Abstract
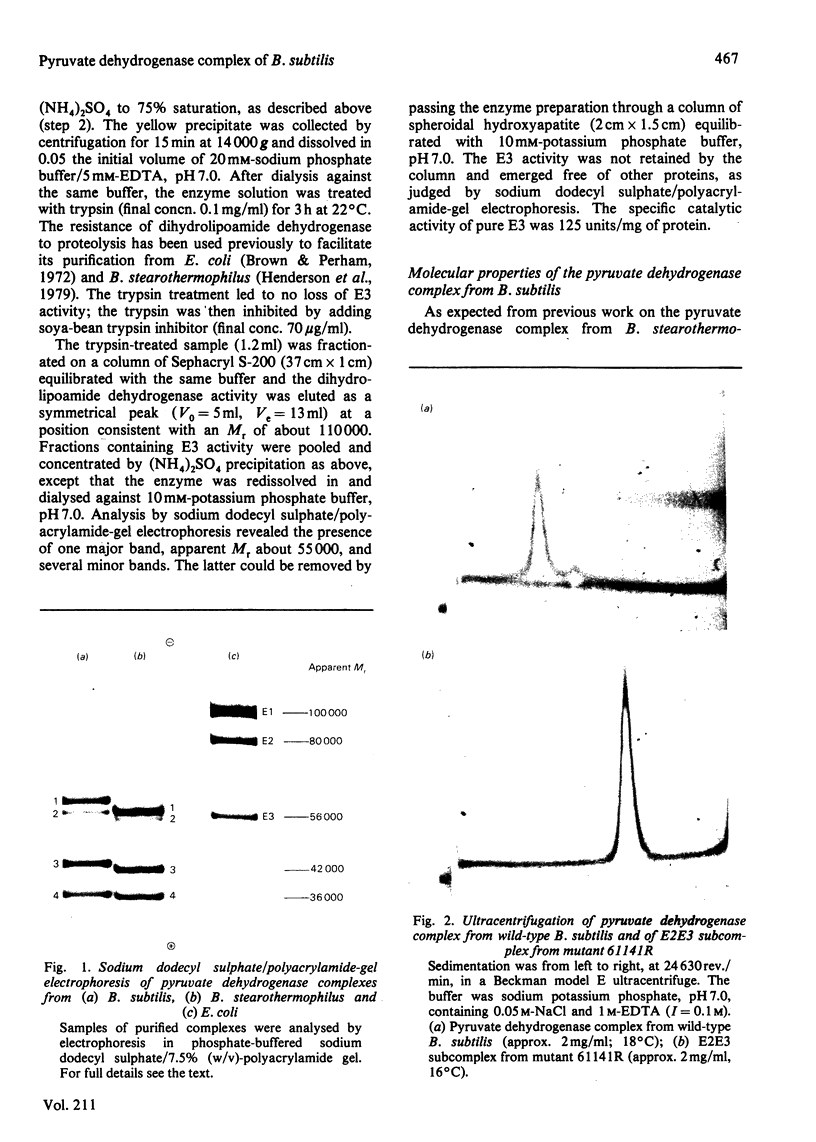
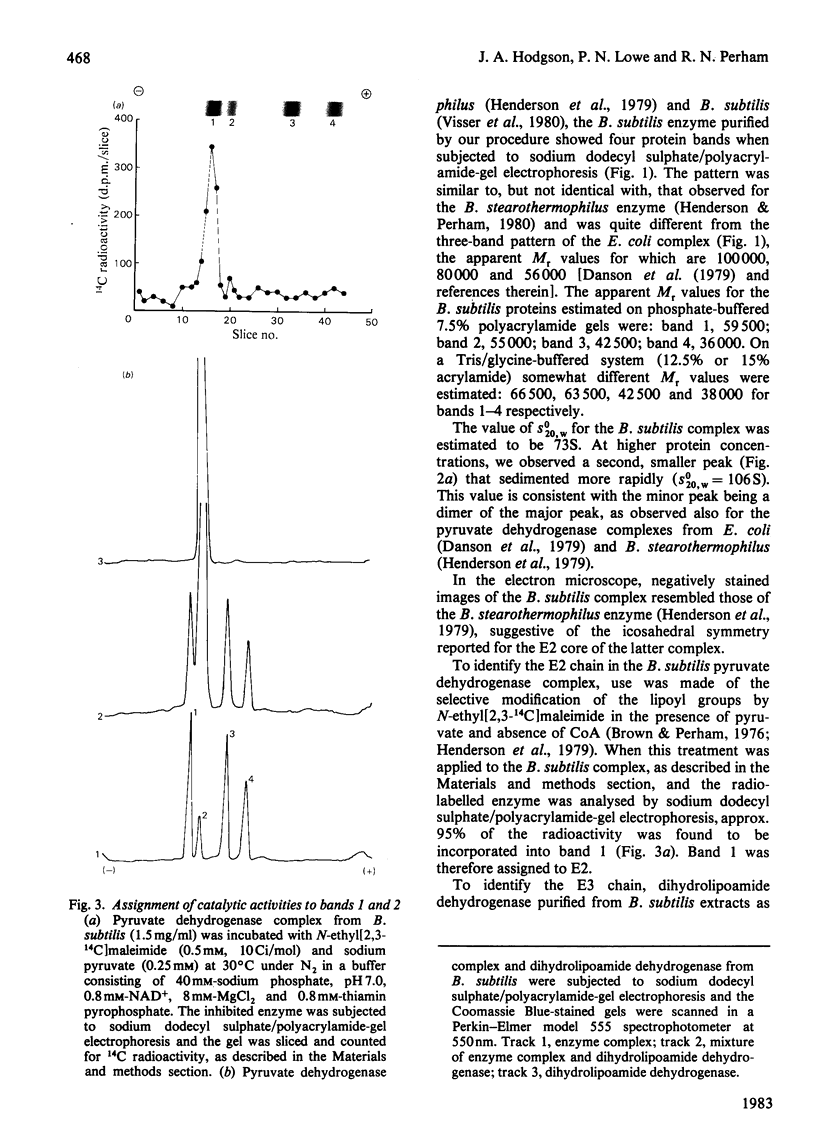
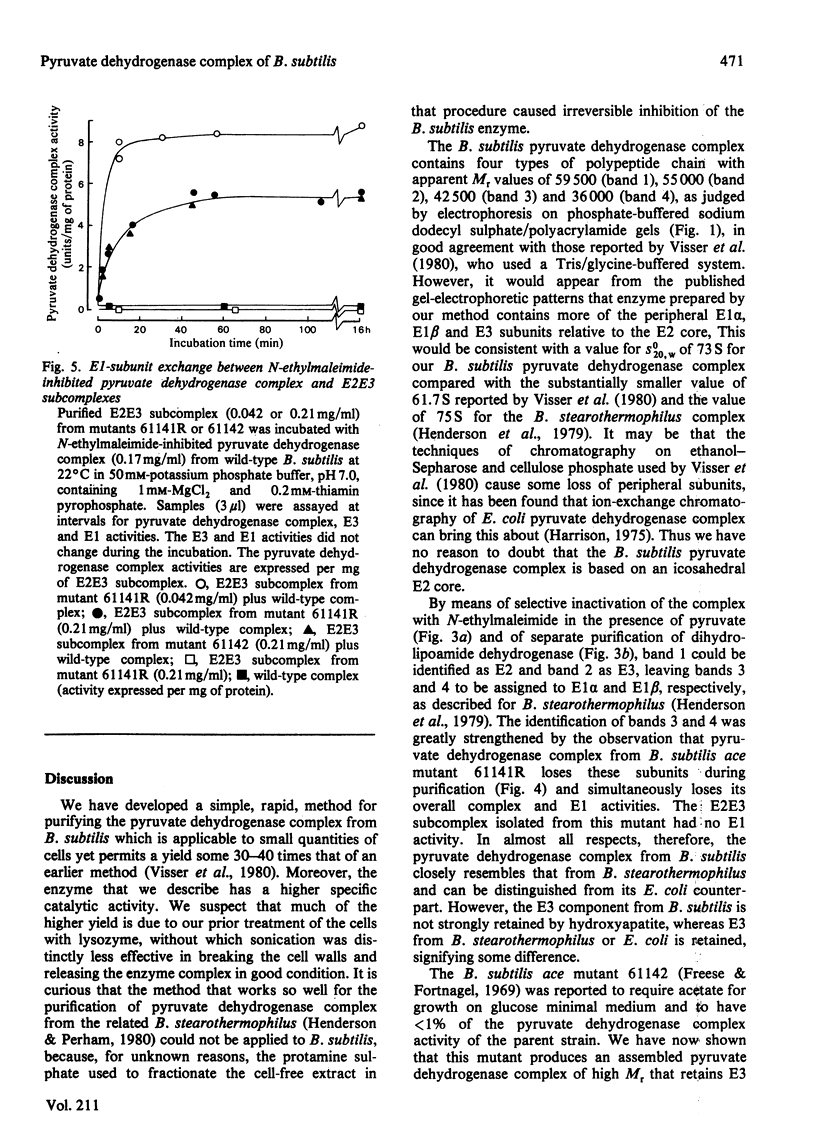
A simple procedure is described for the purification of the pyruvate dehydrogenase complex and dihydrolipoamide dehydrogenase from Bacillus subtilis. The method is rapid and applicable to small quantities of bacterial cells. The purified pyruvate dehydrogenase complex (s0(20),w = 73S) comprises multiple copies of four different types of polypeptide chain, with apparent Mr values of 59 500, 55 000, 42 500 and 36 000: these were identified as the polypeptide chains of the lipoate acetyltransferase (E2), dihydrolipoamide dehydrogenase (E3) and the two types of subunit of the pyruvate decarboxylase (E1) components respectively. Pyruvate dehydrogenase complexes were also purified from two ace (acetate-requiring) mutants of B. subtilis. That from mutant 61142 was found to be inactive, owing to an inactive E1 component, which was bound less tightly than wild-type E1 and was gradually lost from the E2E3 subcomplex during purification. Subunit-exchange experiments demonstrated that the E2E3 subcomplex retained full enzymic activity, suggesting that the lesion was limited to the E1 component. Mutant 61141R elaborated a functional pyruvate dehydrogenase complex, but this also contained a defective E1 component, the Km for pyruvate being raised from 0.4 mM to 4.3 mM. The E1 component rapidly dissociated from the E2E3 subcomplex at low temperature (0-4 degrees C), leaving an E2E3 subcomplex which by subunit-exchange experiments was judged to retain full enzymic activity. These ace mutants provide interesting opportunities to analyse defects in the self-assembly and catalytic activity of the pyruvate dehydrogenase complex.