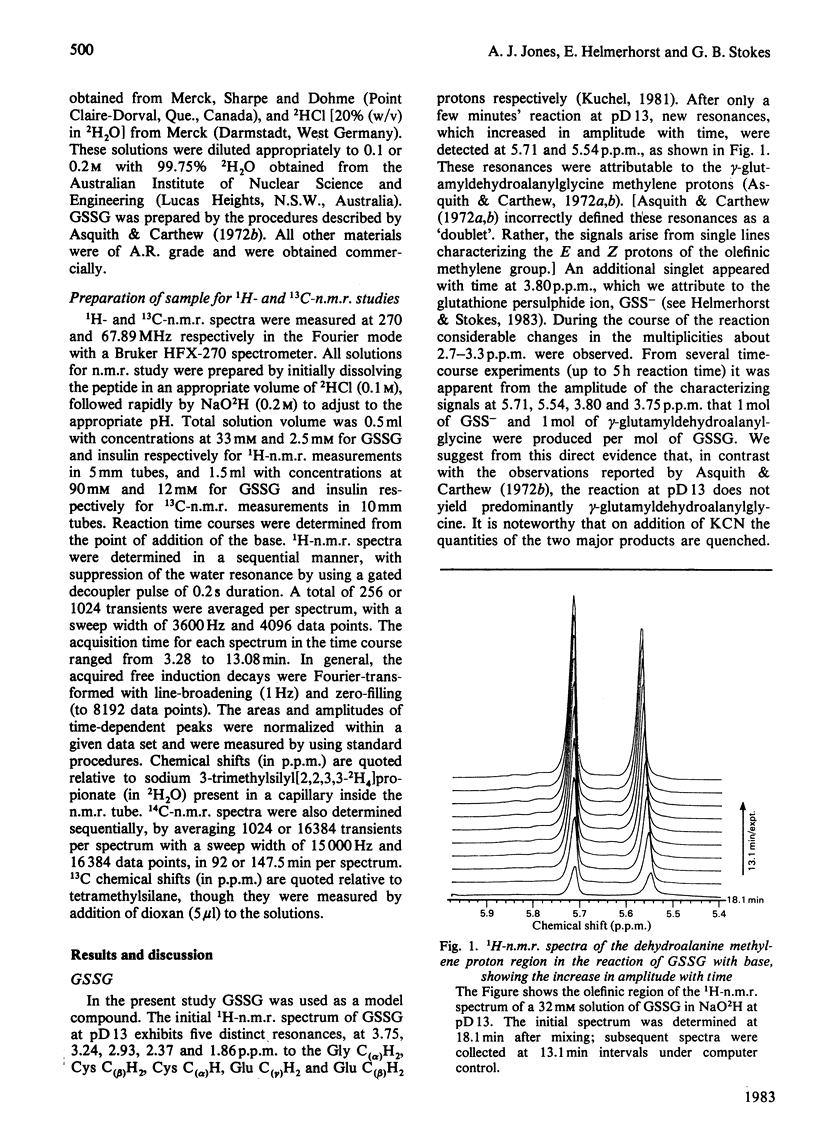
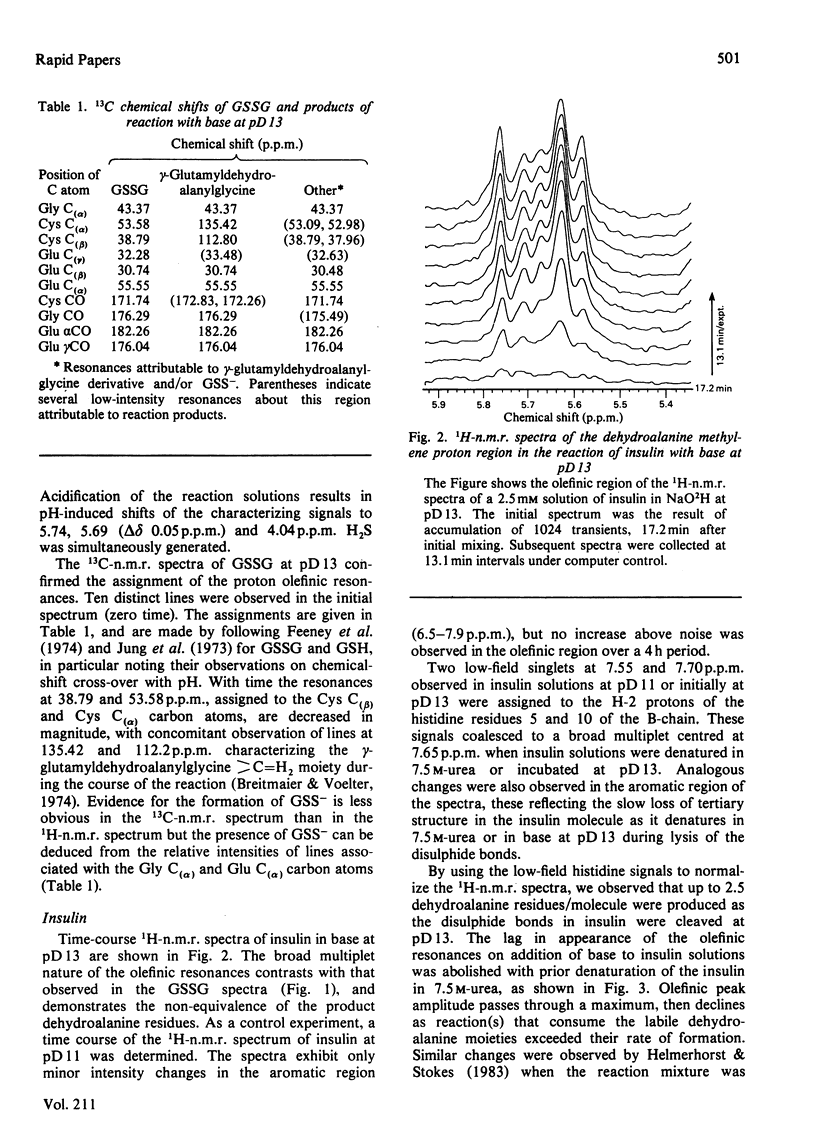
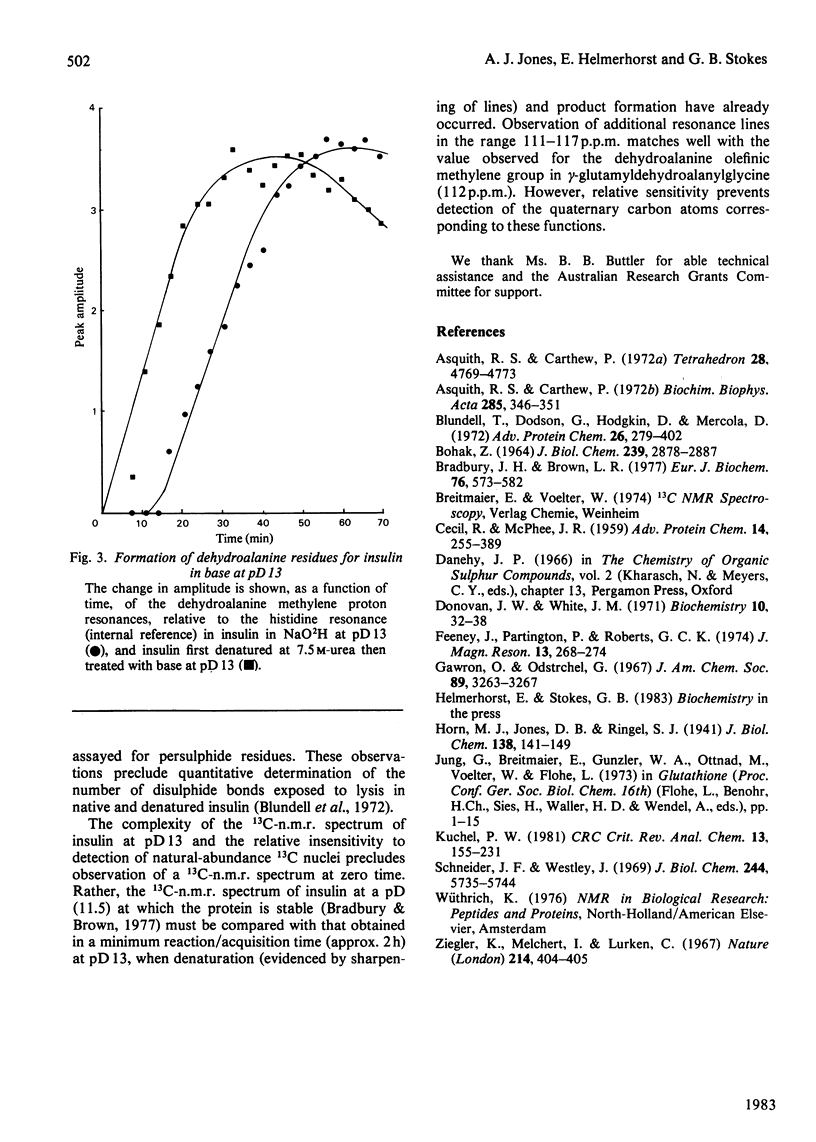
Abstract
1H- and 13C-n.m.r. measurements enable direct observation of the rate of formation of dehydroalanine residues resulting from lysis of the disulphide bonds of insulin and oxidized glutathione in base at pD13. The data provide clear evidence for the beta-elimination mechanism for this reaction. The dehydroalanine-containing products from the lysis of insulin undergo secondary reactions.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asquith R. S., Carthew P. The preparation and subsequent identification of a dehydroalanyl peptide from alkali-treated oxidised glutathione. Biochim Biophys Acta. 1972 Dec 28;285(2):346–351. doi: 10.1016/0005-2795(72)90319-4. [DOI] [PubMed] [Google Scholar]
