Summary
The NLRP3 inflammasome is a key multi-protein complex controlling inflammation, particularly interleukin-1β (IL-1β) production. Here, we present a protocol to profile spatially resolved NLRP3 inflammasome complexes using ascorbic peroxidase 2 (APEX2)-based proximity labeling combined with liquid chromatography-tandem mass spectrometry (LC-MS/MS). We describe steps for design and generation of the fusion construct, characterization of the stable FLAG-NLRP3-APEX2 expression cell line by western blotting/imaging, biotinylated proteome enrichment, and mass spectrometry analysis.
For complete details on the use and execution of this protocol, please refer to Liang et al.1
Subject areas: immunology, mass spectrometry, molecular biology, proteomics
Graphical abstract

Highlights
-
•
Step-by-step APEX2-based proximity labeling protocol for capturing molecular snapshots
-
•
Procedure to probe protein complexes at different functional stages
-
•
Instructions for profiling the molecular environment during NLRP3 inflammasome assembly
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The NLRP3 inflammasome is a key multi-protein complex controlling inflammation, particularly interleukin-1β (IL-1β) production. Here, we present a protocol to profile spatially resolved NLRP3 inflammasome complexes using ascorbic peroxidase 2 (APEX2)-based proximity labeling combined with liquid chromatography-tandem mass spectrometry (LC-MS/MS). We describe steps for design and generation of the fusion construct, characterization of the stable FLAG-NLRP3-APEX2 expression cell line by western blotting/imaging, biotinylated proteome enrichment, and mass spectrometry analysis.
Before you begin
Defining the molecular environment of dynamic protein interaction networks at a molecular level is experimentally challenging due to frequent weak and transient interaction stoichiometries between proteins, adaptor molecules, enzymes and substrates. Co-immunoprecipitation (co-IP) and affinity-purification mass spectrometry (AP-MS)2,3 with fusion tags, such as Halo-tag, utilizing a reversible binding interaction with a specific ligand,4,5 have been applied. However, these approaches are suffering from limitations in capturing preferentially higher affinity interactions6 and from potential steric hindrance due to tagging. Recently, a promising proximity labeling (PL)-based approach has been widely utilized to label proximal proteins with biotin, followed by isolation via streptavidin-based enrichment and identification by tandem mass spectrometry (LC-MS/MS). To achieve this, proteins of interest (POIs) are fused to biotin ligase derivates, such as BioID and TurboID,7,8 or peroxidases (HRP, APEX and APEX2).9,10,11 The peroxidase-based biotinylation reaction using hydrogen peroxide and biotin-phenol bears the advantage of short labeling times, usually enabling molecular snapshots within sub-minute resolution.12,13,14
Here, we present a detailed APEX2-based experimental approach for defining the molecular profile of the active and inactive NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome as an addendum to our original report.1 We describe critical parameters that need to be optimized in order to maximize signal-to-noise information, complementing other reports describing the NLRP3 and caspase-1 complex.15 In particular, concentrations of hydrogen peroxide exposed for around 1 min to cells, as well as concentrations used to preload cells with a biotin-phenol substrate are critical for the efficient APEX2-dependent generation of biotin-phenoxyl radicals that covalently tag proximal endogenous proteins. Biotinylated proteins captured by NLRP3-APEX2 proximity labeling can be classified into i) proteins that physically bind to NLRP3 and or other components of the complex; ii) compartmental or proximal proteins in the local milieu of NLRP3; iii) proteins that non-specifically stick to streptavidin beads and iv) physically non-existing proteins detected as a methodological error. The first two types provide insights into the protein-protein interaction (PPI) network and local milieu of NLRP3. However, others are considered false positives. To establish a high-confidence proximity proteome resource for NLRP3, a variety of approaches were used to filter out contaminant proteins.
In summary, we have optimized an APEX2-based experimental approach for defining the molecular details of the NLRP3 inflammasome complex. Furthermore, this technology is adaptable to other cellular protein targets of interest, in particular for those that are associated with larger sub-cellular compartments, such as organelles or membranes.
Design and generation of the flag-NLRP3 and flag-NLRP3-APEX2 fusion construct
Timing: 3–4 weeks
This section describes steps for the design and generation of appropriate plasmids needed for an APEX2 proximity labeling experiment in a Flp-In stable cell line system.
The proximity labeling (PL) enzymes consist of biotin ligase derivates and peroxidases. Due to rapid labeling kinetics, the peroxidase is preferentially used for the profiling of fast biological processes, such as enzyme-substrate interactions, ligand-receptor pairings, and signaling complex assembly. The comparison of distinct proximity labeling enzymes and the key factors to consider when choosing them have been comprehensively reviewed.7,16 Here, in this protocol, we utilize APEX2-based technology (Figure 1) for the proximity labeling of NLRP3 inflammasome. To ensure the normal biological function of NLRP3 and enzymatic activity of APEX2, we insert APEX2 to the C-terminus of NLRP3 with an appropriate linker between them, and flag tag to the N-terminus for western blotting and immunofluorescence. To assess the tolerance of the APEX2 tagging, flag-NLRP3 fusion construct was used as a control.
Note: In terms of APEX2 tagging, a flexible linker, such as Glycine/Serine (Gly/Ser) linker, is recommended. The length of the linker can be adjusted to suit specific experimental needs. It has been suggested that the size of the linker can affect the biotinylation radius.17 Thus, by varying the linker length, the biotinylation range during proximity labeling can be modulated.
Note: In terms of epitope tagging, V5 and flag have been successfully used for APEX2 fusion proteins.9 Hemagglutinin (HA) epitope should be avoided because biotin-phenoxyl radicals are highly reactive towards the tyrosine present in the HA sequence. This reactivity may interfere with the proper functioning of the HA tag in proximity labeling experiments.
Figure 1.
Schematic representation of APEX2 proximity labeling
(A) Once activated, APEX2 catalyzes the oxidation of a biotin-phenol substrate, generating highly reactive biotin-phenoxyl radicals.
(B) The biotin-phenoxyl radicals quickly interact with nearby electron-rich amino acids, particularly tyrosine (tryptophan and cysteine in some cases). The radical reacts with the aromatic ring of tyrosine through a radical coupling mechanism, resulting in biotinylation of the protein.
Characterization of the flag-NLRP3 and flag-NLRP3-APEX2 fusion construct by imaging
Timing:3 days
This section describes the procedures for the preparation, transfection, and imaging of HEK293T cells to detect NLRP3 puncta formation via confocal microscopy.
-
1.Poly-L-lysine (PLL) coating of the plates.
-
a.Prepare and coat a 96-well plate with 50 μL PLL (0.1 mg/mL) per well for 10 min at 22°C.
-
b.Aspirate the PLL solution, and rinse with 200 μL Milli-Q water for three times.
-
c.Remove the final wash and leave the plate uncovered in the hood for around 2 h to dry.
-
a.
Alternative: this step is only for anchorage-dependent cells, like HEK293T, that weakly adhere to the plastic plate bottom.
-
2.
Seed 1 ×104 HEK293T cells per well on the PLL-pre-coated 96-well plate in 100 μL complete medium and incubate the cells for 16 h at 37°C with 5% CO2.
-
3.
Transfect the HEK293T cells with NLRP3-APEX2 fusion construct using Lipofectamine 3000 according to manufacturer’s instructions (https://www.thermofisher.com/order/catalog/product/L3000001).
-
4.
24 h after the transfection, treat the cells with 10 μM Nigericin and incubate for different time points.
Note: Based on our previous studies, we observed that maximum speck formation occurred after 90 min of Nigericin treatment, after which the response plateaued. Nigericin, a well-established potassium ionophore, is utilized in this protocol as an activator of NLRP3 inflammasome assembly. For the activation of other inflammasomes, it is important to employ appropriate and specific inducers.
