Abstract
Introduction
Osteoarthritis (OA) of the knee affects millions of people with sizable socioeconomic burden. Conventional treatment modalities are prioritized, turning to surgical intervention only when they have failed. However, these traditional modalities have shortcomings, only aiming to reduce pain rather than targeting the underlying pathophysiology. Recently, the use of biologics, including autologous peripheral blood-derived orthobiologics (APBOs), has increased and demonstrated great promise for the management of knee OA. Platelet-rich plasma (PRP) is the most widely used APBO, but its efficacy is still uncertain, attributed to lack of standardized formulation protocols, characterization, and patient variables. To overcome the limitations posed by PRP, the use of other APBOs such as platelet lysate (PL) has been considered. This review summarizes the outcomes of clinical studies involving PL to manage OA of the knee.
Methods
Multiple databases (Scopus, Embase, PubMed, and Web of Science) were searched employing terms “platelet lysate” and “knee osteoarthritis” for articles published in the English language to August 15, 2024, adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Results
Only three clinical studies fulfilled our search and inclusion criteria. Intra-articular injection of three doses of PL injected every 3–4 weeks is safe and efficacious, resulting in statistically significant improvements in different patient-reported outcome measures at 6–12 months follow-up.
Conclusion
The existing published peer-reviewed literature suggests that intra-articular injection of PL is safe and can decrease pain and increase function in patients with knee OA. Nonetheless, given the dearth of pertinent literature, more adequately powered, multicenter, prospective, non-randomized and randomized controlled studies with extended follow-up are needed to confirm the effectiveness of PL in knee OA. Further comparative studies to help clinicians in choosing the best APBO for knee OA treatment are also warranted.
Keywords: Knee osteoarthritis, Autologous blood-derived orthobiologics, Platelet-rich plasma, Platelet lysate, Patient reported outcome measures
Key Summary Points
| Intra-articular injection of three doses of platelet lysate (PL) injected every 3-4 weeks is safe and can decrease pain and increase function in patients with knee osteoarthritis (OA). |
| The clinical results attained with PL are in accordance with the outcomes described for platelet-rich plasma (PRP), while mitigating few PRP-associated shortcomings. More adequately powered, multicenter, prospective, non-randomized and randomized controlled studies with extended follow-up are needed to confirm the effectiveness of PL in knee OA. |
| Comparative studies to help clinicians in choosing the best autologous peripheral blood-derived orthobiologic for knee OA treatment are also warranted. |
Introduction
Osteoarthritis (OA), a chronic health condition, affects over 240 million individuals worldwide [1]. It involves several anatomical and physiological joint alterations, including cartilage deterioration, osteophyte formation, and bone remodeling, causing pain, swelling, stiffness, and limited joint function, thereby impacting quality of sleep, mental health, and work participation, leading to major socioeconomic burden [1]. Conventionally, OA of the knee is initially managed using non-pharmacological modalities such as physiotherapy and weight management, nutraceuticals such as undenatured collagen type II, pharmacological substances such as viscosupplementation, corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs), minimally invasive interventions such as genicular nerve radiofrequency ablation, and surgery, when the conventional modalities have been unresponsive or in advanced stages of OA [2–4]. Nonetheless, these therapies have limitations, aiming to only decrease pain, but being unable to modify the underlying pathophysiology of the condition [2–4].
Recently, the use of autologous peripheral blood-derived orthobiologics (APBOs) to manage musculoskeletal disorders, including knee OA, has significantly increased [5–8]. Platelet-rich plasma (PRP) is the most frequently used APBO, and several level I (systematic review or meta-analysis of all relevant randomized controlled trials, RCTs) and level II (RCT) investigations have shown its safety and effectiveness [5–11]. The effectiveness of PRP remains controversial, given the dearth of uniform preparation protocol and formulation characterization, and patient-related factors [5–11]. To sidestep the limitations presented by PRP, the prospect of utilizing other APBOs such as platelet lysate (PL) has been considered.
