Abstract
Many studies have been carried out along mighty rivers with heavily industrialized watersheds to evaluate pollutants and their effects on freshwater organisms. However, their impact on marine organisms is virtually unknown. In order to cover this gap, Solea solea, one of the most important commercial fish species, together with sediments, were sampled during 2013–2015 offshore from the Ebro Delta river mouth. Fish health indicators (condition indices, histological tissue alterations, and parasite descriptors) were used to assess the potential effect of pollutants, an issue of particular interest in the area following the dredging activities taking place in the river upstream in 2013. No major histopathological alterations were detected, but perivascular inflammatory foci (PIF) were frequently observed, especially in 2014. The most prevalent and abundant parasites were acanthocephalans and digeneans within the digestive tract and copepods on the gills. Levels of trace metals from sediments and fish muscle were below the effects range median and reference levels accepted for human consumption, respectively. However, the lower levels of the hepatosomatic index, higher numbers of PIF, and variations in the abundance of parasites in 2014 and 2015 could suggest a pollutant exposure during these years. These results warn signs of toxicity, which could be associated with sediment leaks during the dredging activities.
Keywords: Solea solea; Ebro river mouth; Sentinel, Fish health; Trace metals; Flix
Introduction
The Ebro river basin is the largest Spanish fluvial system flowing into the Mediterranean Sea, with a course of more than 900 km that ends in a delta of more than 30,000 ha (Ocampo 2008; CHEbro 2009). This riverine system and its runoff are of vital importance for both the existing marine ecosystems in the area, with a large primary and secondary production, and commercial activities (such as fish and shellfish fisheries). Historically, the Ebro river mouth has been an environmentally relevant area due to its natural characteristics (World Health Organization 1999). Nevertheless, anthropogenic pressures arising from industrial and agricultural activities carried out along the river lead to the release and potential accumulation of pollutants in the water, sediments, and organisms and can affect the continental margins once they end up in the sea (Mormede and Davies 2001; Storelli et al. 2002; Koenig et al. 2013). In addition, the industrial wastes from the chlor-alkali plant operating over decades at the vicinity of the town of Flix (Catalonia, Spain) have significantly contributed to the increase in the levels of pollutants such as metals, organic compounds, and radionuclides in the area (Grimalt et al. 2003; Nadal et al. 2011; Palanques et al. 2014). Although the flow retention of the river induced by the Flix Dam contributed to the formation of a sludge deposit at the southern riverbank, some authors pointed out the potential risk of contaminated resuspended fine particles when the flow of the river increases (Bosch et al. 2009; Alcaraz et al. 2011; Palanques et al. 2014) and with periodic artificial flows (Tena and Batalla 2013). This could affect the ecosystems located downstream, in the lower Ebro river, or even reach the river delta, where natural reserves and human activities (fisheries and aquaculture) are very important. In fact, high levels of metals were found in the wildlife around the factory (Carrasco et al. 2008; Faria et al. 2010; Soto et al. 2011) and some invertebrates downstream, especially zebra mussels, indicating a resuspension of polluted sediments in the Flix reservoir was probably taking place (Carrasco et al. 2008; Faria et al. 2010; Alcaraz et al. 2011). Due to the toxicological levels of the polluted mud deposit, a dredging process for its removal was initiated in 2013. Sediments were subsequently processed in a nearby treatment plant and disposed of in a landfill. Dredging activities may represent a considerable release of toxic compounds accumulated in sediments to the river water (Gustavson et al. 2008) and are, consequently, a risk to aquatic organisms. Lower river transects have been monitored in recent years, focusing especially on contaminants in water, sediments, and biota along the river (Vilavert et al. 2015; Blanco et al. 2018). However, despite extensive river monitoring, studies of pollutants from river discharge in the adjacent marine system and their potential effects on marine organisms are scarce (Crespo and Solé 2016).
Marine species are particularly susceptible to pollutants because oceans act as the ultimate sink for most of them. According to different guidelines on surveillance programs in marine strategies (e.g., OSPAR or the European Commission), fish and shellfish are suitable organisms for monitoring hazardous substances, such as metals. Each organism gives us different information because they occupy different levels in the food chain (filter-feeding and predators). For example, mollusks are very useful for assessing water quality (Sarma et al. 2013; Seetharaman et al. 2015), but fish, being higher up in the food chain, integrate information of the whole ecosystem. Both mollusks and fish can be useful in toxicity studies. According to Yancheva et al. (2018) (and references therein), fish could be good sentinel organisms monitoring the accumulation of pollutants and assessing their potential effects on health status as they respond to toxic environmental changes by adapting their metabolite functions. Also, they have well-developed osmoregulatory, endocrine, nervous, and immune systems compared to invertebrates, and they also absorb toxicants directly from the surrounding water and sediments (waterborne exposure) or ingest them along with contaminated food (dietary exposure), enabling the assessment of pollutant transfer through the trophic web. Regarding the best choice of target fish species, flatfish have been proposed as suitable sentinel species to study the effects of exposure to environmental stressors, not only by European guidelines but also by several scientific studies (Myers et al. 1994; Stentiford et al. 2003; Stehr et al. 2004; Dabrowska et al. 2012), due to their benthic behavior and their propensity to develop toxicopathic liver lesions. Species such as Limanda limanda, but also Platichthys flesus or Parophrys vetulus, are the main target species for monitoring purposes in North Europe and America (Kohler et al. 1992; Vethaak and Wester 1996; Myers et al. 2003; Lang et al. 2006; Zorita and Cuevas 2014). Unfortunately, these species are not present in the Mediterranean Sea, and for this reason, other fish species present in the area with similar characteristics have to be used instead, as is the case of the benthic fish sole Solea solea. This species, besides being an important commercial fish in Europe, has been widely used as a sentinel organism both in field and experimental studies (Oliva Ramirez 2011; Fonseca et al. 2011; Ribecco et al. 2012; Sànchez-Nogué et al. 2013; Solé et al. 2013; Jebali et al. 2013; Siscar et al. 2015). Solea spp. have also been used in several ecotoxicological studies, including laboratory exposures to waterborne or contaminated sediments and directly injected contaminants (Arellano et al. 1999; Riba et al. 2004; Costa et al. 2008, 2009, 2012; Oliva et al. 2009). Most of these studies were based on the enzymatic fish response to different kinds of pollutants, and only some of them included other responses such as fish condition indices, histopathology, or gene expression.
Fish health and biological stress are usually used as an integrative reflection of the effects of anthropogenic pollutants (Johnson and Collier 2002). However, in order to assess the overall quality of the aquatic environment, a holistic approach at various biological levels (from fish condition at the individual level to cellular or tissue alteration and parasite infestation) and combining different techniques to assess possible responses in the exposed organisms to pollutants is the most proficient (Dabrowska et al. 2017) since altogether they can give an overview of the population well-being.
Fish condition indices can manifest signs of disease or other physiological characteristics before mortality events take place. The hepatosomatic (HSI) and gonadosomatic (GSI) indices are widely used in ecological studies, as they provide information on physiological status related to short-term reserve/accumulation capacity and reproductive capacity, respectively (Wootton 1989). Especially HSI is frequently related to the exposure to pollutants (Goede and Barton 1990; Khan 2010), generally increasing in hepatocytes’ size, number, or both, in contaminated areas (Goede and Barton 1990). The Fulton’s condition factor is the main indicator of an individual’s/population’s fattening (Nash et al. 2006), and it is known to decrease in fish after exposure to heavy metals (Bervoets and Blust 2003; Merciai et al. 2014)
Enzymatic biomarkers in fish are widely accepted and validated by providing a rapid response to contaminants (Van der Oost et al. 2003). Recently, Crespo and Solé (2016) identified a potentially stress-driven situation in Solea solea in front of the Ebro river mouth in 2015 by using a battery of enzymatic biomarkers. Despite considered more specific to evaluate the responses to certain chemical exposures (e.g., acetylcholinesterases respond to neurotoxic compounds), their use together with other biomarkers is strongly advised.
Histopathological alterations are of great relevance in determining the effects of environmental contamination (Bernet et al. 1999; Stentiford et al. 2003; Reddy and Rawat 2013) since they reflect the true state of health of the organism (Costa et al. 2009). They also allow for identifying not only the early warning signs of disease and injury in cells, tissues, or organs but also the subsequent extrapolations to the level of population/community. Regarding aquatic environments, the fish liver is considered one of the main target organs due to its role in the transformation, storage, and even elimination of xenobiotics, being the gills, kidneys, gonads, and digestive tract other common subjects (Bernet et al. 1999; Feist et al. 2004; Au 2004; Costa et al. 2010). Guidelines for the methodologies used for monitoring histopathological lesions of the liver in flatfish have been well developed by ICES and the Program for Quality Assurance of Biological Effects in Monitoring (BEQUALM) (Feist et al. 2004; BEQUALM, 2005). The histopathological diagnosis of the liver is based on subjective classification and descriptive qualitative criteria. Weighted indices such as those proposed by Bernet et al. (1999) or Costa et al. (2009) and Costa (2018) are of special relevance since they are based on the premise that different histological changes may not reflect the same impact.
At a community level, parasites have emerged during recent decades as an important tool for assessing environmental health, as bioindicators of pollution effects, based on their responses to host-related and environmental factors (MacKenzie 1999; Sures 2001; Marcogliese 2005; Sasal et al. 2007; Vidal-Martínez et al. 2010). Parasite populations can either increase or decrease against environmental changes depending on their life cycle and the nature of the change (Mackenzie et al. 1995; Marcogliese 2005). However, pollution and environmental stress are often associated with a reduction in the parasitic species richness (Carreras-Aubets et al. 2012; Marcogliese 2005), which denotes an impact on the ecosystem’s health.
Integrating all these techniques in target fish species is crucial to infer how certain pollutants can affect marine organisms. The aim of this study is to assess whether impacted rivers influence the health status of the nearby marine environment by studying the health condition of a sentinel edible fish species such as Solea solea, at the continental shelf. Special focus is provided on the possible impact of dredging activities in the river on the health status of the fish living at the nearby continental shelf. This is the first study that infers the health status not along the river course but on the nearby marine environment.
Materials and methods
Sampling and study area
The sampling area was specifically chosen at the southern front of the Ebro river mouth. This area is under the direct influence of the river discharge throughout the year and regardless of wind conditions (Arin et al. 2005; Fernández-Nóvoa et al. 2015), yet the river plume is mixed with the dense waters that emerge from the platform (Font et al. 1986). Thus, it is the marine area where the highest impact of the river and the potential pollutants it transports is expected. Solea solea was chosen as the target species of this study for various reasons: it is a benthic fish, so it is in direct contact with sediments, several studies support that it meets the requirements to be a good bioindicator, and it is one of the most valuable species in the fisheries of the area (Jebali et al. 2013; Ribecco et al. 2012; Siscar et al. 2015).