-
5.
Remove the media and wash the cells with 200 μL DPBS for three times.
-
6.
Remove the final wash and fix the cells with 100 μL 4% (wt/vol) paraformaldehyde (PFA) in DPBS for 10 min at 25°C .
-
7.
Wash the cells with 200 μL DPBS for three times, each wash lasting 5 min.
-
8.
Permeabilize the cells with 100 μL 0.2% (vol/vol) Triton X-100 in DPBS for 10 min at 25°C.
-
9.
Wash the cells with 200 μL cold DPBS for three times, each wash lasting 5 min.
-
10.
Block the cells with 100 μL 1% (wt/vol) BSA in DPBS for 1–2 h at 25°C.
-
11.
Remove the blocking reagent and incubate the cells with 100 μL diluted anti-flag or anti-NLRP3 antibody (1: 1000 dilution for both antibodies) for 2 h at 25°C or 16 h at 4°C.
-
12.
The following day, wash the cells with 200 μL PBST for three times, each wash lasting 5 min.
-
13.
Remove the final wash and incubate the cells with 100 μL AF488-conjugated goat anti-rabbit IgG (H + L) secondary antibody in 1% (wt/vol) BSA for 1 h at 25°C (1: 1000 dilution).
-
14.
Remove the secondary antibody and wash the cells with 200 μL DPBS for three times, each wash lasting 5 min.
Pause point: The cells can be stored for up to one month in the dark at 4°C.
-
15.
Image the plate on a high-content laser-based spinning disk confocal microscope (Opera Phenix Plus, Revvity), using a 60× water objective. Images are collected and analyzed using Harmony imaging and analysis software.
Generation of a stable flag-NLRP3-APEX2 expression HEK293 cell line
Timing: 1–2 weeks
Here we describe steps for the transfection and generation of a stable flag-NLRP3-APEX2 expression HEK293 cell line.
-
16.Maintenance of Flp-In T-REx HEK293 cells.
-
a.The Flp-In T-REx HEK293 cell line is thawed and cultured in complete medium containing 100 μg/mL Zeocin and 15 μg/mL Blasticidin selection antibiotic in a humidified 37°C, 5% CO2 incubator.
-
b.Cells are passaged when they reach confluency of 80%–90%.
-
a.
Note: The Flp-In T-REx HEK293 cells are commercially available and can also be established with the pFRT/lacZeo construct according to the manufacturer’s instructions (https://www.thermofisher.com/us/en/home/references/protocols/proteins-expression-isolation-and-analysis/protein-expression-protocol/generation-of-isogenic-expression-cell-lines-using-flp-in-t-rex-system.html#prot3).
-
17.Co-transfection of HEK293 cell line.
-
a.Seed 5 ×105 cells per well on a 6-well plate in 2 mL complete medium and incubate the cells for 16 h at 37°C with 5% CO2.
-
b.The following day, co-transfect the cells with a 1:9 ratio (wt/wt) of pcDNA 5/FRT construct: pOG44 plasmid using Lipofectamine 3000 according to manufacturer’s instructions (https://www.thermofisher.com/order/catalog/product/L3000001).
-
a.
Note: Include a well of untransfected cells as a negative control and a well without pOG44 as Flp recombination control.
-
18.Polyclonal selection of stable cell line.
-
a.24 h after transfection, change the medium to remove the cell debris.
-
b.48 h after transfection, split the cells to less than 25% confluency in fresh medium containing hygromycin B.Note: In the case of Flp-In T-REx HEK293, the concentration of hygromycin B is 300 μg/mL. For other cell lines, the minimum concentration is to be determined by testing a range of concentrations.
-
c.Replenish the selective medium every 3–5 days until the single cell-formed colonies/foci can be identified.
-
d.Expand the hygromycin-resistant cell colonies and verify the expression of flag-NLRP3 or flag-NLRP3-APEX2 fusion construct by western blotting.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| NLRP3, antibody dilution: 1/1,000 | Cell Signaling Technology | CAT# 15101 |
| β-Actin, antibody dilution: 1/1,000 | Sigma-Aldrich | CAT# A5441 |
| NEK7, antibody dilution: 1/1,000 | Abcam | CAT# ab133514 |
| ASC/TMS1/PYCARD (B-3), antibody dilution: 1/400 | Santa Cruz | CAT# sc-514414 |
| Anti-Flag M1, antibody dilution: 1/1,000 | Sigma-Aldrich | CAT#F3040 |
| IRDye 800CW goat anti-rabbit IgG secondary antibody, antibody dilution: 1/10,000 | LI-COR | CAT# 926-32211 |
| IRDye 800CW goat anti-mouse IgG secondary antibody, antibody dilution: 1/10,000 | LI-COR | CAT# 926-32210 |
| IRDye 680RD goat anti-mouse IgG secondary antibody, antibody dilution: 1/10000 | LI-COR | CAT# 926-68070 |
| IRDye 680RD goat anti-rabbit IgG secondary antibody, antibody dilution: 1/10,000 | LI-COR | CAT# 926-68071 |
| Goat anti-rabbit IgG (H + L) secondary, Alexa Fluor 555-conjugated, antibody dilution: 1/1,000 | Invitrogen | CAT# A-21428 |
| Goat anti-mouse IgG (H + L) secondary, Alexa Fluor 488-conjugated, antibody dilution: 1/1,000 | Invitrogen | CAT# A-11001 |
| IRDye 800CW Streptavidin, antibody dilution: 1/1,000 | LI-COR | CAT# 926-32230 |
| Chemicals, peptides, and recombinant proteins | ||
| L-glutamine (200 mM) | Gibco | 25030149 |
| Fetal bovine serum | Gibco | 10100147 |
| Poly-L-lysine (PLL) solution | Sigma-Aldrich | P4707 |
| GlutaMAX supplement | Gibco | 35050061 |
| Penicillin-Streptomycin (10,000 U/mL) | Gibco | 15140122 |
| Zeocin | InvivoGen | ant-zn-1 |
| Hygromycin B | InvivoGen | ant-hg-1 |
| Dimethyl sulfoxide | Sigma-Aldrich | D8418 |
| Dulbecco’s phosphate-buffered saline | Sigma-Aldrich | D8537 |
| 16% Formaldehyde (w/v), methanol-free | Thermo Scientific | 28906 |
| PhosSTOP phosphatase inhibitor | Roche | 4906837001 |
| cOmplete, Mini, EDTA-free protease inhibitor cocktail | Roche | 11836170001 |
| Pierce DTT (Dithiothreitol) | Thermo Scientific | 20290 |
| Iodoacetamide | Sigma-Aldrich | A3221 |
| Lipofectamine 3000 Transfection Reagent | Invitrogen | L3000001 |
| Nuclease-free water (not DEPC-treated) | Invitrogen | AM9932 |
| Sequencing-grade modified trypsin | Promega | V5111 |
| Tween 20 | Sigma-Aldrich | P1379-1L |
| Triton X-100 | Sigma-Aldrich | T9284-500ML |
| Bromophenol blue | Sigma-Aldrich | B0126 |
| Biotin | Sigma-Aldrich | B4501 |
| PMSF | G-Biosciences | 786–055 |
| Hydrogen peroxide (H2O2), 30% (wt/wt) | Sigma-Aldrich | H1009-100ML |
