972 Oct 18;94(21):7570–7572. doi: 10.1021/ja00776a047. [DOI] [PubMed] [Google Scholar]
- Holt J. C., Creeth J. M. Studies of the denaturation and partial renaturation of ovalbumin. Biochem J. 1972 Sep;129(3):665–676. doi: 10.1042/bj1290665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S., Davison A. J. The role of interactions between O2, H2O2, .OH,e- and O2- in free radical damage to biological systems. Arch Biochem Biophys. 1980 Oct 1;204(1):18–29. doi: 10.1016/0003-9861(80)90003-x. [DOI] [PubMed] [Google Scholar]
- Matheson I. B., Etheridge R. D., Kratowich N. R., Lee J. The quenching of singlet oxygen by amino acids and proteins. Photochem Photobiol. 1975 Mar;21(3):165–171. doi: 10.1111/j.1751-1097.1975.tb06647.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- Meyer R. A. Comparison of structural glycoproteins from mucus of different sources. Biochim Biophys Acta. 1977 Aug 23;493(2):272–282. doi: 10.1016/0005-2795(77)90183-0. [DOI] [PubMed] [Google Scholar]
- Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun. 1975 Mar 17;63(2):463–468. doi: 10.1016/0006-291x(75)90710-x. [DOI] [PubMed] [Google Scholar]
- Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973 Mar 15;74(4):653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Allen A., Parry S. A 70000-molecular-weight protein isolated from purified pig gastric mucus glycoprotein by reduction of disulphide bridges and its implication in the polymeric structure. Biochem J. 1981 Jul 1;197(1):155–162. doi: 10.1042/bj1970155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P. Isolation and characterisation of glycoproteins from sputum. Eur J Biochem. 1974 Dec 16;50(1):265–280. doi: 10.1111/j.1432-1033.1974.tb03895.x. [DOI] [PubMed] [Google Scholar]
- Roberts G. P. The role of disulfide bonds in maintaining the gel structure of bronchial mucus. Arch Biochem Biophys. 1976 Apr;173(2):528–537. doi: 10.1016/0003-9861(76)90289-7. [DOI] [PubMed] [Google Scholar]
- Rovis L., Anderson B., Kabat E. A., Gruenzo F., Liao J. Structures of oligosaccharides produced by base--borohydride degradation of human ovarian cyst blood group H, Le-b and Le-a active glycoproteins. Biochemistry. 1973 Dec 18;12(26):5340–5354. doi: 10.1021/bi00750a018. [DOI] [PubMed] [Google Scholar]
- Schmut O., Hofmann H. Studies on the generation of hydrogen peroxide during some non-enzymic reactions changing the hyaluronic acid molecule. Biochim Biophys Acta. 1975 Dec 5;411(2):231–235. doi: 10.1016/0304-4165(75)90303-7. [DOI] [PubMed] [Google Scholar]
- Snary D., Allen A., Pain R. H. Structural studies on gastric mucoproteins: lowering of molecular weight after reduction with 2-mercaptoethanol. Biochem Biophys Res Commun. 1970 Aug 24;40(4):844–851. doi: 10.1016/0006-291x(70)90980-0. [DOI] [PubMed] [Google Scholar]
- Teller D. C. Characterization of proteins by sedimentation equilibrium in the analytical ultracentrifuge. Methods Enzymol. 1973;27:346–441. doi: 10.1016/s0076-6879(73)27017-9. [DOI] [PubMed] [Google Scholar]
- Tomita M., Irie M., Ukita T. Sensitized photooxidation of histidine and its derivatives. Products and mechanism of the reaction. Biochemistry. 1969 Dec;8(12):5149–5160. doi: 10.1021/bi00840a069. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wong S. F., Halliwell B., Richmond R., Skowroneck W. R. The role of superoxide and hydroxyl radicals in the degradation of hyaluronic acid induced by metal ions and by ascorbic acid. J Inorg Biochem. 1981 Apr;14(2):127–134. doi: 10.1016/s0162-0134(00)80033-1. [DOI] [PubMed] [Google Scholar]