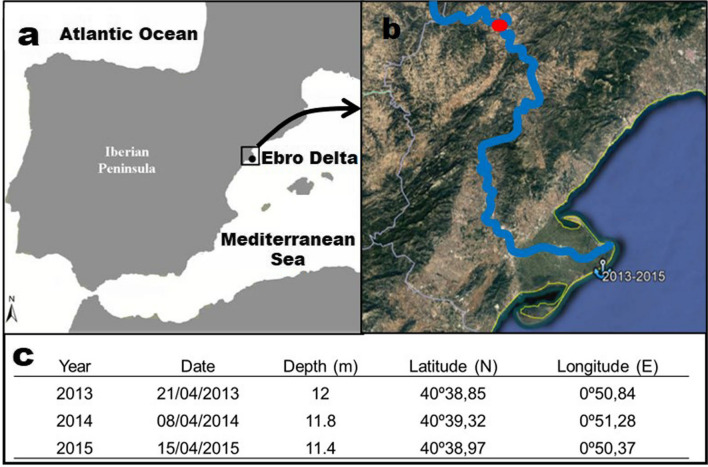
A total of 120 specimens of S. solea were collected aboard the commercial fishing vessels (using a commercial fishing trawl) in 3 consecutive years (2013, 2014, and 2015) (40 fish per year) in the inner continental shelf area at 4.71 miles offshore from the Ebro Delta river mouth (Fig. 1). Only one sampling campaign per year was performed, always in spring, to minimize the confounding effect of seasonality on the health assessment. The sampling in 2013 was conducted before the dredging activities in Flix was initiated in order to establish the previous condition of sediments and fish and assess the potential impact of these activities.
Fig. 1.
Map of the study area. Map of the Iberian Peninsula where the Ebro Delta is located (black dot) (a), detail of the last section of the Ebro river where Flix (red dot) and the sampling point (anchor) in the Ebro Delta from 2013 to 2015 are marked (b), and sampling data for Solea solea and sediments (c)
Captured specimens were processed in different ways: (1) 20 specimens per year were immediately fixed in toto in 10% buffered formalin for histological purposes with the abdominal cavity opened (to help the fixation and preservation of internal organs); (2) 20 specimens per year were frozen (−20°C) for the subsequent parasitological study.
Sediment sampling was carried out thrice per year (same location and time of biotic samples), using a Van Veen grab (KC DENMARK A/S) of 0.05 m2 on sandy bottoms, and samples were stored frozen (−20°C).
Contaminant analyses
Analysis of trace metals, as the term metal is used without distinction between the target metals and the metalloid (arsenic (As), cadmium (Cd), copper (Cu), mercury (Hg), lead (Pb), and zinc (Zn)), was conducted in both fish and sediment samples. These metals have been selected due to their importance in the toxic load of the Flix sludge deposit (Grimalt et al. 2003; Soto et al. 2011). A portion of the axial muscle of the frozen fish was dissected and stored at −20°C. Tissue samples and sediments analyzed for total Hg content were processed by nitric acid digestion in a microwave oven. In sediment, analysis of metals, excluding Hg, involved total digestion of the samples with a hydrofluoric acid and aqua regia mixture in a microwave oven, followed by neutralization with boric acid.
Selected metals were analyzed with a PerkinElmer spectrophotometer equipped with a Zeeman background correction device. Total Hg was determined by the cold vapor technique, employing a PerkinElmer FIMS-400 system (SnCl2 as a reducing agent). More technical details can be found elsewhere (Quelle et al. 2011; Besada et al. 2014).
All samples were included in a quality assurance/quality control (QA/QC) system. Protocols involved the use of certified reference materials, duplicated samples, and procedural blanks. In addition, the laboratory regularly participates in international intercalibration exercises.
In order to identify a toxicological significance, the metal levels in sediments were compared with those of Sediment Quality Guidelines, which may indicate whether adverse effects in living organisms may exist (Long et al. 1995). They identified metals that had ecological or biological effects on organisms and defined two indices: the “effects range low” (ERL, the lowest concentration of metal for which at least 10% of the data reviewed reported adverse effects) and the “effects range median” (ERM, the level at which half of the studies reported harmful effects).
Fish health indicators
Condition indices
The total length (TL), standard length (SL), and total body weight (TW) of each fish were measured rounding to the nearest unit. After dissection, the gonads and liver of each specimen were weighted to the nearest gram before being processed.
Fish condition was evaluated by the condition factor (CF = TW (g) × 100/(SL (cm))3), the hepatosomatic index (HSI = liver weight (g) × 100/TW (g)), and the gonadosomatic index (GSI = gonad weight (g) × 100/TW (g)), in the last case only for females.
Histopathological study
Fixed specimens (n=60, 20 fish per year) were dissected, and the liver, spleen, digestive tract (stomach and intestine), gonads, brain, and gills were processed by routine paraffin histology. One section (4 μm) of each organ was stained with hematoxylin and eosin. All histological samples were screened entirely following the recommendations of Bernet et al. (1999) and Feist et al. (2004) in order to detect histological alterations under the microscope.
Melanomacrophagic centers (MMC) quantitative study was performed in the spleen due to the easiness of ablation of this organ and the possibility of obtaining complete radial sections (Manera et al. 2000; Fournie et al. 2001). Three fields of view (0.23 mm2/screen) were randomly selected from each spleen section at 200× and examined microscopically. Parameters of area (surface of MMC, SC) and abundance (number of MMC, NC) of MMC were measured in each field by using a MicroComp Integrated Image Analysis System. A size discriminator was used to eliminate objects smaller than 100 μm2. The average value of the three field measurements per section was calculated.
Parasitological examination
Frozen fish (n=60) were thawed, dissected, and examined under a stereomicroscope for the presence of parasites. Parasites were collected, counted, and preserved in 70% ethanol. Digeneans, cestodes, and acanthocephalans were stained with iron acetocarmine, dehydrated through a graded ethanol series, cleared in eugenol, and mounted in Canada balsam. Nematodes were cleared in glycerine and mounted on temporary preparations. All parasites were identified to the genus or species level whenever possible.
Parasitological terms (prevalence (P) and mean abundance (MA)) were calculated according to Bush et al. (1997) using data from all thawed specimens. Species with a prevalence > 10% at least in 1 year were considered common. Infracommunity parasite descriptors (mean species richness (MSR), total mean abundance (TMA), and mean diversity index (MDI) (estimated by Brillouin’s index calculated by PRIMER 6 (Anderson et al. 2008)) were also analyzed.
Statistical analyses
Redundancy analyses (RDA)(van den Wollenberg 1977) were used to test the relationships between the response of fish to pollutants, assessed through the condition indices, and the explicative variables. RDAs were also conducted to relate MMC (SC was log-transformed to homogenize this analysis) and TMA, MDI, MSR, and each parasite species abundance with pollutants. The statistical significance of each of the axes derived by each analysis was tested with a Monte Carlo permutation test (Hope 1968). The number of permutations was set at 500.
Differences in the fish condition indices (CF, HSI, and GSI) and SL were tested for the factors MMC parameters (NC and SC), prevalence of MMC, histological alterations (perivascular inflammatory foci (PIF) and cyst of unknown etiology (CUE)), TMA, MDI, MSR, and each parasite species abundance by general linear model (GLM). SL, HSI, CF, and GSI were log-transformed prior to GLM analyses.
Possible effects of the factor year were tested for fish condition indices (CF, HSI, and GSI) and fish standard length (SL) by GLM, with Student-Newman-Keuls (SNK) post hoc pairwise comparisons and using the TMA as a covariate. SL, HSI, CF, and GSI were log-transformed prior to GLM analyses. MMC parameters (NC and SC), infracommunity parasite descriptors, and metals in muscle were also tested by GLM, with SNK post hoc pairwise comparisons, using SL as a covariate to explore the effect of the factor year.
To visualize the patterns in parasite abundance in relation to the year sampled, a factorial correspondence analysis (FCA) was first applied on a data matrix comprising the component population abundance for the six common parasites identified each year. A hierarchical cluster analysis was simultaneously performed based on the parasite coordinates of the first two axes obtained in the FCA to define host groups clearly. Finally, using individual fish as replicate samples, differences in abundance and prevalence among parasite populations were tested by generalized linear models (GZM) for the factor year (applying a log-binomial model for abundance and a binary logistic model for prevalence), using SL as a covariate.
Results
Contaminant analyses
Levels of metals in sediments and fish muscle are detailed in Table 1. In general, no variations in the sediment concentrations of metals were observed among the years sampled, except in 2013, when Cu, Pb, and Zn had the highest values (but no significant trend). In fish muscle, all metal levels were stable among years, except for Cd and Cu, which were significantly higher in 2014 and 2015, respectively (F2,14 = 5.232, p=0.020; F2,14 = 25.688, p < 0.001).
Table 1.
Biometrical parameters, parasite descriptors, and metals present in muscle and sediments of Solea solea collected nearby the Ebro river mouth along the different years of sampling
| 2013 | 2014 | 2015 | |
|---|---|---|---|
| N | 40 (F=11) | 40 (F=23) | 40 (F=21) |
| SL | 20.27(1.81)b | 20.81 (2.05)ab | 21.86 (1.84)a |
| HSI | 1.02 (0.23)a | 0.99 (0.22)ab | 0.90 (0.18)b |
| GSI | 0.10 (0.13) | 0.20 (0.16) | 0.16 (0.16) |
| K | 0.88 (0.13) | 0.88 (0.10) | 0.85 (0.07) |
| MSR | 1.75 (0.72) | 2.00 (0.86) | 2.15 (1.14) |
| TMA | 21.00 (49.75) | 51.65 (60.47) | 35.90 (44.52) |
| MDI | 0.24 (0.22) | 0.24 (0.28) | 0.22 (0.22) |
| NC | 1.45 (1.15)a | 1.02 (1.13)ab | 0.62 (0.74)b |
| SC | 5553 (5502.83)a | 3007 (2712.17)ab | 2672 (2987.45)b |
| As | 4.73 (2.42) | 3.64 (1.32) | 4.09 (1.45) |
| Cd | 0.001 (0.00)b | 0.0028 (0.00)a | 0.001 (0.00)b |
| Cu | 0.22 (0.02)b | 0.14 (0.02)b | 0.46 (0.11)a |
| Hg | 0.04 (0.01) | 0.03 (0.01) | 0.03 (0.01) |
| Pb | 0.02 (0.00) | 0.03 (0.02) | 0.03 (0.01) |
| Zn | 5.60 (0.42) | 5.04 (0.91) | 5.19 (0.97) |
| As_sed | 16.10 (0.14) | 17.97 (0.70) | 15.87 (2.44) |
| Cd_sed | 0.159 (0.003) | 0.148 (0.003) | 0.151 (0.004) |
| Cu_sed | 9.57 (1.32) | 6.81 (0.72) | 6.47 (0.58) |
| Hg_sed | 0.058 (0.005) | 0.067 (0.029) | 0.056 (0.006) |
| Pb_sed | 15.70 (3.11) | 12.07 (0.67) | 12.03 (0.87) |
| Zn_sed | 49.15 (1.34) | 41.87 (3.02) | 40.60 (1.41) |
Values are presented as means and standard deviations. Different superscript letters in each row indicate significant differences among years. Abbreviations: N, total number of specimens (number of females); SL, standard length (cm); HSI, hepatosomatic index; GSI, gonadosomatic index, only for females; K, condition factor; MSR, mean species richness; TMA, total mean abundance; MDI, mean diversity index; NC, number of melanomacrophagic lefts (MMC/mm2) in spleen; SC, area surface of centers (μm2 MMC/mm2) in spleen; chemical pollutants (mg/kg wet weight); sed, sediment samples
Fish health indicators
The total length (TL) of sampled fish ranged from 212 to 262 mm. Fish condition indicators (CF, HSI, and GSI) are shown in Table 1. The RDA relating fish condition indicators with pollutants accumulated 100% of the total variance and shows a negative relationship between HSI and Cu and between GSI and most metals in sediments together with levels of Hg, As, and Zn in fish muscle (Fig. 2A). GSI was also positively related to levels of Pb in fish (Fig. 2A) (p=0.002).