| Trifluoroacetic acid | Sigma-Aldrich | 91707 |
| Formic acid | Sigma-Aldrich | 56302 |
| Biotin-phenol | Sigma-Aldrich | SML2135 |
| Dynabeads MyOne Streptavidin T1 | Invitrogen | 65602 |
| Sodium ascorbate | Sigma-Aldrich | A7631 |
| Trolox | Sigma-Aldrich | 238813-5G |
| Sodium azide | Sigma-Aldrich | S2002 |
| TCEP | Gold Biotechnology | TCEP2 |
| IGEPAL CA-630 | Sigma-Aldrich | I8896 |
| Triton X-100 | Sigma-Aldrich | X100 |
| Benzonase Nuclease | Millipore | E1014 |
| Critical commercial assays | ||
| Pierce BCA Protein Assay Kit | Thermo Scientific | 23225 |
| InstantBlue Coomassie Protein Stain (ISB1L) | Abcam | ab119211 |
| Pierce Silver Stain Kit | Thermo Scientific | 24612 |
| Experimental models: Cell lines | ||
| HEK293T | ATCC | CRL-3216 |
| Flp-In T-REx 293 cell line | Invitrogen | R78007 |
| Recombinant DNA | ||
| pOG44 plasmid vector | Thermo Fisher Scientific | CAT# V600520 |
| pcDNA5/FRT/TO GFP | Addgene | CAT# 19444 |
| pcDNA5/FRT/TO flag-NLRP3 | Generated in this study (Backbone 19444) | N/A |
| pcDNA5/FRT/TO flag-NLRP3-APEX2 | Generated in this study (Backbone 19444) | N/A |
| Software and algorithms | ||
| Prism | GraphPad, version 9 | https://www.graphpad.com/scientific-software/prism/ |
| Perseus | Tyanova et al.18; V2.0.7.0 | https://maxquant.net/perseus/ |
| ImageStudioLite | LI-COR, version 5.2 | https://www.licor.com/bio/image-studio-lite/ |
| FiJi | Schindelin et al.19 | https://imagej.net/software/fiji/ |
| ZEN | Carl Zeiss | RRID: SCR_013672 |
| Illustrator | Adobe | https://www.adobe.com/uk/ |
| Photoshop | Adobe | https://www.adobe.com/uk/ |
| MaxQuant | Tyanova et al.20; V1.6.10.43 | http://www.maxquant.org |
Materials and equipment
| Reagent | Concentration | Amount | Storage/comments |
|---|---|---|---|
| 100 × H2O2 stock buffer | 100 mM | add 100 μL 30% (wt/wt) H2O2 (10 M) in 900 μL of DPBS | freshly made each time before use |
| 1 M Sodium azide stock buffer | 1 M | dissolve 0.65 g sodium azide in 10 mL of distilled H2O | stored at −20°C for at least a month |
| 1 M Sodium ascorbate stock buffer | 1 M | dissolve 198 mg Sodium ascorbate in 1 mL of distilled H2O | freshly made each time before use |
| Trolox stock buffer | 500 mM | dissolve 250 mg Trolox in 1 mL of DMSO | freshly made each time before use |
| KCl | 1 M | dissolve 37.25 g KCl in 500 mL of distilled H2O | stored at RT for months |
| Na2CO3 | 0.1 M | dissolve 5.295 g Na2CO3 in 500 mL of distilled H2O | stored at RT for months |
| Tris-HCl, pH 8.0 | 10 mM | dissolve 605.7 mg Tris base in 500 mL of distilled H2O and adjust the pH to 8.0 using HCl | stored at RT for months |
| 2 M urea stock buffer | 2 M | dissolve 6 g urea in 50 mL 10 mM Tris-HCl, pH 8.0 | freshly made each time before use |
| Biotin stock buffer | 100 mM | dissolve 24.43 mg biotin in 1 mL DMSO | stored at −20°C for at least a month |
| Urea | 8 M | dissolve 4.8 g urea in 10 mL 100 mM TEAB | freshly made each time before use |
| TCEP | 0.5 M | dissolve 1.43 g TCEP in 10 mL 100 mM TEAB | freshly made each time before use |
| Iodoacetamide | 0.5 M | dissolve 924.8 mg iodoacetamide in 10 mL 100 mM TEAB | freshly made each time before use |
Note: Sonicate the Trolox and sodium ascorbate for 5–10 min, until they are thoroughly dissolved.
Quencher Buffer (100 mL)
| Reagent | Stock concentration | Final concentration | Amount |
|---|---|---|---|
| Trolox | 500 mM | 5 mM | 1 mL |
| Sodium ascorbate | 1 M | 10 mM | 1 mL |
| Sodium azide | 1 M | 10 mM | 1 mL |
| DPBS | N/A | N/A | 97 mL |
| Total | N/A | N/A | 100 mL |
CRITICAL: Freshly prepared before use.
RIPA lysis buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris-HCl, pH 7.6 | 25 mM | 2.0 g |
| NaCl | 150 mM | 4.4 g |
| NP-40 | 1% | 5 mL |
| Sodium deoxycholate | 1% | 5.0 g |
| SDS | 0.1% | 0.5 g |
| MilliQ-H2O | N/A | 495 mL |
| Total | N/A | 500 mL |
CRITICAL: Store at 4°C for up to 6 months.
Lysis buffer (10 mL)
| Reagent | Stock concentration | Final concentration | Amount |
|---|---|---|---|
| RIPA lysis buffer | N/A | N/A | 700 μL |
| Trolox | 500 mM | 5 mM | 100 μL |
| Sodium ascorbate | 1 M | 10 mM | 100 μL |
| Sodium azide | 1 M | 10 mM | 100 μL |
| PhosSTOP phosphatase inhibitor | N/A | N/A | 1 tablet |
| cOmplete, Mini, EDTA-free Protease | N/A | N/A | 1 tablet |
| Total | N/A | N/A | 10 mL |
CRITICAL: Should be freshly prepared before use.
DMEM complete medium (500 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| Dulbecco’s Modified Eagle Medium (DMEM) | N/A | 440 mL |
| Fetal bovine serum (FBS) | 10% | 50 mL |
| 100× GlutaMAX Supplement | 2 mM | 5 mL |
| 100× Penicillin/Streptomycin | 1% | 5 mL |
| Total | N/A | 500 mL |
CRITICAL: Store at 4°C for up to 1 month.
Freezing medium (500 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| Dulbecco’s Modified Eagle Medium (DMEM) | N/A | 390 mL |
| Fetal bovine serum (FBS) | 10% | 50 mL |
| 100× GlutaMAX Supplement | 2 mM | 5 mL |
| 100× Penicillin/Streptomycin | 1% | 5 mL |
| DMSO | 10% | 50 mL |
| Total | N/A | 500 mL |
CRITICAL: Store at −20°C for up to 6 months.
Note: Avoid multiple freeze-thaw cycles. Mixed well before used.
DPBS (500 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| KCl | 0.2 g/L | 0.1 g |
| NaCl | 8.0 g/L | 4.0 g |
| KH2PO4 | 0.2 g/L | 0.1 g |
| Na2HPO4 (anhydrous) | 1.15 g/L | 0.6 g |
| MilliQ-H2O | N/A | 500 mL |
| Total | N/A | 500 mL |
CRITICAL: Store at 22°C for up to a year.
PBST (500 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| KCl | 0.2 g/L | 0.1 g |
| NaCl | 8.0 g/L | 4.0 g |
| KH2PO4 | 0.2 g/L | 0.1 g |
| Na2HPO4 | 1.15 g/L | 0.6 g |
| MilliQ-H2O | N/A | 450 mL |
| Tween 20 | 0.1% | 500 μL |
| Total | N/A | 500 mL |
CRITICAL: Store at 22°C up to a year.
Solution A (30 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| CH3CN | 2% | 600 μL |
| Trifluoroacetic acid (TFA) | 0.1% | 30 μL |
| MilliQ-H2O | 98% | 29.4 mL |
| Total | N/A | 30 mL |
CRITICAL: Store at 4°C for up to 1 month.