PL is an acellular preparation rich in platelet-derived bioactive molecules, including growth factors, formulated by lysing platelets in a PRP formulation following multiple freeze/thawing cycles [12]. In vitro studies have shown the potential of PL as a non-xenogeneic alternative to fetal bovine serum to culture mesenchymal stem cells (MSCs), cultured and/or expanded to assess their potential for regenerative medicine applications, including management of OA of the knee [13–15]. In vitro, PL can promote proliferation of chondrocytes and inhibit apoptosis, improve cartilage matrix metabolism and promote cartilage repair, and increase chondrocyte autophagy and improve senescence [15–20]. Preclinical studies have also demonstrated the potential of PL for the management of knee OA [19, 21, 22]. Specifically, Yan et al. investigated the effect of PL on OA in an animal model (Sprague Dawley rats) of arthritis induced by intra-articular injection of monoiodoacetate [19]. Administration of PL resulted in significant restoration of mechanical allodynia, thermal hyperalgesia, and pain-related behavior (spontaneous activity and gait parameters) towards normal levels compared to the model levels [19]. Histopathology showed significant reversal in articular cartilage degeneration (assessed via number of chondrocytes, collagen matrix mass, and cartilage surface), with significantly decreased Mankin and OARSI (The Osteoarthritis Research Society International) scores in a dose-dependent manner [19]. Hsieh et al. prepared porcine PL and assessed whether its administration can avoid inflammatory reaction in the knee joint of (New Zealand) rabbits, and then treat osteochondral defects and arthritis [21]. The tissue sections from rabbit knee joint showed no inflammatory reaction, and administration of PL can result in better cartilage growth and delay occurrence of arthritis [21]. Forteza-Genestra et al. evaluated the efficacy of PL-derived extracellular vesicles (EVs) compared to MSC-derived EVs in an OA cartilage animal (Wistar rats) model (monoiodoacetate injection-induced OA) [22]. PL-derived EVs showed better subchondral bone integrity parameters in cone beam computed tomography as well as better OARSI score compared to the MSC-derived EVs or OA group, with values being close to the healthy group [22].
Despite the promising outcomes of these aforementioned in vitro and preclinical studies, there are limited studies evaluating the safety and efficacy of PL in patients with knee OA. Moreover, there are inadequate studies summarizing the outcomes of clinical studies investigating the effect of PL for the management of knee OA. Thus, the primary goal of the present work is to recapitulate the outcomes of clinical studies involving PL to manage knee OA. The secondary aim is to document the ongoing clinical studies listed on different clinical trial protocol archives involving PL for knee OA treatment.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by either of the authors.
Methods
Ethics Approval and Search Criteria
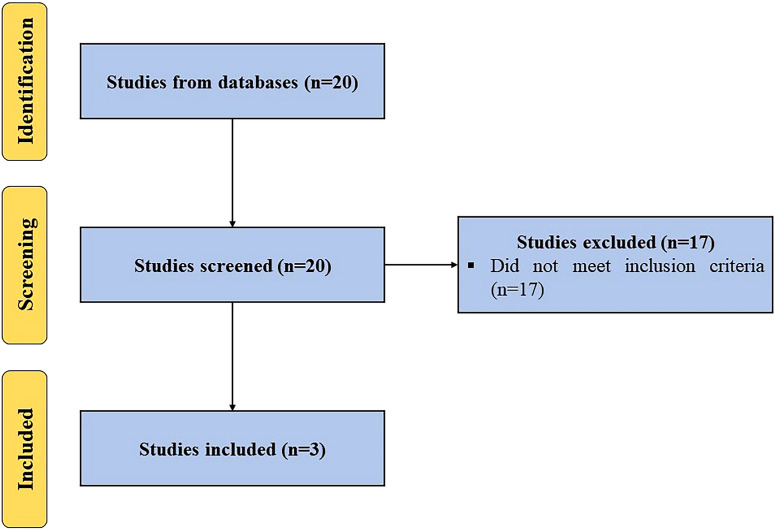
A systematic search was performed utilizing keyword terms, (“platelet lysate” OR “PL”) AND (“knee osteoarthritis” OR “knee”), in different databases (Scopus, PubMed, Embase, and Web of Science) for articles published to August 15, 2024, in the English language, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. All clinical studies employing PL to manage knee OA were included. Studies not using PL alone or not targeting OA of the knee were excluded (Fig. 1). The PICO (population, intervention, comparison, and outcome) of this study are as follows:
Population: patients with knee OA
Intervention: PL alone
Comparison: baseline, placebo, and/or active comparators (e.g., corticosteroids, viscosupplementation, APBOs, etc.)