Fig. 2.
Redundant analysis (RDA) to related responses against metals in fish health indicators: a metals and fish condition indices; b metals and number of melanomacrophagic centers (NC) and surface area of melanomacrophagic centers (SC); c metals with the parasite descriptors total mean abundance (TMA), mean diversity index (MDI), and mean species richness (MSR); d metals and the abundance of each parasite. Abbreviations: CF, condition factor; HSI, hepatosomatic index; GSI, gonadosomatic index; bosp, Bomolochus bellones; cuhe, Cucullanus heterochrous; gasp, Galactosomum sp.; hyad, Hysterothylacium aduncum; hyfa, Hysterothylacium fabri; disp, Dichelyne sp.; pssp, Pseudorhadinorhynchus sp.; scpl, Tetraphyllidea gen. sp.; tryp, Trypanorhyncha gen. sp.; metals abbreviated are those present within the tissue, while metals abbreviated and followed by –sed are metal abundance measured at sediment samples
Regarding differences among years, HSI showed significantly lower values in 2015 than that in 2013 (HSI, F2,117 = 3.324, p=0.039) (Table 1).
The extensive histological study revealed the presence of MMC in the spleen, PIF in the liver, and CUEs in the gills, liver, and spleen (Table 2, Fig. 3A, B). In addition, acanthocephalans in the lumen of the intestine, digenean metacercariae mostly in the tectum opticum of the brain tissue, coccidian within the intestinal epithelium, and some ciliates in the gills were detected (Table 2, Fig. 3C).
Table 2.
Prevalence (P%) and mean abundance (MA) of the parasites and histopathological alterations of Solea solea collected nearby the Ebro river mouth along the different years
| 2013 | 2014 | 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parasite taxa | Code | P (%) | MA | P (%) | MA | P (%) | MA | |||
| Ciliates(*) | cili | 10 | X | 5 | X | 10 | X | |||
| Coccidia(*) | cocc | 14 | X | - | - | - | - | |||
| Digenea | ||||||||||
| Galactosomum sp. | gasp | 35 | 18.00 (50.22)a | 15 | 5.65 (21.56)b | 30 | 13.15 (38.83)a | |||
| Cestoda | ||||||||||
| Tetraphyllidea gen. sp. | scpl | 5 | 0.05 (0.22)b | 5 | 0.45 (2.01)ab | 15 | 0.70 (2.47)a | |||
| Trypanorhyncha gen. sp. | tryp | - | - | - | - | 5 | 0.05 (0.22) | |||
| Acanthocephala | ||||||||||
| Pseudorhadinorhynchus sp. | pssp | 65 | 1.55 (2.06)c | 100 | 44.15 (60.20)a | 80 | 20.30 (27.32)b | |||
| Nematoda | ||||||||||
| Cucullanus heterochrous | cuhe | - | - | - | - | 5 | 0.10 (0.45) | |||
| Dichelyne sp. | disp | - | - | - | - | 5 | 0.05 (0.22) | |||
| Hysterothylacium aduncum | hyad | - | - | 5 | 0.10 (0.45) | - | - | |||
| Hysterothylacium fabri | hyfa | 10 | 0.15 (0.49) | 15 | 0.15 (0.37) | - | - | |||
| Copepoda | ||||||||||
| Bomolochus bellones | bosp | 60 | 1.25 (1.45) | 60 | 1.15 (1.42) | 70 | 1.55 (1.76) | |||
| Melanomacrophage centers(*) | MMC | 80 | X | 65 | X | 55 | X | |||
| Cysts of unknown etiology(*) | CUE | 15 | X | - | - | 25 | X | |||
| Perivascular inflammatory foci(*) | PIF | 15 | X | 40 | X | 30 | X | |||
MA values are presented as means and standard deviations. Different superscript letters in each row indicate significant differences among years. (*) Data from the histological study. Abbreviations: N, total number of specimens; X, no available data; -, no parasite or histopathological alteration
Fig. 3.
Sections of different organs from Solea solea revealing different histopathological alterations and parasites. a and b Cysts of unknown etiology from the spleen and liver, respectively. c Ciliates between gill lamellae. d Perivascular inflammatory focus in liver
MMC were distributed homogeneously in the splenic tissue of most of the specimens and presented an irregular shape with variable size. Higher values of SC were significantly found in smaller specimens (GZM, χ2 = 4.755, p= 0.029), whereas higher NC values were significantly correlated with lower levels of GSI (GZM, χ2 = 4.000, p= 0.046). The RDA relating MMC with pollutants accumulated 100% of the total variance, and no relationships were observed among the number or size of MMC with pollutants (Fig. 2B).
PIF were mainly composed of lymphocytes associated with blood vessels in the liver (Fig. 3D).
CUEs were spherical in shape, with a homogeneous core of acellular, amorphous, and eosinophilic material, surrounded by acellular basophilic material (Fig. 3A, B). Connective tissue and capillaries were observed around the cyst. Usually, only one cyst per organ was detected.
Intestinal coccidian oocysts were found within the epithelial cells of the intestine with low prevalence (Table 2). Due to the methodology used in this study, the identification of coccidians to species or even family level was unfeasible. Oocysts were still undivided, and the observation of sporozoites was not possible. Ciliates presented a crown of cilia on one side and were found between gill filaments. Their morphology apparently corresponds to species of the family Trichodinidae.
None of these histopathological alterations were related to size or fish condition indicators (GZM, p > 0.05).
The prevalence and average values for size and number of MMC and histological alterations among years are shown in Table 1 and Table 2, respectively. Significant higher values for both NC and SC were detected in 2013 (GLM, F2,57 = 3.320, p= 0.043; GLM, F2,57 = 3.200, p=0.048, respectively). PIF were present at all years, but with higher prevalences in 2014 and 2015 (not a significant trend) (GZM, p > 0.05), whereas CUEs were only detected in 2013 and 2015, with a higher prevalence in 2015 (not a significant trend) (GZM, p > 0.05) (Table 2).
Regarding parasitological studies, a total of 2171 metazoan parasites belonging to nine different taxa were found: one digenean, two cestodes, one acanthocephala, four nematodes, and one copepod (Table 2). The most prevalent and abundant parasite was the acanthocephalan Pseudorhadinorhynchus sp., followed by the copepod Bomolochus bellones and the digenean Galactosomum sp. (Fig. 4).
Fig. 4.

Total mean abundance (TMA, left axis) and total prevalence (TP, right axis) of the different parasite taxa found in Solea solea during all study long. Abbreviations: Bosp, Bomolochus bellones; Cuhe, Cucullanus heterochrous; Gasp, Galactosomum sp.; Hyad, Hysterothylacium aduncum; Hyfa, Hysterothylacium fabri; Disp, Dichelyne sp.; Pssp, Pseudorhadinorhynchus sp.; Scpl, Tetraphyllidea gen. sp.; Tryp, Trypanorhyncha gen. sp.
Parasitological descriptors (MSR, TMA, MDI) are shown in Table 1. None of them showed an interaction or relation with fish size (GLM, p>0.05), except for TMA, with a negative correlation (F1,58=5.685, p=0.020). Contrary, the abundance of the cestode Tetraphyllidea gen. sp was positively correlated with SL (F1,58=7.609, p=0.008). Lower levels of HSI were significantly correlated with the abundance of the acanthocephalan Pseudorhadinorhynchus sp. (F1,58=5.776, p=0.019).
The RDA relating parasite descriptors and abundances with pollutants accumulated 100% of the total variance in the first two axes (Fig. 2C and D), and no relationships were observed between them.
None of the parasitological descriptors (MSR, TMA, MDI) showed significant differences among years (GLM, p>0.05).
Figure 5 shows a plot of the first factorial plane of co-inertia analysis explaining 100% of the variance, predominantly along the first axis (97.54% of the total inertia) of the FCA, carried out using component population data for the parasite species in S. solea. From this figure and a cluster analysis of the years (not shown), two different groups of S. solea were identified depending on their parasite load: group A (samples from D0-2013) and group B (samples from D1-2014 and D2-2015). Samples from group A were characterized by Galactosomum sp. and Hysterothylacium fabri. Interactions between host size and year were found for Galactosomum sp. (GZM interaction). Less abundance of this parasite was found in both the smallest and largest fish in 2014 (GZM, χ2 =11,986, p=0.002, and χ2 =15.890, p<0.001, respectively) (Table 2). Samples from group B were especially characterized by Pseudorhadinorhynchus sp., but also by Bomolochus bellones and Tetraphyllidea gen. sp. The abundance of Pseudorhadinorhynchus sp. was significantly higher in 2014 (GZM, χ2 =79,465, p<0.001) (Table 2). A higher abundance of Tetraphyllidea gen. sp. was observed in 2014 in the largest specimens (GZM, χ2 = 17.020, p<0.001) and in 2015 in both the largest and smallest specimens (GZM, χ2 =5945, p=0.015; GZM, χ2 =5492, p=0.049; respectively) (Table 2).
Fig. 5.