Solution B (30 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| CH3CN | 65% | 6.5 mL |
| Trifluoroacetic acid (TFA) | 0.1% | 10 μL |
| MilliQ-H2O | 35% | 3.5 mL |
| Total | N/A | 10 mL |
CRITICAL: Store at 4°C for up to 1 month.
Liquid chromatography (LC) and mass spectrometer (MS) settings
An Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific) paired with an Ultimate 3000 UHPLC system (Thermo Fisher Scientific) was utilized to analyze purified tryptic peptides through LC-MS/MS, as previously described.21 Approximately 200 ng of the tryptic peptides were loaded onto a PepMap C18 trap column (300 μm × 5 mm, 5 μm particle size) and separated on a 50 cm EasySpray column using a 60-min linear gradient from 2% to 35% buffer B (5% DMSO, 0.1% formic acid in acetonitrile) at a flow rate of around 250 nL/min. The mass spectrometer operated in data-dependent acquisition mode with Advanced Peak Detection enabled, capturing survey scans in the Orbitrap at a resolution of 120,000 over an m/z range of 400–1500, with an AGC target of 4e5 and S-lens RF of 30. Fragment ion spectra were obtained in the ion trap using rapid scan mode, with a quadrupole isolation window of 1.6 m/z, a 40% AGC target, and a maximum injection time of 35 ms, employing higher-energy collisional dissociation at 28% collision energy for fragmentation.
Liquid chromatography (LC) settings
| Time intervals (min) | Gradient (percentage of buffer B) | Flow rate (uL/min) |
|---|---|---|
| 0 | 2% | 0.25 |
| 3 | 2% | 0.25 |
| 6 | 5% | 0.25 |
| 63 | 35% | 0.25 |
| 70 | 99% | 0.25 |
| 75 | 99% | 0.25 |
| 78 | 2% | 0.25 |
| 82 | 99% | 0.25 |
| 88 | 2% | 0.25 |
| 89 | 2% | 0.25 |
| 103 | stop | N/A |
Analysis sequence for NLRP3 speck properties in Columbus
| Step | Building block | Method | Input | Output |
|---|---|---|---|---|
| 1 | Input image | Flat-field correction: basic Bright-field correction: yes Stack Processing: Individual plane Create Global Image: No | Channel: HOECHST 3334 Channel: Alexa 488 | Output image |
| 2 | Find nuclei | Method: B Common Threshold: 0.4 Area > 30 μm2 Splitting coefficient: 7.0 Individual threshold: 0.4 Contrast>0.1 | Channel: HOECHST 3334 ROI population: None | Population: Nuclei |
| 3 | Find cytoplasm | Method: A Individual threshold: 0.15 | Channel: Alexa 488 Nuclei: Nuclei | Output population: Cytoplasm |
| 4 | Find spots | Method: A Relative spot intensity >0.18 Splitting sensitivity: 0.910 Calculate spot properties | Channel: Alexa 488 ROI population: Nuclei ROI region: Cytoplasm | Output population: NLRP3 speck |
| 5 | Define results | Standard output: spots number Formula output: spots number/nuclei number | N/A | Spots number/cell Relative spot intensity Spot Area [px2] Roundness |
Step-by-step method details
Characterization of the stable flag-NLRP3-APEX2 expression HEK293 cell line by western blotting
Timing: 3 days
This section describes the characterization of a stable HEK293 cell line expressing flag-NLRP3-APEX2. Protein expression is induced using tetracycline, followed by treatment with Nigericin and proximity labeling with biotin-phenol and H2O2. The cells are then lysed, and the samples are prepared for analysis via western blotting to evaluate protein expression and labeling efficiency (Figures 1, 2, and 3).
-
1.
PLL coating of the plates. Prepare and coat the 6-well plate as described above. Refer to Table 1 for the appropriate volumes.
-
2.
Add tetracycline (tet) to achieve a final concentration of 1 μg/mL to induce the expression of flag-NLRP3-APEX2. Incubate the cells 16 h at 37°C with 5% CO2.
-
3.
The following day, add Nigericin to the culture medium at a final concentration of 10 μM and incubate cells for different time points.
-
4.
Replace the Nigericin-containing medium with complete medium supplemented with 500 μM biotin-phenol and incubate the cells for 30 min at 37°C with 5% CO2.
CRITICAL: The medium must be warmed up prior to the addition of phenol-biotin to prevent precipitation. If precipitation occurs, sonicate the medium containing phenol-biotin in a water sonicator for 5 min to resuspend. Additionally, incubation periods should be followed strictly.
-
5.
While waiting, prepare the quencher buffer (Trolox 5 mM, sodium ascorbate 10 mM, sodium azide 10 mM).
CRITICAL: The quencher buffer should be freshly made immediately before use.
-
6.
After the incubation, add H2O2 directly to the biotin-phenol -containing medium to achieve a final concentration of 1 mM. Incubate the cells for 1 min at 37°C with 5% CO2.
-
7.
Decant the medium and incubate the cells with 2 mL quencher buffer for 1 min at 25°C.
-
8.
Wash with 2 mL quencher buffer per well for another two times, each wash lasting 1 min.
-
9.
Decant the final wash thoroughly and lyse the cells by pipetting in 100 μL RIPA lysis buffer supplemented with quencher buffer (Trolox 5 mM, sodium ascorbate 10 mM, sodium azide 10 mM).
-
10.
Collect and sonicate the lysate using a microtip at 15 W output with 3 bursts of 15 s each.
-
11.
Cool the samples on ice for 1 min between each burst. After sonication, leave the lysates on ice for 5 min. This process will help reduce the viscosity of the sample.
-
12.
Clarify the samples via centrifugation at 10,000 g for 10 min at 4°C to remove the cell debris.
Note: 5% of the whole cell lysate will be saved for subsequent western blot analysis. The remaining lysate can either be frozen at −80°C for future use or immediately proceed to streptavidin pull-down.
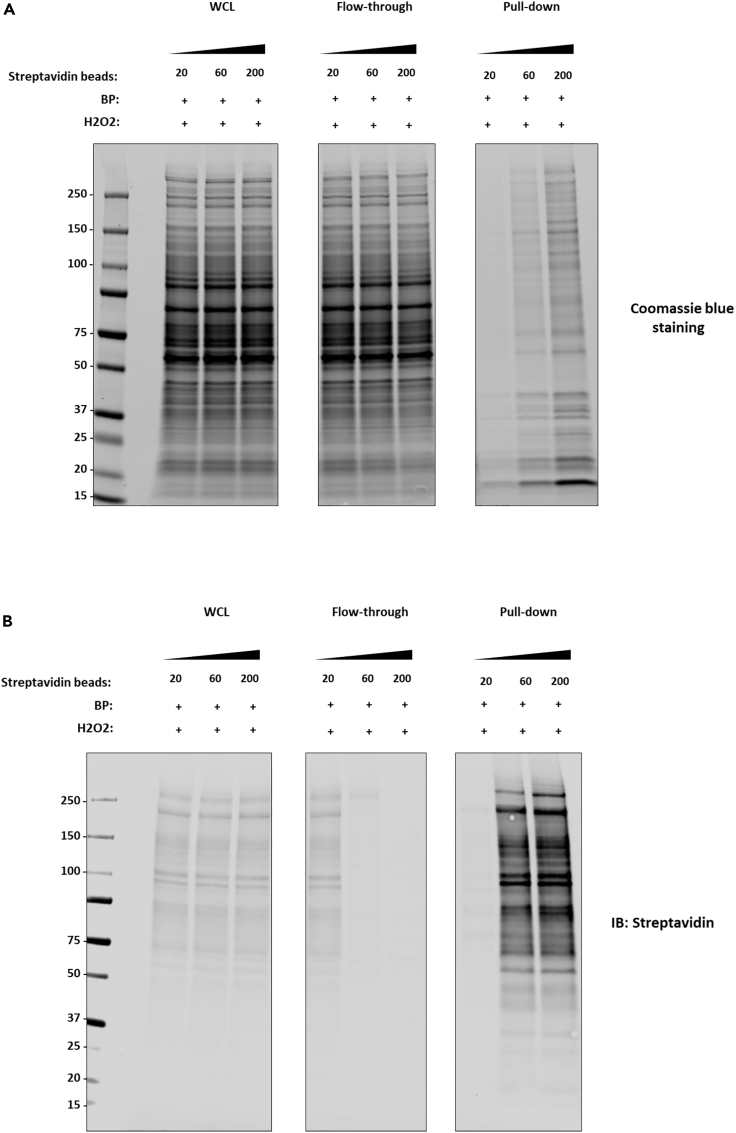
Figure 2.