- BOHAK Z. N-EPSILON-(DL-2-AMINO-2-CARBOXYETHYL)-L-LYSINE, A NEW AMINO ACID FORMED ON ALKALINE TREATMENT OF PROTEINS. J Biol Chem. 1964 Sep;239:2878–2887. [PubMed] [Google Scholar]
- Bradbury J. H., Brown L. R. Nuclear-magnetic-resonance-spectroscopic studies of the amino groups of insulin. Eur J Biochem. 1977 Jun 15;76(2):573–582. doi: 10.1111/j.1432-1033.1977.tb11627.x. [DOI] [PubMed] [Google Scholar]
- CECIL R., McPHEE J. R. The sulfur chemistry of proteins. Adv Protein Chem. 1959;14:255–389. doi: 10.1016/s0065-3233(08)60613-0. [DOI] [PubMed] [Google Scholar]
- Donovan J. W., White T. M. Alkaline hydrolysis of the disulfide bonds of ovomucoid and of low molecular weight aliphatic and aromatic disulfides. Biochemistry. 1971 Jan 5;10(1):32–38. doi: 10.1021/bi00777a005. [DOI] [PubMed] [Google Scholar]
- Gawron O., Odstrchel G. Kinetic studies on the alkaline decomposition of cystine derivatives and peptides. J Am Chem Soc. 1967 Jun 21;89(13):3263–3267. doi: 10.1021/ja00989a029. [DOI] [PubMed] [Google Scholar]
- Schneider J. F., Westley J. Metabolic interrelations of sulfur in proteins, thiosulfate, and cystine. J Biol Chem. 1969 Oct 25;244(20):5735–5744. [PubMed] [Google Scholar]
- Ziegler K., Melchert I., Lürken C. N-delta-(2-amino-2-carboxyethyl)-ornithine, a new amino-acid from alkali-treated proteins. Nature. 1967 Apr 22;214(5086):404–405. doi: 10.1038/214404a0. [DOI] [PubMed] [Google Scholar]