Plot of the first factorial plane of co-inertia analysis of the factorial correspondence analysis (FCA) on component population data for the abundance of parasite species in Solea solea. A and B indicate the groups established in the parasite fauna description. Abbreviations: D0, 2013; D1, 2014; D2, 2015; Bosp, Bomolochus bellones; Cuhe, Cucullanus heterochrous; Gasp, Galactosomum sp.; Hyad, Hysterothylacium aduncum; Hyfa, Hysterothylacium fabri; Disp, Dichelyne sp.; Pssp, Pseudorhadinorhynchus sp.; Scpl, Tetraphyllidea gen. sp.; Tryp, Trypanorhyncha gen. sp.
Discussion
Many studies have been carried out focusing on the pollution of mighty rivers with large industrial activities and their impact on the organisms inhabiting them, as is the case of the Ebro river. However, very little is known about their impact on the adjacent marine environments, particularly the continental shelves under the influence of these rivers’ discharge. In this study, this potential impact is assessed not only considering the levels of metals in sediments of the continental shelf but also in a holistic way, taking into account the health status of S. solea, a demersal species of great relevance in fisheries, by integrating its biological condition, histological alterations, and parasite communities. As a result, S. solea is used as a sentinel of potential changes in marine environmental pollution carried out by the river, such as dredging activities.
Fish health status assessment
Fish are suitable organisms for biomonitoring water pollution for many reasons, including their special biological characters, their top position in the aquatic food chain, and because most of them have an interest as food for human beings and hence they could directly affect our health (Zhou et al. 2008). Many fish studies about pollutants in the Ebro river have been carried out, confirming their bioaccumulation and impact in different biota (Lavado et al. 2006; Navarro et al. 2009; Benejam et al. 2010; Crespo and Solé 2016; Blanco et al. 2018). Solea solea, in addition, is an ideal species for assessing impacts from sediment pollution due to its close relation with sediments. Some metals, such as Cu or Zn, are essential elements for organisms since they play an important role in biological systems but can have a detrimental effect once they reach a certain threshold. Other metals, Hg or Pb, for example, are toxic even in trace amounts and have no known biological functions (US Environmental Protection Agency (USEPA)2006). Hg and Pb pollution in the Ebro river has been known to affect the organisms living in the Flix reservoir and downstream, as reported in the case of worms (Ramos et al. 1999), zebra mussels (Carrasco et al. 2008), crayfish (Suárez-Serrano et al. 2010), carps (Navarro et al. 2009), and catfish (Carrasco et al. 2011). The levels of such metals have been attributed to the inappropriate waste disposal from the Flix industrial complex (Bosch et al. 2009; Grimalt et al. 2003; Palanques et al. 2014), and metal accumulation patterns in oysters and crayfish from the Ebro delta could have a similar explanation. However, other sources of contamination might play a role too. For instance, the extensive hunting in the area may also contribute to the high Pb levels (Mateo et al. 1999), and As, Cd, Cu, and Zn could come from agricultural activities (Suárez-Serrano et al. 2010; Ochoa et al. 2013).
In the present study, the levels of trace metals analyzed in fish tissue do not exceed the reference levels accepted for human consumption in the regulations of the European Union for hazardous metals (European Union 2006 and following updates). In addition, they are lower than the levels observed in freshwater fish species from the river basin (Terrado et al. 2006; Navarro et al. 2009; Soto et al. 2011). This fact may suggest a dilution process and the potential lesser impact of these metals on the health of biota on the marine continental shelf (compared to those living in the river), and so to human health when these fish are consumed. Ochoa et al. (2013) also detected lower metal levels in oysters from both bays of the Ebro Delta, similar to those found in reference unpolluted sites, and far below the maximum limits of tolerance recommended by the European Commission.
The lower levels of almost all metal concentrations in sediments from the continental shelf of the Ebro Delta compared to those detected in riverine sediments (Terrado et al. 2006; Ferré-Huguet et al. 2009; Roig et al. 2011; Vilavert et al. 2015) are also in agreement with this potential dilution process. Generally, metals are naturally present at low concentrations in rivers and show variability over the river course (Hölemann et al. 2005; Sekhar et al. 2005). Arsenic is the only trace metal analyzed with higher values than those obtained from other authors at the Ebro river basin (Roig et al. 2011; Vilavert et al. 2015). Different natural and anthropogenic inputs received from the tributaries of the Ebro river may be contributing to the metal levels in the water (Gallo et al. 2006).
The main sources of arsenic are natural processes, mainly from existing natural minerals. However, anthropogenic activities such as mining, metal acquisition and processing, or the use of pesticides (among others), which are very abundant in the area, may also contribute to increasing its concentration (Eisler 2004). Arsenic values exceed the ERL in all sampled years, although they could be considered non-harmful levels for fish on the grounds that they are fairly homogeneous and remain far away from the ERM threshold. Zinc is the metal with the highest values in sediments from the continental shelf, coinciding with patterns observed in other studies from the Ebro basin, but with much lower values than in the river (49.15 mg/kg in the present study vs. 292.58 mg/kg, mean values in (Terrado et al. 2006), respectively). Environmental levels of this metal are commonly raised by agricultural practices, which are also very widespread in the area, due to the application of manure or inorganic fertilizers (Rodríguez et al. 2008). Comparing current levels of metals, including arsenic, with national guidelines, they are inside the safety range (Generalitat de Catalunya 2009). The rest of the trace metals analyzed are below the ERL, so they are not expected to cause adverse effects on marine organisms.
The absence of DELT anomalies (external deformities, erosion, lesions, and tumors) in S. solea would further support the suitable health status of fish. This set of pathological alterations has been used to characterize aquatic resources (Sanders et al., 1999) even in the Ebro river. A high prevalence of DELT was reported in freshwater fish species from the heavily polluted Flix reservoir (Benejam et al. 2010). Hepatic lesions such as tumors, cellular and nuclear pleomorphism, and different kinds of inflammation processes are considered indicators of previous contaminant exposure (Feist et al. 2004; Myers et al. 2008). In the present study, unspecific PIFs are found with moderate prevalence, indicating some sort of biological effect. Specifically, leukocyte infiltration is a morphological alteration in the liver that has been frequently related to exposure to contaminants. Its function is usually associated with neutralizing and destroying the causative agent, cleaning the tissue, removing dead cells, and inducing the recovery of damaged tissue (Bernet et al. 1999). CUEs are the only other histological alteration found in fish from this study and are reported for the first time in S. solea. The morphology and structure of CUEs are similar to those described by other authors (Constenla et al. 2015; MacKenzie 1979; Munday and Brand 1992; Nowak 1996) in several fish species. They are usually reported in the gills but have also been identified in other internal organs (Heidel et al. 2002; Kent et al. 2005) as in the present study. Although they were described for the first time 50 years ago (MacKenzie 1979), their etiology is still unknown. No pathogen has ever been isolated or identified associated with these alterations, and most recent studies point to a type of cartilaginous metaplasia, probably related to an inflammatory response (Heidel et al. 2002; Kent et al. 2005). Some studies coincide in relating them to environmental pollution (Munday and Brand 1992; Constenla et al. 2015), but others disagree (Heidel et al. 2002; Kent et al. 2005). No relationship seems to exist between PIFs or CUEs and metals in the present study, but other forms of environmental pollution should not be ruled out.
The number and size of MMC are known to increase in size with fish age (Stentiford et al. 2003) since MMC are sites where the residues of effete and damaged cells end up. If we consider that the age in wild fish can be reflected by their size (Pauly 1998), in the present study, the variation in number and size of MMC does not seem to be related to fish age. Other studies have associated MMC with sex (Blazer et al. 1987), which is not the case in the present study either, or the spawning phase (Kumar et al. 2016), which also disagrees with the higher numbers of MMC associated with lower levels of GSI we observed. Several studies have also related MMC to the destruction, recycling, and storage of endogenous and exogenous materials, environmental stress, and infections and parasite-related processes (Agius and Roberts 2003; Carrassón et al. 2008). Steinel and Bolnick (2017) concluded that the huge number of factors (sex, pollution exposure, stress, environmental factors) that should be considered confounding variables underscore the difficulties in interpreting MMC metrics in wild populations.
The parasite fauna of S. solea is quite diverse and especially abundant, which is expectable in near-bottom fishes due to the high availability of intermediate hosts in the benthos (Klimpel et al. 2010; Marcogliese 2002). The most prevalent and abundant parasites are acanthocephalans, which are located within the intestine. They are present in a very high abundance in some individuals, even obliterating the intestinal lumen and potentially affecting the health of infected fish, as reported by other authors (Jithendran and Kannappan 2010). Such abundance of parasites within the intestine may interfere in nutrient absorption, thus decreasing the energy status of fish over time. Since HSI is known to be an indirect indicator of energy status (Busacker et al. 1990), the observed lower levels of this biological indicator can be interpreted as a result of the high infestation. Bomolochus bellones, found with a high prevalence in the gills, is the first crustacean ectoparasite described in S. solea. Metacercariae of Galactosomum usually encyst in the nervous tissue of different fish species, waiting to be transmitted via food web to their final host, fish-eating birds and mammals (Pearson 1973; Dailey et al. 2002). The parasite location in the host’s nervous system makes fish swim closer to the surface, where they are more likely to be eaten by aquatic birds. As a result, the parasite increases its chances of success to complete its life cycle. The significantly higher infection values in larger specimens of these parasites and also of Tetraphyllidean larvae Scolex pleuronectis agree with a number of studies. This relationship with the host’s size is related to the host’s vagility and longevity and the space available to parasites (Sasal et al. 1997; Constenla et al. 2015).