Optimization of the streptavidin beads for the enrichment of biotinylated proteins
(A and B) Coomassie blue staining (A) and streptavidin blotting (B) of whole cell lysates (WCL), flow-through, and pull-down fractions after streptavidin bead enrichment. Different amounts of streptavidin beads (20, 60, 200 μL) were used for the pull-down experiments as indicated.
Figure 3.
Capture and biotinylation of ASC in HEK293 cells stably expressing Flag-NLRP3-APEX2
(A) Schematic overview of the experimental design for treatment conditions and APEX2 induced biotinylation. Flp-In T-REx HEK293 cell lines stably expressing flag-NLRP3-APEX2 were treated with either DMSO or Nigericin (10 μM) for 90 min, followed by incubation with 500 μM biotin-phenol (BP) and 1 mM H2O2 for 20 s. The diagram illustrates the biotinylation process and the potential interaction with ASC protein under different treatment conditions.
(B and C) Streptavidin blotting of whole cell lysates (B) and Immunoblotting of the streptavidin beads-pulled down samples (C) from HEK293 cells stably expressing flag-NLRP3-APEX2 under various conditions as indicated. Figure reprinted with permission from Liang et al.1
Table 1.
Reagent volumes and cell density for different plate formats
| Number of cells | Tetracycline | Medium | Biotin-phenol (BP) | H2O2 | Quencher buffer | |
|---|---|---|---|---|---|---|
| 96-well plate | 1×104 cells/well | 0.1 μL | 100 μL | 0.1 μL | 1 μL | 200 μL |
| 6-well plate | 1×105 cells/well | 2 μL | 2 mL | 1 μL | 10 μL | 2 mL |
| 100 mm dish | 1×106 cells/well | 10 μL | 10 mL | 5 μL | 50 μL | 10 mL |
Characterization of the stable flag-NLRP3-APEX2 expression HEK293 cell line by imaging
Timing: 2 days
This section describes steps for the characterization of the speck formation upon Nigericin activation in a stable flag-NLRP3-APEX2 expression HEK293 cell line (Figure 4).
-
13.Pre-coat the 96-well plate with PLL and seed the stable flag-NLRP3-APEX2 HEK293 cells as described above. Perform the proximity labeling as described in steps 4–8.Note: Refer to Table 1 for the appropriate volumes.
-
a.Treat the cells with tetracycline and Nigericin for distinct time-points as described in steps 2–3.
-
b.Replace the Nigericin-containing medium with warmed medium with 500 μM biotin-phenol (BP).
-
c.Incubate for 30 min at 37°C, add H2O2 to 1 mM and incubate for 1 min at 25oC.
-
d.Decant and wash twice with freshly prepared quencher buffer, each wash lasting 1 min.
-
a.
-
14.
Immediately decant the final wash and fix the cells with 4% (wt/vol) paraformaldehyde (PFA), followed by permeabilization and blocking as described above.
-
15.
After blocking, incubate the cells with 100 μL diluted anti-NLRP3 or anti-flag antibody (1:1000 dilution) and IRDye 800CW Streptavidin (1: 10000 dilution) for 16 h at 4°C.
-
16.
The following day, wash the cells with 200 μL cold PBST for three times, each wash lasting 5 min.
-
17.
Remove the final wash and incubate the cells with 100 μL AF555-conjugated goat anti-rabbit IgG (H + L) secondary antibody (1: 1000 dilution) in 1% (wt/vol) BSA for 1 h at 25°C.
-
18.
Remove the secondary antibody and wash the cells with 200 μL cold DPBS for three times, each wash lasting 5 min.
-
19.
Image the plate on a high-content laser-based spinning disk confocal microscope (Opera Phenix Plus, Revvity), using a 60× water objective.
-
20.
Determine the spatial specificity of the NLRP3-APEX2 induced biotinylation via the confocal immunofluorescence results.
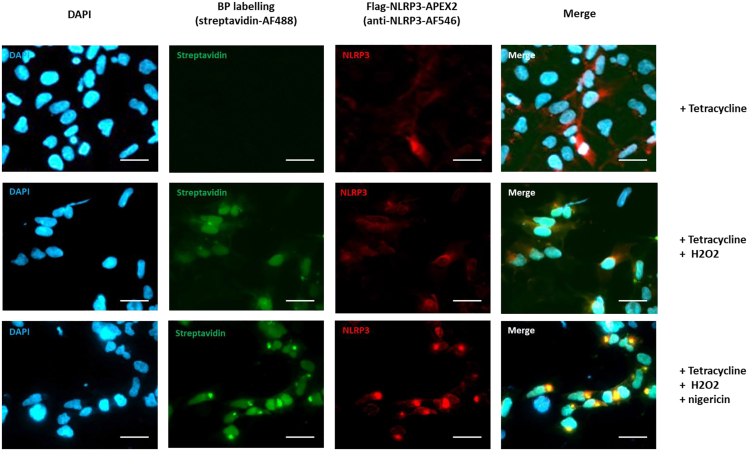
Figure 4.
APEX2 labeling is able to capture the formation of NLRP3 puncta upon activation
Confocal fluorescence imaging of NLRP3 APEX2 labeling was conducted in HEK293 cell lines. NLRP3-APEX2 HEK293 stable cell lines were treated with tetracycline 16 h, followed by a 90-min treatment with either Nigericin (10 μM) or DMSO (as a control). Subsequently, the cells were incubated with biotin phenol and then H2O2, as specified. After incubation, the cells were fixed and stained with a streptavidin-Alexa Fluor 488 (AF488) conjugate to visualize biotinylated proteins and with an anti-NLRP3 antibody to determine the localization of NLRP3-APEX2. Biotinylated proteins (BP) were visualized, and scale bars represent 50 μm. Figure reprinted with permission from Liang et al.1
Streptavidin pull-down
Timing: 12–15 h
This section describes the streptavidin pull-down of biotinylated proteins from the lysate of flag-NLRP3-APEX2 expressing HEK293 cells. After incubating the lysate with streptavidin beads 16 h, the beads are washed to remove non-specific proteins. The biotinylated proteins are then eluted for SDS-PAGE, followed by western blot and Coomassie or silver staining analysis (Figures 2, 3, and 5).
-
21.
Prepare the streptavidin magnetic beads. Take 80 μL streptavidin magnetic beads and wash with 1 mL RIPA lysis buffer twice at 25°C.
-
22.
Remove the final wash and add the clarified lysate from step-10 to the beads. Incubate and rotate the magnetic beads 16 h at 4°C.
-
23.