Outcome: patient-reported outcome measures (e.g., visual analogue scale (VAS), Knee Injury and Osteoarthritis Outcome Score (KOOS), Western Ontario and McMaster Universities Arthritis Index (WOMAC) score, etc.), clinical outcomes (e.g., range of motion (ROM), etc.)
Fig. 1.
PRISMA flow diagram outlining the record identification and selection process
To minimize the risk of bias, both authors discussed and reviewed all the selected articles, references, and excluded articles from the study; any disagreements were resolved after thorough discussion. All the data were extracted and analyzed by the first author, and then reviewed and approved by the second author.
Additionally, we identified ongoing clinical studies concerning the utilization of PL for managing knee OA, registered on ClinicalTrials.gov, Clinical Trials Registry—India (CTRI), and Chinese Clinical Trial Register (ChiCTR) using the search terms mentioned above.
Results
Clinical Studies
Al-Ajlouni et al., in an open-label prospective study, investigated the safety and effectiveness of autologous intra-articular administration of PL in early and intermediate OA of the knee [23]. Inclusion criteria included patients 35–70 years old, swelling or pain in one or both knees for at least 4 months, and radiographic or magnetic resonance imaging (MRI) confirmation of joint degeneration (Kellgren-Lawrence (KL) grade I/II). Exclusion criteria included varus or valgus knee deformity, uncontrolled diabetes, autoimmune diseases, cancer, infections, and use of NSAIDs 5 days prior to blood draw. PL was prepared by drawing 20 mL blood in sterile citrate tubes. The double-spin method was used to obtain a platelet pellet. This pellet was then suspended in 5 mL platelet-poor plasma (PPP), freeze-thawed (− 80 ℃ for 10 min) twice, and the final suspension was centrifuged. The supernatant was collected, passed through a 0.2-µm filter, and the filtered product was used for intra-articular administration at days 0, 21, and 42. The patient-reported outcome measure (PROM) included KOOS, administered at the baseline and at 3, 6, 12, 32, and 52 weeks follow-up post-injection. A total of 48 patients were enrolled in this study. The platelet count in the final formulation (1000–1700 × 109/L) was 5.6 times higher than the baseline whole blood (180–302 × 109/L) levels. No major adverse events were reported. Statistically significant improvements were observed in the overall KOOS and all KOOS subscales (symptoms, stiffness, pain, daily living, and sport score) at 32 and 52 weeks follow-up compared to the baseline. The limitations of this study include brief follow-up, small cohort size, lack of a control group, and lack of radiographic and MRI analysis. Injection of PL is safe and potentially efficacious in patients with knee OA (Table 1).
Table 1.