Potential impact of dredging activities in the river on the health status of fish
Dredging activities of contaminated sediments in Flix began in 2013, after the first sampling of this study was conducted, and continued in the following years. However, prior to these activities, a retaining wall was constructed to isolate the contaminated sludge from the surrounding waters. Both activities are expected to have an impact on biota, and in fact, Blanco et al. (2018) detected an increase of organic pollutants in the muscle of fish from the river during the wall construction (2012) that then decreased in 2013 (together with levels of EROD activities in fish). Blanco et al. (2018) attributed the decrease in the levels of pollutants and EROD activity in 2013 to the effectiveness of the retaining wall once built. In our results, the significant decrease in the number and size of MMC in subsequent years could also reflect this effectiveness. Thus, the high levels of MMC in 2013 would reflect a chronic exposure to contaminants before the construction of the wall or during its construction, when contaminated sediments might have been resuspended and not significantly retained yet. However, in our study, light increases of PIF are detected in fish in 2014, which could also be indicative of a contaminant exposure (Bernet et al. 1999; Feist et al. 2004) during the following years. Higher levels of Cd (one of the most important metals accumulated in the contaminated sludge of Flix (Huertas 2015)) and Cu are also detected in the muscle of fish in 2014 and 2015, respectively. A slight increase in Hg levels in seagull feathers from the Ebro Delta was also detected in the period 2014–2019 (Sánchez-Fortún et al. 2020), which concluded that an effect of the Flix dredging could not be ruled out. Therefore, these observations may contradict the effectiveness of the wall during dredging activities in preventing the contaminants to reach the main river flow and being then transported towards the sea. However, in no case the presence of PIF is associated with the levels of metals reported; thus, other forms of contamination, such as radionuclides and organochlorine compounds that were also present in the contaminated sludge of the Flix reservoir ((Bosch et al. 2009; Casacuberta et al. 2009), may be playing an important role. Moreover, other sources and pathways of contamination may not be dismissed. For instance, temporal fluctuations of PIF could be attributed to different river discharges which have been long associated to the transport of terrestrial contaminants into the sea. Flash floods, which are common in Mediterranean systems, might be particularly relevant for the arrival of pulses of contaminants with an increased risk of impact on organisms in the following months (Herrero et al. 2018; Palanques et al. 2020). 2013 has been the rainiest year of the last decade in the regions of Catalonia in the Ebro river catchment area (Instituto de Estadística de Cataluña 2020), and, as a result, an increase in the river flow throughout the year is to be expected. However, the increase of enzymatic biomarkers throughout the years 2013 to 2015 in fish from the Ebro river mouth observed by Crespo and Solé (2016) suggested an acute toxic exposure in this area in 2015 rather than in 2013. The lower HSI values we observed in 2015 further support a potential acute exposure taking place that year, contrary to chronic toxic responses where the liver is enlarged due to the increased contribution of biotransformation enzymes. Extremely acute physiological responses following the start of the dredging activities and which may have been later neutralized by the buffering action of coastal water bodies may have gone unnoticed in the yearly sampling conducted (Menon et al. 2021). Similarly, seasonal fluctuations in fish health, such as those related to parasite infestations that are expected to vary throughout the year due to the parasites’ life cycle, may not be dismissed either. A more extended sampling could provide further information on the potential drivers of the trends observed. Nonetheless, it must be taken into account that the continental shelf receives a variable input of pollutants mainly from the river throughout the year. While these impacts may be time-restricted and highly influenced by local hydrodynamics in planktonic systems (Shafeeque et al. 2019), pollutants eventually sink and accumulate in sediments (rate of sediment deposition, approximately 2 cm per year (Maldonado et al. 1983)), and thus benthic environments can integrate the potential impacts over larger temporal scales. Therefore, the study of benthic organisms, especially through condition indices and histological alterations that can reflect conditions developed through a long period or long after the pollutant exposure took place, may better assess the potential impacts throughout the year.
Using parasites as effect indicators is not an easy task since parasites respond to anthropogenic pollution in a variety of ways. As a rule, ectoparasites with direct single-host life cycles (MacKenzie 1999) and with indirect contact with the external environment are to resist/endure certain environmental changes and adverse conditions. Thus, they might be more tolerant than their hosts to certain types of environmental changes and, consequently, are expected to increase with increasing levels of pollution (Lafferty 1997). Ectoparasites affecting fish from the Ebro river are no exception. Benejam et al. (2010) found a higher prevalence of ectoparasites (copepods and leeches) in common carp and other fish species at the polluted Flix reservoir. However, no relationship is found between ectoparasites (copepods and ciliates) and metal levels in the present study. Contrary to ectoparasites, endoparasitic helminths with complex indirect life cycles usually tend to decrease with pollutants since they can be affected directly (by free-living transmission stages or adult forms in the alimentary tract) or indirectly (through adverse effects on any of the hosts in the parasite’s life cycle) (MacKenzie 1999; Sures 2001). However, an enormous variation in the responses of different parasite taxa to different types of pollution exists (Lafferty 1997). Metacercariae of the digenean Galactosomum sp. reach their fish host after a free-living larval stage. Their drastic decrease in 2014, which does not correlate with the decreasing trends in environmental levels of metal contamination, again points out to other sources or forms of contamination during this year. Contrary, higher levels of Pseudorhadinorhynchus sp. and Tetraphyllidea gen. sp are detected in 2014 and 2015, respectively. These parasites are known to be trophically transmitted, so they do not have free-living larval stages, being protected inside the intermediate hosts if levels of pollutants are not high enough. Thus, moderate pollutant stressors on their hosts can decrease their immune response, leading to higher parasitizing rates (Sures 2001).
Conclusions
The health status of S. solea on the continental shelf does not show significant alterations that hamper the survival of fish. Metal concentrations in sediments from the continental shelf of the Ebro Delta are below ERL (except for As) but remain far away from the ERM and so are considered non-harmful levels for fish. Their levels in fish muscle, in addition, are lower than in freshwater fish species from the river basin and do not exceed reference levels accepted for human consumption. Unspecific PIF in liver of S. solea might be indicative of a contaminant exposure (although not linked to metals), but the absence of tumors or other relevant histological alterations points to a low impact of pollutants in fish from the continental shelf. The only parasites that seem to affect fish health are acanthocephalans, which are also the most prevalent and abundant parasites, and the digenean metacercariae of Galactosomum, which could be leading to higher predation of infected S. solea by aquatic birds.
Although there is no evidence of significant disease in fish, some histological and parasitological alterations could be attributable to changes in the contributions of pollutants by the nearby river. Higher levels of Cd in fish muscle, PIF in liver, and acanthocephalan abundance and lower abundance of Galactosomum sp. in 2014 could point out increasing levels of contamination in this year. Furthermore, higher levels of Cu in fish muscle and lower levels of HSI could also suggest an acute exposure in 2015. These results suggest that, although dredging activities seem to be not severely affecting the health status of this sentinel fish species through the increase in metal exposure, there are warnings of some type of acute toxicity taking place in 2014 and 2015 that should be studied in more detail.
Acknowledgements
The authors would like to thank the staff at the Centro Oceanográfico de Vigo- IEO (F. Schultze and M. López) for their help and collaboration in sample preparation and the staff of the Universitat Autònoma de Barcelona (F. Padrós, S. Dallarés and D. Pérez-i-Garcia) and students of the Department of Animal Biology, Vegetal Biology and Ecology that helped during the campaigns and the laboratory work. Authors are also grateful to E. Carreras for critically reading the manuscript, for her suggestions and contributions to improve the discussion, and for the English revision of the manuscript.
Author contribution
MC1 conceived and designed the experiments, contributed to sample collection, performed the lab procedures, analyzed and interpreted the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper, and final approved the submitted version of the ms; AS-M contributed to sample collection, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper, and final approved the submitted version of the ms; VB performed the lab procedures, analyzed the data, reviewed drafts of the paper, and final approved the submitted version of the ms; and MC2 analyzed and interpreted the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, reviewed drafts of the paper, and final approved the submitted version of the ms.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All applicable institutional, national, and international guidelines for the care and use of animals were followed.
Consent for publication
All authors have agreed to be listed and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised due to a retrospective Open Access order.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/7/2024
A Correction to this paper has been published: 10.1007/s11356-024-35278-3
References
- Agius C, Roberts RJ (2003) Melano-macrophage centres and their role in fish pathology. J Fish Dis 26:499–509 [DOI] [PubMed] [Google Scholar]
- Alcaraz C, Caiola N, Ibáñez C (2011) Bioaccumulation of pollutants in the zebra mussel from hazardous industrial waste and evaluation of spatial distribution using GAMs. Sci Total Environ 409:898–904. 10.1016/j.scitotenv.2010.11.015 [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E Ltd, Plymouth, UK pp 1–214
- Arellano JM, Storch V, Sarasquete C (1999) Histological changes and copper accumulation in liver and gills of the senegales sole, Solea senegalensis. Ecotoxicol Environ Saf 44:62–72. 10.1006/eesa.1999.1801 [DOI] [PubMed] [Google Scholar]
- Arin L, Estrada M, Salat J, Cruzado A (2005) Spatio-temporal variability of size fractionated phytoplankton on the shelf adjacent to the Ebro river (NW Mediterranean). Cont Shelf Res 25:1081–1095. 10.1016/J.CSR.2004.12.011 [Google Scholar]
- Au DWT (2004) The application of histo-cytopathological biomarkers in marine pollution monitoring: A review. Mar Pollut Bull 48:817–834 [DOI] [PubMed] [Google Scholar]