The following day, pellet the beads on the magnetic rack and collect the supernatant (flow-through) for further analysis.
-
24.
Wash the beads twice with 1 mL RIPA lysis buffer per samples (in each wash step, rotate the tube 2–3 times and pellet the beads on magnetic rack for 1 min incubation time).
-
25.
Wash the beads as described in step-24 but with the following reagents in this order: once with 1 M KCL, 0.1 M Na2CO3 and 2 M Urea (in 10 mM Tris-HCL, pH 8) and finally twice with RIPA buffer again.
Note: The beads can be frozen at −80°C for future use, or you can proceed directly to step-26 for western blot analysis, or on-beads trypsin digestion.
-
26.For SDS-PAGE, place the samples on the magnetic rack.
-
a.Once all the beads have adhered to the rack, carefully remove the final RIPA buffer thoroughly.
-
b.Elute the biotinylated proteins via boiling the beads in 60 μL 3 x Laemmli loading buffer (supplemented with 2 mM biotin and 20 mM DTT) at 98°C for 10 min.
-
c.For western blot analysis, use 12 μL of the whole cell lysate and 15 μL of the eluted biotinylated proteins.
-
a.
CRITICAL: A negative control without biotinylation is necessary to evaluate the effectiveness of the washes in removing non-specifically bound proteins by the beads.
-
27.
Coomassie or silver staining can be used to visualize the total proteins, including biotinylated proteins and non-specifically bound proteins by the streptavidin beads.
Figure 5.
Characterization of the NLRP3-APEX2 fusion construct by western blotting
(A) Schematics of the experimental workflow. Cells are lysed in RIPA buffer and cleared by centrifugation. Biotinylated proteins are captured using streptavidin beads, followed by washes with RIPA, KCl, Na2CO3, and 2 M Urea before being resuspended in RIPA. Enriched biotinylated proteins are either digested on beads with trypsin or eluted by boiling.
(B–D) Western blotting of whole cell lysate (B), streptavidin-captured proteins (C) and flow-through (D) using the antibodies as indicated.
Figure reprinted with permission from Liang et al.1
On-beads trypsin digestion
Timing: 2 days
This section describes the on-beads trypsin digestion of biotinylated proteins from the lysate of flag-NLRP3-APEX2 expressing HEK293 cells. Following protein denaturation, reduction, alkylation, and dilution, the proteins are digested with trypsin and then prepared for subsequent purification and mass spectrometry analysis.
-
28.
Remove the final wash of RIPA lysis buffer from step-25, add 200 μL of 8 M urea to the beads, and incubate for 30 min at 25°C to fully denature the proteins.
-
29.
Add 4 μL of 0.5 M TCEP per sample (final conc. = 10 mM) and incubate for 30 min at 25°C.
-
30.
Alkylate the samples by adding 20.4 μL of 0.5 M iodoacetamide (IAA) per sample (final conc. = 50 mM) and incubate in darkness for 30 min at 25°C.
-
31.
Quench the reaction by adding 4.5 μL of 1 M dithiothreitol (DTT) (final conc. = 20 mM).
-
32.
Dilute the urea concentration to 1.5 M by adding 972.8 μL of 50 mM Triethylammonium bicarbonate (TEAB).
-
33.
Digest the biotinylated proteins by incubation with trypsin for 16 h at 37°C (1 μg trypsin / 20 μL streptavidin beads).
CRITICAL: The amount of trypsin required can vary depending on the initial material and the efficiency of the IP-Streptavidin process. We recommend using 1 μg of trypsin per 20 μL of streptavidin beads. Resuspend the lyophilized sequencing grade modified Trypsin in resuspension Buffer (50 mM acetic acid) to achieve a final concentration of 0.1 mg/mL.
-
34.
The following day, pellet the beads and collect the supernatant for further purification and mass spectrometry analysis.
C18 peptide desalting
Timing: 1 day
This section describes C18 peptide desalting of trypsin-digested biotinylated proteins from flag-NLRP3-APEX2 expressing HEK293 cells, followed by preparation for mass spectrometry analysis.
-
35.
Attach the syringes to the Sep-Pak column and attach both to a vacuum manifold.
Note: It is important to avoid air bubbles in the column at any step during the protocol.
-
36.
Prepare and equilibrate the column by flushing first with 5 mL of solution B (65% CH3CN, 35% MilliQ-H2O, 0.1% TFA) and 10 mL of solution A (98% MilliQ-H2O, 2% CH3CN, 0.1% TFA).
-
37.
Add the peptide digest from step-34 into the 10 mL of solution A and let it into the Sep-Pak column slowly.
-
38.
Wash the sample with 10 mL of solution A.
-
39.
Elute twice with 600 μL of solution B and combine the purified sample in 2 mL Eppendorf tubes.
-
40.
Dry the purified peptides by a speed-vac concentrator at 25°C. This may take several hours dependent on vacuum performance.
-
41.
Resuspend the peptides in 20 μL of solution A and store at −80°C until further analysis.
-
42.
Transfer the purified peptide into the glass vial and place it in the LC autosampler.
-
43.
Set up the orbitrap fusion Lumos mass spectrometry instrument with the parameters described in the materials and equipment setup section. Inject between 1 - 5 μL for each sample dependent on test injections, where the ion chromatogram intensity should be kept < 1E9.
Mass spectrometry (MS) data analysis
Timing: 1 week
This section describes the analysis of mass spectrometry (MS) data derived from proximity-labeled proteins in HEK293 cells expressing flag-NLRP3-APEX2. The process includes data processing using MaxQuant, filtering, SAINT scoring, cutoff determination, pathway enrichment analysis, and the construction of protein-protein interaction networks.
-
44.Analyze Raw MS Data.
-
a.Analyze the raw MS data using MaxQuant software.
-
b.Database Search: Search the raw MS files against the UniProtKB human sequence database (UP000005640).
-
c.Label-Free Quantification Parameters.
-
i.Fixed Modification: Set Carbamidomethyl (C) as the fixed modification.
-
ii.Variable Modifications: Set Oxidation (M) and Deamidation (NQ) as variable modifications.
-
iii.Missed Cleavages: Allow a maximum of two missed cleavages.
-
iv.Match Between Runs (MBR): Enable the Match Between Runs (MBR) function to align features across different runs.
-
i.
-
a.
-
45.
Data Filtering: Filter the MS/MS data set to remove the proteins that are potential contaminants, only identified by peptides or those that match to the reversed peptide sequences.
-
46.
SAINT probabilistic scoring for PL-MS data. Assign confidence scores to the proximity labeling-MS data using the computational tool-SAINTexpress via the online Contaminant Repository for Affinity Purification (CRAPome) platform at www.crapome.org.
-
47.
To assess the enrichment specificity for NLRP3 proximal proteins, generate a list of experimentally validated NLRP3-interacting or -co-localizing proteins via BioGRID database or literature curation.
-
48.Cutoff Determination: To denoise the proximity proteome, perform the receiver operating characteristic (ROC) analysis.
-
a.Rank Proteins: Rank the proteins that pass the FDR<0.05 threshold in descending order by log2 [fold change (stimulated/unstimulated)].
-
b.True Positive Rate (TPR): Calculate the TPR, defined as the fraction of experimentally validated NLRP3-interacting proteins identified in the BioGRID or literature that are detected in your dataset.
-
c.False Positive Rate (FPR): Calculate the FPR, defined as the proportion of nucleolus and secreted proteins (based on Gene Ontology Cellular Component (GO-CC)) detected in your dataset.
-
d.Plot ROC Curve: Plot TPR against FPR to generate a ROC curve.
-
e.Determine Cutoff: Set the cutoff where the difference (TPR - FPR) is maximal to achieve the greatest signal-to-background ratio, effectively denoising the proximity proteome.