Summary of main findings of included clinical studies
| References | Main findings |
|---|---|
| Al-Ajlouni et al. [23] | Intra-articular administration of three doses of PL injected every 3 weeks is safe and led to statistically significant improvement in the overall KOOS and all KOOS subscales at 32 and 52 weeks follow-up compared to the baseline |
| Jeyaraman et al. [24] | Intra-articular administration of three doses of PL injected every 4 weeks is safe and led to statistically significant improvements in the VAS and WOMAC scores at 12 months follow-up compared to the baseline and PRP group |
| Hosseini et al. [25] | Intra-articular administration of three doses of PL injected every 21 days is safe and led to statistically significant improvements in the VAS and WOMAC scores and ROM at 6 months follow-up compared to the baseline and PRP group |
PL platelet lysate, KOOS Knee Injury and Osteoarthritis Outcome Score, VAS visual analogue scale, WOMAC Western Ontario and McMaster Universities Arthritis Index, PRP platelet-rich plasma, ROM range of motion
Jeyaraman et al., in a prospective cohort study, evaluated the effectiveness of homologous PL compared to PRP in patients with early OA of the knee [24]. Inclusion criteria included 30–70-year-old patients, radiographic confirmation of KL grade I or II knee OA, pain for at least 3 months, and lack of response to anti-inflammatory treatment. Exclusion criteria included history of corticosteroid injection in the last 3 months, advanced OA of the knee (KL grade III or IV), presence of infectious diseases and rheumatoid arthritis. PRP was formulated by drawing 20 mL blood in tubes containing sodium citrate. The double-spin method was used to prepare 3–4 mL of PRP and this was activated using 10% calcium chloride solution (PRP/calcium chloride 10:1). The homologous PL was formulated by using O-positive blood procured from a blood bank. A PL preparation was obtained using three cycles of freeze–thaw. This formulation was stored as lyophilized powder and was reconstituted in normal saline prior to use. A total of 121 patients were allotted into two groups. Patients in group A (n = 57) and group B (n = 64) received three doses of 3 mL PRP and PL injections, respectively, at day 0 and at the end of weeks 4 and 8. The PROMs included the VAS and WOMAC scores, assessed at baseline and at 1, 2, 3, and 12 months follow-up post-injection.
No adverse events were observed. Both PRP and PL groups showed statistically significant improvements in the VAS and WOMAC scores at 12 months follow-up compared to the baseline. The PL group also showed significantly greater improvement compared to the PRP group. The limitations of this study included small cohort size, short follow-up, being a single-center study, and the lack of radiographic and MRI analysis. Injection of PL is safe and effective and offers advantage over PRP in patients with knee OA (Table 1).
Hosseini et al., in a randomized clinical study, compared the effectiveness of PL and PRP in patients with knee OA [25]. Inclusion criteria included 38–67-year-old patients with bilateral OA (grade II or III on KL scale) of the knee, with symptoms for at least 4 months, and BMI of 18–32.5. Exclusion criteria included age less than 30 years old or greater than 70 years old, BMI > 32, KL grade greater than III, systemic disorders such as rheumatoid arthritis, history of knee surgery in the last 6 months, and use of NSAIDs 1 week prior to and during the treatment. PRP was formulated by drawing 20 mL blood in tubes containing 2.5 mL citrate dextrose-A (ACD-A). The double-spin method was used to formulate leukocyte-poor PRP with platelet count at 4.2–4.6-fold compared to the baseline whole blood. No external activator was used. PL was prepared by drawing 20 mL blood in sterile citrate tubes. PRP was first prepared using the double-spin method and a platelet count of about 1 × 107/µL was attained. This PRP was then subjected to the double freeze–thaw technique to obtain the required PL.
Twenty-five female patients with bilateral OA of the knee were recruited. These patients were given three doses every 21 days of PRP and PL in the right and left knee, respectively. The outcome measures included VAS score, WOMAC scores (overall and subscales), and ROM assessed at baseline and at 1 and 6 months follow-up post-administration. No major adverse events were observed. Both groups, PRP and PL, showed statistically significant improvements in the VAS score, overall WOMAC and all WOMAC subscale scores, and ROM at 1 and 6 months follow-up compared to the baseline. Additionally, statistically significant improvements were observed for all outcome measures in the PL group at 6 months follow-up compared to the PRP group. The limitations of this study included small cohort size, short follow-up, and lack of radiographic and MRI analysis. Injection of PL is safe and effective and offers advantages over PRP in patients with knee OA (Table 1).