- Benejam L, Benito J, García-Berthou E (2010) Decreases in condition and fecundity of freshwater fishes in a highly polluted reservoir. Water Air Soil Pollut 210:231–242. 10.1007/s11270-009-0245-z [Google Scholar]
- Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34. 10.1046/j.1365-2761.1999.00134.x [Google Scholar]
- Bervoets L, Blust R (2003) Metal concentrations in water, sediment and gudgeon (Gobio gobio) from a pollution gradient: relationship with fish condition factor. Environ Pollut 126:9–19. 10.1016/S0269-7491(03)00173-8 [DOI] [PubMed] [Google Scholar]
- Besada V, Sericano JL, Schultze F (2014) An assessment of two decades of trace metals monitoring in wild mussels from the Northwest Atlantic and Cantabrian coastal areas of Spain, 1991-2011. Environ Int 71:1–12. 10.1016/j.envint.2014.05.024 [DOI] [PubMed] [Google Scholar]
- Blanco M, Fernandes D, Rizzi J, Huertas D, Caiola N, Fernández P, Porte C (2018) The combined use of chemical and biochemical markers in Rutilus rutilus to assess the effect of dredging in the lower course of the Ebro river. Ecotoxicol Environ Saf 155:9–16. 10.1016/j.ecoenv.2018.02.060 [DOI] [PubMed] [Google Scholar]
- Blazer VS, Wolke RE, Brown J, Powell CA (1987) Piscine macrophage aggregate parameters as health monitors: effect of age, sex, relative weight, season and site quality in largemouth bass (Micropterus salmoides). Aquat Toxicol 10:199–215. 10.1016/0166-445X(87)90012-9 [Google Scholar]
- Bosch C, Olivares A, Faria M, Navas JM, del Olmo I, Grimalt JO, Piña B, Barata C (2009) Identification of water soluble and particle bound compounds causing sublethal toxic effects. A field study on sediments affected by a chlor-alkali industry. Aquat Toxicol 94:16–27. 10.1016/j.aquatox.2009.05.011 [DOI] [PubMed] [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. Revisited. J Parasitol 83:575–583. 10.2307/3284227 [PubMed] [Google Scholar]
- Carrasco L, Díez S, Soto DX et al (2008) Assessment of mercury and methylmercury pollution with zebra mussel (Dreissena polymorpha) in the Ebro river (NE Spain) impacted by industrial hazardous dumps. Sci Total Environ 407:178–184. 10.1016/j.scitotenv.2008.07.031 [DOI] [PubMed] [Google Scholar]
- Carrasco L, Barata C, García-Berthou E, Tobias A, Bayona JM, Díez S (2011) Patterns of mercury and methylmercury bioaccumulation in fish species downstream of a long-term mercury-contaminated site in the lower Ebro river (NE Spain). Chemosphere 84:1642–1649. 10.1016/j.chemosphere.2011.05.022 [DOI] [PubMed] [Google Scholar]
- Carrassón M, Curell J, Padrós F, Crespo S (2008) Evaluation of splenic macrophage aggregates of red mullet (Mullus barbatus) as biomarkers of degraded environments in the western Mediterranean using image analysis. Bull Eur Assoc Fish Pathol 28:46–51 [Google Scholar]
- Carreras-Aubets M, Montero FE, Kostadinova A, Carrassón M (2012) Parasite communities in the red mullet, Mullus barbatus L., respond to small-scale variation in the levels of polychlorinated biphenyls in the Western Mediterranean. Mar Pollut Bull 64:1853–1860. 10.1016/j.marpolbul.2012.06.008 [DOI] [PubMed] [Google Scholar]
- Casacuberta N, Masqué P, Garcia-Orellana J, Bruach JM, Anguita M, Gasa J, Villa M, Hurtado S, Garcia-Tenorio R (2009) Radioactivity contents in dicalcium phosphate and the potential radiological risk to human populations. J Hazard Mater 170:814–823. 10.1016/J.JHAZMAT.2009.05.037 [DOI] [PubMed] [Google Scholar]
- CHEbro (2009) Portal de CHEbro. Confederación Hidrográfica del Ebro, Spain [Google Scholar]
- Constenla M, Montero FE, Padrós F, Cartes JE, Papiol V, Carrassón M (2015) Annual variation of parasite communities of deep-sea macrourid fishes from the western Mediterranean Sea and their relationship with fish diet and histopathological alterations. Deep Sea Res Part I Oceanogr Res Pap 104:106–121. 10.1016/j.dsr.2015.07.002 [Google Scholar]
- Costa PM (2018) The handbook of histopathological practices in aquatic environments: Guide to Histology for Environmental Toxicology. Academic Press. 292 pp
- Costa PM, Lobo J, Caeiro S, Martins M, Ferreira AM, Caetano M, Vale C, DelValls TÁ, Costa MH (2008) Genotoxic damage in Solea senegalensis exposed to sediments from the Sado estuary (Portugal): effects of metallic and organic contaminants. Mutat Res Genet Toxicol Environ Mutagen 654:29–37. 10.1016/j.mrgentox.2008.04.007 [DOI] [PubMed] [Google Scholar]
- Costa PM, Diniz MS, Caeiro S, Lobo J, Martins M, Ferreira AM, Caetano M, Vale C, DelValls TÁ, Costa MH (2009) Histological biomarkers in liver and gills of juvenile Solea senegalensis exposed to contaminated estuarine sediments: a weighted indices approach. Aquat Toxicol 92:202–212. 10.1016/j.aquatox.2008.12.009 [DOI] [PubMed] [Google Scholar]
- Costa CAX, Brito KNO, Gomes MA, Caliari MV (2010) Histopathological and immunohistochemical study of the hepatic lesions experimentally induced by Entamoeba dispar. Eur J Histochem 54:170–174. 10.4081/ejh.2010.e39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PM, Chicano-Gálvez E, Caeiro S, Lobo J, Martins M, Ferreira AM, Caetano M, Vale C, Alhama-Carmona J, Lopez-Barea J, DelValls TÀ, Costa MH (2012) Hepatic proteome changes in Solea senegalensis exposed to contaminated estuarine sediments: a laboratory and in situ survey. Ecotoxicology 21:1194–1207. 10.1007/s10646-012-0874-7 [DOI] [PubMed] [Google Scholar]
- Crespo M, Solé M (2016) The use of juvenile Solea solea as sentinel in the marine platform of the Ebre delta: in vitro interaction of emerging contaminants with the liver detoxification system. Environ Sci Pollut Res 23:19229–19236. 10.1007/s11356-016-7146-7 [DOI] [PubMed] [Google Scholar]
- Dabrowska H, Ostaszewska T, Kamaszewski M, Antoniak A, Napora-Rutkowski, Kopko O, Lang T, Fricke NF, Lehtonen KK (2012) Histopathological, histomorphometrical, and immunohistochemical biomarkers in flounder (Platichthys flesus) from the southern Baltic Sea. Ecotoxicol Environ Saf 78:14–21. 10.1016/j.ecoenv.2011.10.025 [DOI] [PubMed] [Google Scholar]
- Dabrowska H, Kopko O, Lehtonen KK, Lang T, Waszak I, Balode M, Strode E (2017) An integrated assessment of pollution and biological effects in flounder, mussels and sediment in the southern Baltic Sea coastal area. Environ Sci Pollut Res 24:3626–3639. 10.1007/s11356-016-8117-8 [DOI] [PubMed] [Google Scholar]
- Dailey MD, Demaree RS, Critchfield RL (2002) Galactosomum stelleri sp. n. (Trematoda: Heterophyidae) from the northern sea-lion, Eumetopias jubatus (Schreber, 1776) (Carnivora: Otariidae). Comp Parasitol 69:58–61. 10.1654/1525-2647(2002)069[0058:gssnth]2.0.co;2 [Google Scholar]
- Generalitat de Catalunya, 2009. Diari Oficial de la Generalitat de Catalunya: Decret legislatiu 1/2009. Anex II: Nivells genèrics de referència per a metalls i metal·loides a Catalunya. DOGC núm. 5430, 28/07/2009
- Eisler R (2004) Arsenic hazards to humans, plants, and animals from gold mining reviews of environmental contamination and toxicology. Springer, New York, pp 133–165 [DOI] [PubMed] [Google Scholar]
- European Union. 2006. Comission Regulation (EC) n° 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs.
- Faria M, Huertas D, Soto DX, Grimalt JO, Catalan J, Riva MC, Barata C (2010) Contaminant accumulation and multi-biomarker responses in field collected zebra mussels (Dreissena polymorpha) and crayfish (Procambarus clarkii), to evaluate toxicological effects of industrial hazardous dumps in the Ebro river (NE Spain). Chemosphere 78:232–240. 10.1016/j.chemosphere.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Feist S, Lang T, Stentiford G, et al (2004) ICES Techniques in Marine Environmental Sciences Biological effects of contaminants: use of liver pathology of the European flatfish dab (Limanda limanda L.) and flounder (Platichthys flesus L.) for monitoring International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer. ICES Tech Mar Environ Sci 42:pp. 10.17895/ices.pub.5062
- Fernández-Nóvoa D, Mendes R, deCastro M, Dias JM, Sánchez-Arcilla A, Gómez-Gesteira M (2015) Analysis of the influence of river discharge and wind on the Ebro turbid plume using MODIS-Aqua and MODIS-Terra data. J Mar Syst 142:40–46. 10.1016/J.JMARSYS.2014.09.009 [Google Scholar]
- Ferré-Huguet N, Bosch C, Lourencetti C, Nadal M, Schuhmacher M, Grimalt JO, Domingo JL (2009) Human health risk assessment of environmental exposure to organochlorine compounds in the catalan stretch of the Ebro river, Spain. Bull Environ Contam Toxicol 83:662–667. 10.1007/s00128-009-9871-9 [DOI] [PubMed] [Google Scholar]
- Fonseca VF, França S, Vasconcelos RP, Serafim A, Company R, Lopes B, Bebianno MJ, Cabral HN (2011) Short-term variability of multiple biomarker response in fish from estuaries: influence of environmental dynamics. Mar Environ Res 72:172–178. 10.1016/j.marenvres.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Font J, Julia A, Rovira J, et al (1986) Circulación marina en la plataforma continental del Ebro determinada a partir de la distribución de masas de agua y los microcontaminantes orgánicos en el sedimento. 483–489
- Fournie JW, Summers JK, Courtney LA, Engle VD, Blazer VS (2001) Utility of splenic macrophage aggregates as an indicator of fish exposure to degraded environments. J Aquat Anim Health 13:105–116. 10.1577/1548-8667(2001)013<0105:UOSMAA>2.0.CO;2 [Google Scholar]
- Gallo M, Trento A, Alvarez A, Beldoménico H, Campagnoli D (2006) Dissolved and particulate heavy metals in the Salado River (Santa FE, Argentina). Water Air Soil Pollut 174:367–384. 10.1007/s11270-006-9128-8 [Google Scholar]
- Goede R, Barton B (1990) Organismic indices and an autopsy-based assessment as indicators of health and condition in fish. In: Adam S (ed) Biological Indicators of Stress in Fish. American Fisheries Society, Scientific Research Publishing, Bethesda, pp 93–108 [Google Scholar]
- Grimalt JO, Sánchez-Cabeza JA, Palanques A, Catalan J, 2003. Estudi de la dinàmica dels compostos organoclorats persistents I alters contaminants en els sistemes aquatics continentals. Agència catalana de l’aigua, Comissió interdepartamental de recerca i tecnologia. Government of Catalonia. http://aca.gencat.cat/web/.content/20_Aigua/08_consulta_de_dades/05_altres_dades/01_descontaminacio-de-lembassament-de-flix/Flix_ResumEstudi_2003.pdf
- Gustavson KE, Burton GA, Francingues NR et al (2008) Evaluating the effectiveness of contaminated-sediment dredging. Environ Sci Technol 42:5042–5047 [DOI] [PubMed] [Google Scholar]
- Heidel JR, Nowak BF, Fischer KA, Watral VG, Kent ML (2002) Visceral nodular cartilaginous metaplasia in rockfishes (Sebastes spp.) in the eastern North Pacific Ocean. J Vet Diagn Investig 14:495–497. 10.1177/104063870201400608 [DOI] [PubMed] [Google Scholar]
- Herrero A, Vila J, Eljarrat E, Ginebreda A, Sabater S, Batalla RJ, Barceló D (2018) Transport of sediment borne contaminants in a Mediterranean river during a high flow event. Sci Total Environ 633:1392–1402. 10.1016/J.SCITOTENV.2018.03.205 [DOI] [PubMed] [Google Scholar]
- Hölemann JA, Schirmacher M, Prange A (2005) Seasonal variability of trace metals in the Lena River and the southeastern Laptev Sea: impact of the spring freshet. Glob Planet Chang 48:112–125. 10.1016/j.gloplacha.2004.12.008 [Google Scholar]
- Hope ACA (1968) A simplified Monte Carlo significance test procedure. J R Stat Soc Ser B 30:582–598. 10.1111/j.2517-6161.1968.tb00759.x [Google Scholar]
- Huertas D, 2015. PhD Thesis: Estudi de la distribució des compostos organoclorats en els organismes fluvials sota la influència dels efluents d'una planta clor-àlcali (riu Ebre, entre Mequinensa i el Delta). Universitat de Barcelona.