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma H. J., de Graaf-Hess A. C., de Kok A., Veeger C., Visser A. J., Voordouw G. Pyruvate dehydrogenase complex from Azotobacter vinelandii: structure, function, and inter-enzyme catalysis. Ann N Y Acad Sci. 1982;378:265–286. doi: 10.1111/j.1749-6632.1982.tb31202.x. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Perham R. N. An amino acid sequence in the active site of lipoamide dehydrogenase from the 2-oxoglutarate dehydrogenase complex of E. coli (Crookes strain). FEBS Lett. 1972 Oct 1;26(1):221–224. doi: 10.1016/0014-5793(72)80577-5. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Perham R. N. Selective inactivation of the transacylase components of the 2-oxo acid dehydrogenase multienzyme complexes of Escherichia coli. Biochem J. 1976 May 1;155(2):419–427. doi: 10.1042/bj1550419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate R. L., Roche T. E. Function and regulation of mammalian pyruvate dehydrogenase complex. Acetylation, interlipoyl acetyl transfer, and migration of the pyruvate dehydrogenase component. J Biol Chem. 1979 Mar 10;254(5):1659–1665. [PubMed] [Google Scholar]
- Danson M. J., Hale G., Johnson P., Perham R. N., Smith J., Spragg P. Molecular weight and symmetry of the pyruvate dehydrogenase multienzyme complex of Escherichia coli. J Mol Biol. 1979 Apr 25;129(4):603–617. doi: 10.1016/0022-2836(79)90471-6. [DOI] [PubMed] [Google Scholar]
- Danson M. J., Hooper E. A., Perham R. N. Intramolecular coupling of active sites in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochem J. 1978 Oct 1;175(1):193–198. doi: 10.1042/bj1750193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel U. Growth and sporulation of Bacillus subtilis mutants blocked in the pyruvate dehydrogenase complex. J Bacteriol. 1969 Sep;99(3):745–756. doi: 10.1128/jb.99.3.745-756.