-
a.
-
49.Pathway Enrichment Analysis.
-
a.Filter Data: Focus on proximity proteomes with SAINT probability >0.8 and fold change (FC-A) ≥1 in either unstimulated or stimulated samples.
-
b.Import to STRING Database: Import the filtered data into the STRING database search portal.
-
c.Plot Top Enriched Terms.
-
i.Retrieve the top 20 enriched biological terms.
-
ii.Plot these terms based on their strength (Log10 [observed/expected]) and false discovery rate (FDR) (interaction score ≥0.7).
-
i.
-
d.Clustering.
-
i.Cluster the top enriched terms based on the similarity of their enrichment strength patterns across different experimental groups.
-
ii.Visualize the results using the clusterProfiler 4.0 package in R.
-
i.
-
a.
-
50.Protein-Protein Interaction (PPI) Network Construction.
-
a.Upload the filtered data to the STRING portal (https://string-db.org/).
-
b.Physical subnetwork type is chosen to extract the physical (direct) protein-protein associations from the database.
-
c.Active interaction sources, including text mining, experiments, and databases, and an interaction score > 0.7 (high confidence interaction) are applied to construct the PPI networks.
-
d.Markov clustering algorithm (MCL) is used to group the interaction subnetworks with the inflation value of 3.0.
-
a.
Expected outcomes
The outcomes of APEX2 proximity labeling can be assessed by multiple experiments, including immunofluorescence (spatial specificity), immunoblotting and silver staining (labeling robustness). Initially, to confirm the efficacy of the biotinylating efficiency achieved by APEX2 labeling, biotinylated proteins can be visualized as a band profile by SDS-PAGE and streptavidin blotting. We recommend optimizing the biotin-phenol concentration and incubation time to ensure robust biotinylation and low background noise. In our study case on NLRP3, we confirmed successful APEX2 labeling (Figures 2A and 2B). However, this does not ensure the ability to detect subtle changes in the microenvironment of NLRP3 upon different physiological stages (this limitation may apply to other target proteins). Therefore, we performed western blotting to confirm the recruitment of ASC, a known NLRP3 interactor (Figures 3A–3C) and immunofluorescence following APEX2 proximity labeling, comparing activated and inactivated stages of NLRP3 (Figure 4). Under unstimulated conditions (inactivated NLRP3), biotinylated proteins and NLRP3-APEX2 are diffused across the cytosol (Figure 4). Upon Nigericin treatment (activated NLRP3), NLRP3-APEX2 oligomerizes to form puncta close to the nucleus, with corresponding specks formed by the biotinylated proteins. This reflects a crucial experiment to investigate the ability of APEX2 labeling to capture changes in the microenvironment of activated versus inactivated NLRP3. Confirming successful APEX2 labeling (Figures 5A–5D) and the ability to capture known interactors at different stages (Figures 3A–3C) provides evidence that the APEX2-MS experiment will reflect an adequate physiological condition. Due to the wealth of MS data being generated, a SAINT (Significance Analysis of INTeractome) score analysis can be used to determine the proteins in close proximity to your bait protein under different conditions. Of note is that one may detect more than 700 proteins to be determined as significantly enriched. A fraction only of these proteins may reflect direct interactors of NLRP3, and the majority may be in its close proximity. To mitigate such a limitation, a comparison of different physiological stages, such as unstimulated versus Nigericin-stimulated NLRP3, can be advantageous to reveal physiologically relevant associated factors.
Limitations
This APEX2 PL-MS study has certain limitations, particularly in the use of HEK293 cells instead of appropriate immune cell types where the NLRP3 inflammasome assembly naturally occurs. Although our research and others' studies have successfully shown that the NLRP3 inflammasome can be reconstituted in HEK293T cells,22 indicating the presence of essential components, it is possible that some regulatory factors present in myeloid-derived cell lineages may be absent in HEK293 cells. During the preparation of this protocol, Hollingsworth et al. employed APEX2 proximity labeling in immortalized bone marrow-derived macrophages (iBMDMs) to investigate the NLRP3 trafficking upon stimulation.23 This study provided additional insights into the NLRP3 proximity networks in a more appropriate cellular system. Also, there are limitations in the degree of complexity that mass spectrometry experiments are now providing, often leading to extensive interaction maps that need rigorous curation to identify truly associated proteins from non-specific counterparts.24 Furthermore, the PL approach exerts limitations in choosing adequate proteins that are amenable to this type of analysis. In particular, PL works best when POIs are associated with large intracellular structures, organelles or membranes are chosen1,25,26,27,28 and biotinylation sites are identified to reveal more structural information about interactions.29
Troubleshooting
Problem 1
Insufficient biotinylation during proximity labeling can occur. This protocol can be applied to different cell lines, including primary cells and iPSCs. However, APEX2 labeling efficiency may vary between cell lines. If you apply this protocol to a different cell line, you may experience insufficient biotinylation.
Potential solution
Optimize the concentration of biotin-phenol (BP) and the BP incubation time. Evaluate the biotinylation efficiency with a range of BP concentrations by western blotting and silver staining (or Coomassie staining). It is noted that higher BP concentration sometimes only increases the biotinylation of the same protein at multiple sites, which may not change the total yield.
Problem 2
High background in the negative control during streptavidin pull-down (Figure 6).
Figure 6.
High background in the negative control during streptavidin pull-down
Streptavidin blotting (left) and Coomassie blue staining (right) of the pull-down fraction after streptavidin bead enrichment from an APEX2 labeling.
Potential solution
Optimize the amount of beads used in Streptavidin IP (Figures 2A and 2B). Using enough beads to capture the biotinylated proteins without excess can help minimize the background and reduce the cost of the experiment. Additional washes can be applied in this protocol to remove non-specific background from the beads, including washes with 2% SDS.
Problem 3
High background noise for the immunofluorescence (Figure 4).
Potential solution
-
•
Optimize the dilution of both the primary and secondary antibodies to reduce background noise and improve the signal specificity.
-
•
Additional washes after applying the secondary antibody to ensure the removal of non-specific bindings.
-
•
Use the minimum effective concentration of phenol biotin can further decrease the background noise.
Resource availability
Lead contact
For further information and requests for resources and reagents, please contact the lead contact, Professor Benedikt Kessler (benedikt.kessler@ndm.ox.ac.uk).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contacts, Zhu Liang (zhu.liang@ndm.ox.ac.uk) and Andreas Damianou (andreas.damianou@ndm.ox.ac.uk).
Materials availability
Plasmids generated in this study will be available from the lead contact upon request.
Data and code availability
-
•
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD045862.
-
•
This study does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
Z.L., A.D., I.V., and B.M.K. were supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Science (CIFMS), China (grant number: 2018-I2M-2-002), awarded to B.M.K. B.M.K. and A.D. were supported by Pfizer. Z.L. was supported by the China Scholarship Council. E.D.D. was supported by Alzheimer’s Research UK grant ARUK2015DDI-OX. We thank members of the Discovery Proteomics Facility, the Kessler lab, and Oxford Drug Discovery Institute groups for constructive discussions. We would also like to thank Raphael Heilig for his assistance with mass spectrometry and Dr. Val Millar and Daniel Ebner’s group for their expert help in the use of PerkinElmer Opera Phenix/High Content Imaging. The graphical abstract/figures were created using BioRender.com.
Author contributions
B.M.K., Z.L., E.D.D., and A.D. conceptualized the study. Z.L., A.D., A.G., H.B.L.J., and V.S. conducted the experiments, and I.V., Z.L., and F.L. analyzed samples and results by mass spectrometry. B.M.K., Z.L., E.D.D., and A.D. wrote the manuscript, and A.G., H.B.L.J., V.S., F.L., and I.V. contributed to text editing.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2024.103417.