- Instituto de Estadística de Cataluña. 2020. Anuario estadístico de Cataluña. https://www.idescat.cat/pub/?id=aec&n=217&lang=es. (Update date 24/01/2020)
- Jebali J, Sabbagh M, Banni M, Kamel N, Ben-Khedher S, M’hamdi N, Boussetta H (2013) Multiple biomarkers of pollution effects in Solea solea fish on the Tunisia coastline. Environ Sci Pollut Res 20:3812–3821. 10.1007/s11356-012-1321-2 [DOI] [PubMed] [Google Scholar]
- Jithendran KP, Kannappan S (2010) A short note on heavy infection of acanthocephalan worm (Neoechinorhynchus agilis) in grey mullet, Mugil cephalus. J Parasit Dis 34:99–101. 10.1007/s12639-010-0019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Collier T (2002) Assessing contaminant-induced stress across levels of biological organization. In: Adams S (ed) Biological Indicators of Aquatic Ecosystem Stress. American Fisheries Society, Bethesda, Maryland, pp 533–563 [Google Scholar]
- Kent ML, Heidel JR, Marie A, Moriwake A, Moriwake V, Alexander B, Watral V, Kelley CD (2005) Diseases of Opakapaka Pristipomoides filametosus. In: Walker P, Lester RG, Bondad-Rentaso MG (eds) Diseases in Asian aquaculture V. Asian Fisheries Society, Manila, pp 183–195
- Khan RA (2010) Two species of commercial flatfish, winter flounder, pleuronectes americanus, and American Plaice, Hippoglossoides platessoides, as sentinels of environmental pollution. Bull Environ Contam Toxicol 85:205–208. 10.1007/s00128-010-0050-9 [DOI] [PubMed] [Google Scholar]
- Klimpel S, Busch MW, Sutton T, Palm HW (2010) Meso- and bathy-pelagic fish parasites at the Mid-Atlantic Ridge (MAR): low host specificity and restricted parasite diversity. Deep Sea Res Part I Oceanogr Res Pap 57:596–603. 10.1016/j.dsr.2010.01.002 [Google Scholar]
- Koenig S, Solé M, Fernández-Gómez C, Díez S (2013) New insights into mercury bioaccumulation in deep-sea organisms from the NW Mediterranean and their human health implications. Sci Total Environ 442:329–335. 10.1016/j.scitotenv.2012.10.036 [DOI] [PubMed] [Google Scholar]
- Kohler A, Deisemann H, Lauritzen B (1992) Histological and cytochemical indices of toxic injury in the liver of dab Limanda limanda. Mar Ecol Prog Ser 91:141–153. 10.3354/meps091141 [Google Scholar]
- Kumar R, Joy KP, Singh SM (2016) Morpho-histology of head kidney of female catfish Heteropneustes fossilis: seasonal variations in melano-macrophage centers, melanin contents and effects of lipopolysaccharide and dexamethasone on melanins. Fish Physiol Biochem 42:1287–1306. 10.1007/s10695-016-0218-2 [DOI] [PubMed] [Google Scholar]
- Lafferty KD (1997) Environmental parasitology: what can parasites tell us about human impacts on the environment? Parasitol Today 13:251–255 [DOI] [PubMed] [Google Scholar]
- Lang T, Wosniok W, Baršiene J et al (2006) Liver histopathology in Baltic flounder (Platichthys flesus) as indicator of biological effects of contaminants. Mar Pollut Bull 53:488–496. 10.1016/j.marpolbul.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Lavado R, Ureña R, Martin-Skilton R, Torreblanca A, del Ramo J, Raldúa D, Porte C (2006) The combined use of chemical and biochemical markers to assess water quality along the Ebro river. Environ Pollut 139:330–339. 10.1016/j.envpol.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97. 10.1007/BF02472006 [Google Scholar]
- MacKenzie K (1979) Some parasites and diseases of blue whiting, Micromesistius poutassou (Risso), to the North and West of Scotland and the Faroe Islands. Scott Fish Res Rep 17:14 [Google Scholar]
- MacKenzie K (1999) Parasites as pollution indicators in marine ecosystems: a proposed early warning system. Mar Pollut Bull 38:955–959. 10.1016/S0025-326X(99)00100-9 [Google Scholar]
- Mackenzie K, Williams HH, Williams B et al (1995) Parasites as indicators of water quality and the potential use of helminth transmission in marine pollution studies. Adv Parasitol 35:85–144. 10.1016/S0065-308X(08)60070-6 [DOI] [PubMed] [Google Scholar]
- Maldonado A, Swift DJP, Young RA, Han G, Nittrouer CA, DeMaster DJ, Rey J, Palomo C, Acosta J, Ballester A, Castellvi J (1983) Sedimentation on the Valencia continental shelf: preliminary results. Cont Shelf Res 2:195–211. 10.1016/0278-4343(83)90016-X [Google Scholar]
- Manera M, Sera R, Isani G, Carpenea E (2000) Macrophage aggregates in gilthead sea bream fed copper, iron and zinc enriched diets. J Fish Biol 57:457–465. 10.1111/j.1095-8649.2000.tb02184.x [Google Scholar]
- Marcogliese DJ (2002) Food webs and the transmission of parasites to marine fish. Parasitology 124:83–99. 10.1017/s003118200200149x [DOI] [PubMed] [Google Scholar]
- Marcogliese DJ (2005) Parasites of the superorganism: are they indicators of ecosystem health? Int J Parasitol. 35: 705–716. 10.1016/j.ijpara.2005.01.015 [DOI] [PubMed]
- Mateo R, Estrada J, Paquet JY, Riera X, Domínguez L, Guitart R, Martínez-Vilalta A (1999) Lead shot ingestion by marsh harriers Circus aeruginosus from the Ebro delta, Spain. Environ Pollut 104:435–440. 10.1016/S0269-7491(98)00169-9 [Google Scholar]
- Menon N, George G, Ranith R, Sajin V, Murali S, Abdulaziz A, Brewin RJW, Sathyendranath S (2021) Citizen science tools reveal changes in estuarine water quality following demolition of buildings. Remote Sens 13(9):1683. 10.3390/RS13091683
- Merciai R, Guasch H, Kumar A, Sabater S, García-Berthou E (2014) Trace metal concentration and fish size: variation among fish species in a Mediterranean river. Ecotoxicol Environ Saf 107:154–161. 10.1016/j.ecoenv.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Mormede S, Davies IM (2001) Trace elements in deep-water fish species from the rockall trough. Fish Res 51:197–206. 10.1016/S0165-7836(01)00245-4 [Google Scholar]
- Munday BL, Brand DG (1992) Apparently embolic, enigmatic bodies in the gill filaments of perch. Bull Eur Assoc Fish Pathol 12:127–130 [Google Scholar]
- Myers MS, Stehr CM, Olson OP, Johnson LL, McCain BB, Chan SL, Varanasi U (1994) Relationships between toxicopathic hepatic lesions and exposure to chemical contaminants in English sole (Pleuronectes vetulus), starry flounder (Platichthys stellatus), and white croaker (Genyonemus lineatus) from selected marine sites on the Pacific Coast, USA. Environ Health Perspect 102:200–215. 10.1289/ehp.94102200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MS, Johnson LL, Collier TK (2003) Establishing the causal relationship between polycyclic aromatic hydrocarbon (PAH) exposure and hepatic neoplasms and neoplasia-related liver lesions in English sole (Pleuronectes vetulus). Hum Ecol Risk Assess 9(1):67–94
- Myers MS, Anulacion BF, French BL, Reichert WL, Laetz CA, Buzitis J, Olson OP, Sol S, Collier TK (2008) Improved flatfish health following remediation of a PAH-contaminated site in Eagle Harbor, Washington. Aquat Toxicol 88:277–288. 10.1016/j.aquatox.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Nadal M, Casacuberta N, Garcia-Orellana J, Ferré-Huguet N, Masqué P, Schuhmacher M, Domingo JL (2011) Human health risk assessment of environmental and dietary exposure to natural radionuclides in the Catalan stretch of the Ebro river, Spain. Environ Monit Assess 175:455–468. 10.1007/s10661-010-1543-z [DOI] [PubMed] [Google Scholar]
- Navarro A, Quirós L, Casado M, Faria M, Carrasco L, Benejam L, Benito J, Díez S, Raldúa D, Barata C, Bayona JM, Piña B (2009) Physiological responses to mercury in feral carp populations inhabiting the low Ebro river (NE Spain), a historically contaminated site. Aquat Toxicol 93:150–157. 10.1016/j.aquatox.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Nowak BF (1996) Health of red morwong, Cheilodactylus fuscus, and rock cale, Crinodus lophodon, from Sydney cliff-face sewage outfalls. Mar Pollut Bull 33:281–292. 10.1016/S0025-326X(96)00122-1 [Google Scholar]
- Ocampo D (2008) PhD thesis on the development of decision-making systems based on fuzzy models to assess water quality in rivers Universitat Rovira i Virgili on the development of decision-making systems based on fuzzy models to assess water quality in rivers.