Ongoing Clinical Studies
As of August 15, 2024, one clinical trial is listed on ClinicalTrials.gov, CTRI, or ChiCTR to evaluate the safety and/or effectiveness of PL to manage knee OA (Table 2).
Table 2.
Ongoing clinical trials registered on ClinicalTrials.gov, Clinical Trials Registry—India, and Chinese Clinical Trial Register till August 15, 2024, evaluating the safety and/or efficacy of platelet lysate for the management of osteoarthritis of the knee
| Study identifier | Biologic | Study phase; estimated enrollment (N) | Primary outcome measure(s) | Recruitment status | Study location(s) |
|---|---|---|---|---|---|
| NCT03734900 | Autologous platelet lysate vs. autologous platelet-rich plasma vs. saline injection | Phase IV; N = 150 | Pain score of the patient—Using visual analog score (from 0 to 10, 0 indicate no pain and 10 indicate maximal pain) to evaluate pain of surgical wound of the patient [time frame: up to 24 weeks after surgery] | Unknown | Taiwan |
Discussion
The prevalence of knee OA will grow with the aging population and rise in obesity, leading to major increase in global healthcare costs [23]. The conventional treatment modalities for knee OA treatment have evident flaws and side effects [2–4]. The last 20 years have seen a substantial increase in the use of APBOs to manage knee OA [5–8]. PRP is the most frequently used APBO, and many studies have shown its safety and effectiveness in patients with knee OA [9, 11, 26]. The effectiveness of PRP is attributed to its secretome, consisting of growth factors, cytokines, chemokines, and extracellular vesicles/exosomes, released from the platelet’s alpha and dense granules, lysosomes, and microparticles [12, 23, 27–29]. Nevertheless, the efficacy of PRP is debatable given the lack of optimized formulation protocols [5–11]. This results in diverse PRP compositions, leading to varying outcomes [5–8]. Notably, studies have demonstrated that a platelet concentration of 5- to 7-fold compared to the baseline whole blood levels and mean platelet dose of at least 5 billion is necessary to increase cell proliferation and migration, tissue regeneration, and attain positive clinical outcomes [11, 30, 31]. Additionally, too much inflammation led by pro-inflammatory neutrophils can produce deleterious effects on osteoarthritic pain [30]. Moreover, intra-articular injection of red blood cells is detrimental, and they should be eliminated in PRP preparations [32]. Thus, formulations which are acellular, enriched with bioactive molecules, and have a standardized preparation protocol are needed for managing OA of the knee.
The present study examined the effectiveness of PL for managing knee OA. All clinical studies using PL alone to manage knee OA were incorporated. Three clinical studies fulfilled our search and inclusion conditions.