Contributor Information
Zhu Liang, Email: zhu.liang@ndm.ox.ac.uk.
Andreas Damianou, Email: andreas.damianou@ndm.ox.ac.uk.
Benedikt M. Kessler, Email: benedikt.kessler@ndm.ox.ac.uk.
Supplemental information
References
- 1.Liang Z., Damianou A., Vendrell I., Jenkins E., Lassen F.H., Washer S.J., Grigoriou A., Liu G., Yi G., Lou H., et al. Proximity proteomics reveals UCH-L1 as an essential regulator of NLRP3-mediated IL-1beta production in human macrophages and microglia. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2024.114152. [DOI] [PubMed] [Google Scholar]
- 2.Burckstummer T., Bennett K.L., Preradovic A., Schutze G., Hantschel O., Superti-Furga G., Bauch A. An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nat. Methods. 2006;3:1013–1019. doi: 10.1038/nmeth968. [DOI] [PubMed] [Google Scholar]
- 3.Gregan J., Riedel C.G., Petronczki M., Cipak L., Rumpf C., Poser I., Buchholz F., Mechtler K., Nasmyth K. Tandem affinity purification of functional TAP-tagged proteins from human cells. Nat. Protoc. 2007;2:1145–1151. doi: 10.1038/nprot.2007.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.S N.P., Kwon K. The HaloTag: Improving Soluble Expression and Applications in Protein Functional Analysis. Curr Chem Genomics. 2012;6:8–17. doi: 10.2174/1875397301206010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stagge F., Mitronova G.Y., Belov V.N., Wurm C.A., Jakobs S. SNAP-CLIP- and Halo-tag labelling of budding yeast cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keilhauer E.C., Hein M.Y., Mann M. Accurate protein complex retrieval by affinity enrichment mass spectrometry (AE-MS) rather than affinity purification mass spectrometry (AP-MS) Mol. Cell. Proteomics. 2015;14:120–135. doi: 10.1074/mcp.M114.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho K.F., Branon T.C., Udeshi N.D., Myers S.A., Carr S.A., Ting A.Y. Proximity labeling in mammalian cells with TurboID and split-TurboID. Nat. Protoc. 2020;15:3971–3999. doi: 10.1038/s41596-020-0399-0. [DOI] [PubMed] [Google Scholar]
- 8.May D.G., Scott K.L., Campos A.R., Roux K.J. Comparative Application of BioID and TurboID for Protein-Proximity Biotinylation. Cells. 2020;9 doi: 10.3390/cells9051070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung V., Udeshi N.D., Lam S.S., Loh K.H., Cox K.J., Pedram K., Carr S.A., Ting A.Y. Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nat. Protoc. 2016;11:456–475. doi: 10.1038/nprot.2016.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang J., Espenshade P.J. Proximity-dependent biotin labelling in yeast using the engineered ascorbate peroxidase APEX2. Biochem. J. 2016;473:2463–2469. doi: 10.1042/BCJ20160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee H.W., Zou P., Udeshi N.D., Martell J.D., Mootha V.K., Carr S.A., Ting A.Y. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science. 2013;339:1328–1331. doi: 10.1126/science.1230593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paek J., Kalocsay M., Staus D.P., Wingler L., Pascolutti R., Paulo J.A., Gygi S.P., Kruse A.C. Multidimensional Tracking of GPCR Signaling via Peroxidase-Catalyzed Proximity Labeling. Cell. 2017;169:338–349.e11. doi: 10.1016/j.cell.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobingier B.T., Huttenhain R., Eichel K., Miller K.B., Ting A.Y., von Zastrow M., Krogan N.J. An Approach to Spatiotemporally Resolve Protein Interaction Networks in Living Cells. Cell. 2017;169:350–360.e12. doi: 10.1016/j.cell.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damianou A., Liang Z., Lassen F., Vendrell I., Vere G., Hester S., Charles P.D., Pinto-Fernandez A., Santos A., Fischer R., Kessler B.M. Oncogenic mutations of KRAS modulate its turnover by the CUL3/LZTR1 E3 ligase complex. Life Sci. Alliance. 2024;7 doi: 10.26508/lsa.202302245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamilloux Y., Lagrange B., Di Micco A., Bourdonnay E., Provost A., Tallant R., Henry T., Martinon F. A proximity-dependent biotinylation (BioID) approach flags the p62/sequestosome-1 protein as a caspase-1 substrate. J. Biol. Chem. 2018;293:12563–12575. doi: 10.1074/jbc.RA117.000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin W., Cho K.F., Cavanagh P.E., Ting A.Y. Deciphering molecular interactions by proximity labeling. Nat. Methods. 2021;18:133–143. doi: 10.1038/s41592-020-01010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D.I., Jensen S.C., Noble K.A., Kc B., Roux K.H., Motamedchaboki K., Roux K.J. An improved smaller biotin ligase for BioID proximity labeling. Mol. Biol. Cell. 2016;27:1188–1196. doi: 10.1091/mbc.E15-12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyanova S., Temu T., Sinitcyn P., Carlson A., Hein M.Y., Geiger T., Mann M., Cox J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods. 2016;13:731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- 19.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyanova S., Temu T., Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016;11:2301–2319. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- 21.Davis S., Charles P.D., He L., Mowlds P., Kessler B.M., Fischer R. Expanding Proteome Coverage with CHarge Ordered Parallel Ion aNalysis (CHOPIN) Combined with Broad Specificity Proteolysis. J. Proteome Res. 2017;16:1288–1299. doi: 10.1021/acs.jproteome.6b00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao L., Magupalli V.G., Wu H. Cryo-EM structures of the active NLRP3 inflammasome disc. Nature. 2023;613:595–600. doi: 10.1038/s41586-022-05570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollingsworth L.R., Veeraraghavan P., Paulo J.A., Harper J.W., Rauch I. Spatiotemporal proteomic profiling of cellular responses to NLRP3 agonists. bioRxiv. 2024 doi: 10.1101/2024.04.19.590338. Preprint at. [DOI] [Google Scholar]
- 24.Trinkle-Mulcahy L. Recent advances in proximity-based labeling methods for interactome mapping. F1000Res. 2019;8 doi: 10.12688/f1000research.16903.1. F1000 Faculty Rev-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumrongprechachan V., Salisbury R.B., Soto G., Kumar M., MacDonald M.L., Kozorovitskiy Y. Cell-type and subcellular compartment-specific APEX2 proximity labeling reveals activity-dependent nuclear proteome dynamics in the striatum. Nat. Commun. 2021;12:4855. doi: 10.1038/s41467-021-25144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehus A.A., Anderson R.H., Roux K.J. BioID Identification of Lamin-Associated Proteins. Methods Enzymol. 2016;569:3–22. doi: 10.1016/bs.mie.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei X.F., Li S., Hu J.L. A TurboID-based proximity labelling approach for identifying the DNA-binding proteins. STAR Protoc. 2023;4 doi: 10.1016/j.xpro.2023.102139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rees J.S. Proteomic Proximity Labeling to Reveal Interactions Between Biomolecules. Methods Mol. Biol. 2019;2008:13–28. doi: 10.1007/978-1-4939-9537-0_2. [DOI] [PubMed] [Google Scholar]
- 29.Shin S., Lee S.Y., Kang M.G., Jang D.G., Kim J., Rhee H.W., Kim J.S. Super-resolution proximity labeling with enhanced direct identification of biotinylation sites. Commun. Biol. 2024;7:554. doi: 10.1038/s42003-024-06112-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD045862.
-
•
This study does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.