- Ochoa V, Barata C, Riva MC (2013) Heavy metal content in oysters (Crassostrea gigas) cultured in the Ebro delta in Catalonia, Spain. Environ Monit Assess 185:6783–6792. 10.1007/s10661-013-3064-z [DOI] [PubMed] [Google Scholar]
- Oliva Ramirez M (2011) Estudio de la contaminación por metales pesados y hidrocarburos aromáticos policíclicos en especimenes de lenguado senegalés, solea senegalensis. Aplicación de biomarcadores. 1
- Oliva M, Garrido MC, Sales Márquez D, González de Canales ML (2009) Sublethal and lethal toxicity in juvenile Senegal sole (Solea senegalensis) exposed to copper: a preliminary toxicity range-finding test. Exp Toxicol Pathol 61:113–121. 10.1016/j.etp.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Palanques A, Grimalt J, Belzunces M, Estrada F, Puig P, Guillén J (2014) Massive accumulation of highly polluted sedimentary deposits by river damming. Sci Total Environ 497–498:369–381. 10.1016/j.scitotenv.2014.07.091 [DOI] [PubMed] [Google Scholar]
- Palanques A, López L, Guillén J, Puig P (2020) Trace metal variability controlled by hydrodynamic processes in a polluted inner shelf environment (Besòs prodelta, NW Mediterranean). Sci Total Environ 735:139482. 10.1016/J.SCITOTENV.2020.139482 [DOI] [PubMed] [Google Scholar]
- Pauly D (1998) Tropical fishes: patterns and propensities*. J Fish Biol 53:1–17. 10.1111/j.1095-8649.1998.tb01014.x [Google Scholar]
- Pearson JC (1973) A revision of the subfamily Haplorchinae Looss, 1899 (Trematoda: Heterophyidae). II. Genus Galactosomum. Philos Trans R Soc Lond Ser B Biol Sci 266:341–447. 10.1098/rstb.1973.0052 [DOI] [PubMed] [Google Scholar]
- Quelle C, Besada V, Andrade JM, Gutiérrez N, Schultze F, Gago J, González JJ (2011) Chemometric tools to evaluate the spatial distribution of trace metals in surface sediments of two Spanish rías. Talanta 87:197–209. 10.1016/j.talanta.2011.09.062 [DOI] [PubMed] [Google Scholar]
- Ramos L, Fernández MA, González MJ, Hernández LM (1999) Heavy metal pollution in water, sediments, and earthworms from the Ebro river, Spain. Bull Environ Contam Toxicol 63:305–311. 10.1007/s001289900981 [DOI] [PubMed] [Google Scholar]
- Reddy B, Rawat S (2013) Assessment of aquatic pollution using histopathology in fish as a protocol. Int Res J Environ Sci ISSN Int Res J Environ Sci 2:2319–1414 [Google Scholar]
- Riba I, Casado-Martínez MC, Blasco J, DelValls TA (2004) Bioavailability of heavy metals bound to sediments affected by a mining spill using Solea senegalensis and Scrobicularia plana. Mar Environ Res 58(2-5):395–399 [DOI] [PubMed]
- Ribecco C, Hardiman G, Šášik R, Vittori S, Carnevali O (2012) Teleost fish (Solea solea): a novel model for ecotoxicological assay of contaminated sediments. Aquat Toxicol 109:133–142. 10.1016/j.aquatox.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JA, Nanos N, Grau JM, Gil L, López-Arias M (2008) Multiscale analysis of heavy metal contents in Spanish agricultural topsoils. Chemosphere 70:1085–1096. 10.1016/j.chemosphere.2007.07.056 [DOI] [PubMed] [Google Scholar]
- Roig N, Nadal M, Sierra J, Ginebreda A, Schuhmacher M, Domingo JL (2011) Novel approach for assessing heavy metal pollution and ecotoxicological status of rivers by means of passive sampling methods. Environ Int 37:671–677. 10.1016/j.envint.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Sánchez-Fortún M, Ouled-Cheikh J, Jover C, García-Tarrasón M, Carrasco JL, Sanpera C (2020) Following up mercury pollution in the Ebro Delta (NE Spain): Audouin’s gull fledglings as model organisms to elucidate anthropogenic impacts on the environment. Environ Pollut 266:115232. 10.1016/j.envpol.2020.115232 [DOI] [PubMed] [Google Scholar]
- Sànchez-Nogué B, Varó I, Solé M (2013) Comparative analysis of selected biomarkers and pesticide sensitivity in juveniles of Solea solea and Solea senegalensis. Environ Sci Pollut Res 20:3480–3488. 10.1007/s11356-012-1355-5 [DOI] [PubMed] [Google Scholar]
- Sarma K, Kumar AA, George G et al (2013) Impact of coastal pollution on biological, biochemical and nutritional status of edible oyster in Phoenix Bay Jetty and North Wandoor of Andaman. Indian J Anim Sci 83:321–325 [Google Scholar]
- Sasal P, Morand S, Guégan JF (1997) Determinants of parasite species richness in Mediterranean marine fishes. Mar Ecol Prog Ser 149:61–71. 10.3354/meps149061 [Google Scholar]
- Sasal P, Mouillot D, Fichez R, Chifflet S, Kulbicki M (2007) The use of fish parasites as biological indicators of anthropogenic influences in coral-reef lagoons: a case study of Apogonidae parasites in New Caledonia. Mar Pollut Bull 54:1697–1706. 10.1016/j.marpolbul.2007.06.014 [DOI] [PubMed] [Google Scholar]
- Seetharaman P, Sarma K, George G, Krishnan P, Roy SD, Sankar K (2015) Impact of coastal pollution on microbial and mineral profile of edible oyster (Crassostrea rivularis) in the coastal waters of Andaman. Bull Environ Contam Toxicol 95:599–605. 10.1007/s00128-015-1601-x [DOI] [PubMed] [Google Scholar]
- Sekhar C, Chary NS, Kamala CT, Shanker, Frank H (2005) Environmental pathway and risk assessment studies of the Musi River’s heavy metal contamination - a case study. Hum Ecol Risk Assess 11:1217–1235. 10.1080/10807030500278594 [Google Scholar]
- Shafeeque M, Shah P, Platt T, Sathyendranath S, Menon NN, Balchand AN, George G (2019) Effect of precipitation on chlorophyll-a in an upwelling dominated region along the west coast of India. J Coast Res 86:218–224. 10.2112/SI86-032.1 [Google Scholar]
- Siscar R, Varó I, Solé M (2015) Hepatic and branchial xenobiotic biomarker responses in Solea spp. from several NW Mediterranean fishing grounds. Mar Environ Res 112:35–43. 10.1016/j.marenvres.2015.09.001 [DOI] [PubMed] [Google Scholar]
- Solé M, Manzanera M, Bartolomé A, Tort L, Caixach J (2013) Persistent organic pollutants (POPs) in sediments from fishing grounds in the NW Mediterranean: ecotoxicological implications for the benthic fish Solea sp. Mar Pollut Bull 67:158–165. 10.1016/j.marpolbul.2012.11.018 [DOI] [PubMed] [Google Scholar]
- Soto DX, Roig R, Gacia E, Catalan J (2011) Differential accumulation of mercury and other trace metals in the food web components of a reservoir impacted by a chlor-alkali plant (Flix, Ebro River, Spain): implications for biomonitoring. Environ Pollut 159:1481–1489. 10.1016/j.envpol.2011.03.017 [DOI] [PubMed] [Google Scholar]
- Stehr CM, Myers MS, Johnson LL, Spencer S, Stein JE (2004) Toxicopathic liver lesions in English sole and chemical contaminant exposure in Vancouver Harbour, Canada. Mar Environ Res 57(1-2):55–74. 10.1016/S0141-1136(03)00060-6 [DOI] [PubMed]
- Steinel NC, Bolnick DI (2017) Melanomacrophage centers as a histological indicator of immune function in fish and other poikilotherms. Front Immunol 8:827 (1-8). https://doi.org/10.3389/fimmu.2017.00827 [DOI] [PMC free article] [PubMed]
- Stentiford GD, Longshaw M, Lyons BP, Jones G, Green M, Feist SW (2003) Histopathological biomarkers in estuarine fish species for the assessment of biological effects of contaminants. Mar Environ Res 55:137–159. 10.1016/S0141-1136(02)00212-X [DOI] [PubMed] [Google Scholar]
- Storelli MM, Giacominelli-Stuffler R, Marcotrigiano GO (2002) Total and methylmercury residues in cartilaginous fish from Mediterranean Sea. Mar Pollut Bull 44:1354–1358. 10.1016/S0025-326X(02)00223-0 [DOI] [PubMed] [Google Scholar]
- Suárez-Serrano A, Alcaraz C, Ibáñez C, Trobajo R, Barata C (2010) Procambarus clarkii as a bioindicator of heavy metal pollution sources in the lower Ebro river and delta. Ecotoxicol Environ Saf 73:280–286. 10.1016/j.ecoenv.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Sures B (2001) The use of fish parasites as bioindicators of heavy metals in aquatic ecosystems: a review. Aquat Ecol 35:245–255. 10.1023/A:1011422310314 [Google Scholar]
- Tena A, Batalla RJ (2013) The sediment budget of a large river regulated by dams (the lower river Ebro, NE Spain). J Soils Sediments 13:966–980. 10.1007/S11368-013-0681-7 [Google Scholar]
- Terrado M, Barceló D, Tauler R (2006) Identification and distribution of contamination sources in the Ebro river basin by chemometrics modelling coupled to geographical information systems. Talanta 70:691–704. 10.1016/j.talanta.2006.05.041 [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) (2006) Freshwater sediment screening benchmarks. https://www.epa.gov/risk/freshwater-sediment-screening-benchmarks. Accessed 28 Jan 2020
- van den Wollenberg AL (1977) Redundancy analysis an alternative for canonical correlation analysis. Psychometrika 42:207–219. 10.1007/BF02294050 [Google Scholar]
- Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149 [DOI] [PubMed] [Google Scholar]
- Vethaak AD, Wester PW (1996) Diseases of flounder Platichthys flesus in Dutch coastal and estuarine waters, with particular reference to environmental stress factors. II. Liver histopathology. Dis Aquat Org 26:99–116. 10.3354/dao026099 [Google Scholar]
- Vidal-Martínez VM, Pech D, Sures B, Purucker ST, Poulin R (2010) Can parasites really reveal environmental impact? Trends Parasitol 26:44–51 [DOI] [PubMed] [Google Scholar]
- Vilavert L, Sisteré C, Schuhmacher M, Nadal M, Domingo JL (2015) Environmental concentrations of metals in the Catalan stretch of the Ebro river, Spain: assessment of temporal trends. Biol Trace Elem Res 163:48–57. 10.1007/s12011-014-0140-3 [DOI] [PubMed] [Google Scholar]
- Wootton RJ (1989) Ecology of teleost fishes. Springer, Netherlands [Google Scholar]
- World Health Organization (1999) Identification of priority pollution hot spots and sensitive areas in the Mediterranean identification Des "Points Chauds" Et "Zones Sensibles" De Pollution Prioritaires En Méditer. MAP Technical Reports Series No. 124
- Yancheva VS, Stoyanova SG, Georgieva ES, Velcheva IG (2018) Synopsis mussels in ecotoxicological studies-are they better indicators for water pollution than fish? Ecologia Balkanica.10(1):57–84
- Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–150 [DOI] [PubMed] [Google Scholar]
- Zorita I, Cuevas N (2014) Protocol for fish disease assessment in marine environmental monitoring using common sole (Solea solea, Linnaeus 1758) as sentinel organism: identification of externally visible diseases and liver histopathology. Rev Investig Mar AZTI-Tecnalia 21:1–18 [Google Scholar]
- Nash RDM, Valencia AH, Geffen AJ (2006) The origin of Fulton's condition factor —setting the record straight. Fisheries 31(5):236–238
- BEQUALM, 2005. Biological Effects Quality Assurance in Monitoring Programmes, Available from: https://www.bequalm.org/about/htm
- Sanders RE, Miltner RJ, Yoder CO, Rankin ET (1999) The Use of External Deformities, Erosion, Lesions, and Tumors (DELT Anomalies) in Fish Assemblages for Characterizing Aquatic Resources: A Case Study of Seven Ohio Streams. In: Simpson TP (Ed) Assessing the sustainability and biological integrity of water resources using fish communities. CRC Press
- Busacker GP, Adelman, IR, Goolish EM (1990) Growth. In Schreck CB, Moyle PB (eds) Methods for fish biology. American Fisheries Society, Bethesda, Maryland. p. 363–387
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.