The prospective studies by Al-Ajlouni et al., Jeyaraman et al., and Hosseini et al. showed that intra-articular injection of three doses of PL injected every 3–4 weeks is safe and resulted in statistically significant improvements in different PROMs such as VAS, KOOS, WOMAC, and ROM at 6–12 months follow-up compared to the baseline and/or PRP [23–25]. In addition, Hosseini et al. reported higher concentration of growth factors and cytokines, including platelet-derived growth factor (PDGF), fibroblast growth factors (FGF), vascular endothelial growth factor (VEGF), and transforming growth factor-beta 1 (TGFβ1) in the PL compared to the PRP [25]. These factors may well play a vital role in cartilage development and/or repair. TGFβ1 is a regulator of chondrocyte proliferation and differentiation and can play a role in reducing inflammation in the joint. PDGF is a potent chemotactic factor for MSC migration to the joint, and can induce anabolism in the joint, with proliferation of chondrocytes and synthesis of proteoglycans [33, 34]. PDGF also promotes production of anti-inflammatory cytokines, including interleukin (IL)-4 and IL-1 receptor antagonist (IL-1RA) [35]. IL-4, in turn, can reduce expression of pro-inflammatory tumor necrosis factor alpha (TNFα) and IL-1β (via promoting release of IL-1RA) and can thus help reduce inflammation and pain [33, 34]. FGF activates anabolic pathways, and can diminish the activity of aggrecanase, whereas VEGF promotes vasculogenesis and angiogenesis [36]. Even though the exact mechanism of action of PL is still under investigation, its greater efficacy compared to the PRP [24, 25] can be attributed to higher expression of aforesaid growth factors and cytokines and their effect on cell proliferation, differentiation and migration, and anti-inflammatory potential in the joint microenvironment, responsible for promoting tissue repair. Moreover, the outcomes from included clinical studies [23–25] are in harmony with the published literature, showing the capability of PRP to decrease pain and increase function [9, 11, 26]. This is then in concurrence with the consensus from the European Society of Sports Traumatology, Knee Surgery and Arthroscopy—ORthoBiologics InitiaTive (ESKAA-ORBIT), endorsing APBOs such as PRP as an effective modality to manage knee OA [37]. The clinical results attained with PL are in accordance with the outcomes described for PRP, while mitigating few PRP-associated shortcomings.
Limitations and Future Studies
The presented investigation is limited, including inclusion of only three clinical studies that fulfilled our pre-defined search and inclusion/exclusion criteria. This limits the ability to comprehensively analyze the effectiveness of PL for the management of knee OA. In addition, the included studies have shortcomings, including small cohort size, short follow-up, lack of radiographic and MRI analysis, and lack of commonly used interventions for the management of knee OA, such as corticosteroids, viscosupplementation, or other APBOs as comparators. Additionally, the risk for publication bias remains, as studies with positive outcomes are more likely to be published, possibly resulting in incomplete representation of the overall efficacy of PL for the management of knee OA.
Thus, more adequately powered, multicenter, prospective, non-randomized and randomized controlled studies with extended follow-up are needed to prove the effectiveness of PL in patients with knee OA. Further comparative studies to help clinicians in choosing the best APBO for knee OA treatment are also warranted.
Conclusion
The existing published peer-reviewed literature suggests that intra-articular injection of PL is safe and can decrease pain and increase function in patients with knee OA.
Acknowledgements
We thank the participants of the study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Ashim Gupta conceptualized the study and wrote the initial draft. Ashim Gupta and Nicola Maffulli commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Ashim Gupta is an Editorial Board member of Pain and Therapy. Ashim Gupta was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Ashim Gupta and Nicola Maffulli declares that he has no other competing interests.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30(2):184–95. 10.1016/j.joca.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aratikatla A, Maffulli N, Rodriguez HC, et al. Allogenic perinatal tissue for musculoskeletal regenerative medicine applications: a systematic review protocol. J Orthop Surg Res. 2022;17(1):307. 10.1186/s13018-022-03197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Jeyaraman M, Potty AG. Leukocyte-rich vs. leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis. Biomedicines. 2023;11(1):141. 