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale G., Bates D. L., Perham R. N. Subunit exchange in the pyruvate dehydrogenase complex of Escherichia coli. FEBS Lett. 1979 Aug 15;104(2):343–346. doi: 10.1016/0014-5793(79)80848-0. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Perham R. N., Finch J. T. Structure and symmetry of B. stearothermophilus pyruvate dehydrogenase multienzyme complex and implications for eucaryote evolution. Cell. 1979 May;17(1):85–93. doi: 10.1016/0092-8674(79)90297-6. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Perham R. N. Purificaton of the pyruvate dehydrogenase multienzyme complex of Bacillus stearothermophilus and resolution of its four component polypeptides. Biochem J. 1980 Jul 1;189(1):161–172. doi: 10.1042/bj1890161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keha E. E., Ronft H., Kresze G. B. On the origin of mitochondria: a reexamination of the molecular structure and kinetic properties of pyruvate dehydrogenase complex from brewer's yeast. FEBS Lett. 1982 Aug 23;145(2):289–292. doi: 10.1016/0014-5793(82)80185-3. [DOI] [PubMed] [Google Scholar]
- LUSTY C. J., SINGER T. P. LIPOYL DEHYDROGENASE. FREE AND COMPLEXED FORMS IN MAMMALIAN MITOCHONDRIA. J Biol Chem. 1964 Nov;239:3733–3742. [PubMed] [Google Scholar]
- Lowe P. N., Leeper F. J., Perham R. N. Stereoisomers of tetrahydrothiamin pyrophosphate, potent inhibitors of the pyruvate dehydrogenase multienzyme complex from Escherichia coli. Biochemistry. 1983 Jan 4;22(1):150–157. doi: 10.1021/bi00270a022. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Seckler R., Binder R., Bisswanger H. Purification and properties of the pyruvate dehydrogenase complex from Salmonella typhimurium and formation of hybrids with the enzyme complex from Escherichia coli. Biochim Biophys Acta. 1982 Jul 26;705(2):210–217. doi: 10.1016/0167-4838(82)90180-7. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]