10.3390/biomedicines11010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaya O, Şenel A, Batur OC, Gönder N, Ergen E, Barış PB. Effectiveness of the thermal genicular nerve radiofrequency ablation therapy under fluoroscopy in patients with non-operative advanced stage knee osteoarthritis: 1-year follow-up results. Indian J Orthop. 2022;56(6):1033–9. 10.1007/s43465-022-00642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Jain VK. Autologous peripheral blood-derived orthobiologics: different types and their effectiveness in managing knee osteoarthritis. World J Orthop. 2024;15(5):400–3. 10.5312/wjo.v15.i5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A. Autologous protein solution (APS) and osteoarthritis of the knee: a scoping review of current clinical evidence. Cureus. 2024;16(2):e53579. 10.7759/cureus.53579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Aratikatla A. Hyperacute serum and knee osteoarthritis. Cureus. 2024;16(1): e53118. 10.7759/cureus.53118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Jain V. Autologous conditioned plasma (ACP) and osteoarthritis of the knee: a review of current clinical evidence. Cureus. 2024;16(1):e52693. 10.7759/cureus.52693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong Y, Gong C, Peng X, et al. Efficacy and safety of platelet-rich plasma injections for the treatment of osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Front Med (Lausanne). 2023;10:1204144. 10.3389/fmed.2023.1204144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Migliorini F, Maffulli N. Management of rotator cuff injuries using allogenic platelet-rich plasma. J Orthop Surg Res. 2024;19(1):165. 10.1186/s13018-024-04657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berrigan WA, Bailowitz Z, Park A, Reddy A, Liu R, Lansdown D. A greater platelet dose may yield better clinical outcomes for platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review. Arthroscopy. 2024;S0749–8063(24):00206–8. 10.1016/j.arthro.2024.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca LD, Santos GS, Huber SC, Setti TM, Setti T, Lana JF. Human platelet lysate—a potent (and overlooked) orthobiologic. J Clin Orthop Trauma. 2021;28(21): 101534. 10.1016/j.jcot.2021.101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palombella S, Orfei CP, Castellini G, et al. Systematic review and meta-analysis on the use of human platelet lysate for mesenchymal stem cell cultures: comparison with fetal bovine serum and considerations on the production protocol. Stem Cell Res Ther. 2022;13(1):142. 10.1186/s13287-022-02815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanskii YD, Sergeeva NS, Sviridova IK, et al. Human platelet lysate as a promising growth-stimulating additive for culturing of stem cells and other cell types. Bull Exp Biol Med. 2013;156(1):146–51. 10.1007/s10517-013-2298-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JY, Xiang XN, Yu X, et al. Mechanisms and applications of the regenerative capacity of platelets-based therapy in knee osteoarthritis. Biomed Pharmacother. 2024;178: 117226. 10.1016/j.biopha.2024.117226. [DOI] [PubMed] [Google Scholar]
- 16.Chitchongyingcharoen N, Tawonsawatruk T, Phetfong J, Aroontanee W, Supokawej A. Application of human platelet lysate in chondrocyte expansion promotes chondrogenic phenotype and slows senescence progression via BMP-TAK1-p38 pathway. Sci Rep. 2023;13(1):21106. 10.1038/s41598-023-48544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rikkers M, Levato R, Malda J, Vonk LA. Importance of timing of platelet lysate-supplementation in expanding or redifferentiating human chondrocytes for chondrogenesis. Front Bioeng Biotechnol. 2020;8(8):804. 10.3389/fbioe.2020.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Liu N, Huang Z, Wang W, Hou D, Wang W. Intra-articular injection of loaded sPL sustained-release microspheres inhibits osteoarthritis and promotes cartilaginous repairs. J Orthop Surg Res. 2021;16(1):646. 10.1186/s13018-021-02777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan L, Zhou L, Xie D, et al. Chondroprotective effects of platelet lysate towards monoiodoacetate-induced arthritis by suppression of TNF-α-induced activation of NF-ĸB pathway in chondrocytes. Aging (Albany NY). 2019;11(9):2797–811. 10.18632/aging.101952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao X, Xu J, Wang C, et al. Porcine platelet lysates exert the efficacy of chondroregeneration and SMAD2-mediated anti-chondrofibrosis on knee osteoarthritis. Int Immunopharmacol. 2024;15(128): 111509. 10.1016/j.intimp.2024.111509. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh YH, Chu YC, Hsiao JT, Shu YT, Hsieh MF, Lee HM. Porcine platelet lysate intra-articular knee joint injections for the treatment of rabbit cartilage lesions and osteoarthritis. J Med Biol Eng. 2023;15(43):102–11. 10.1007/s40846-023-00776-1. [Google Scholar]
- 22.Forteza-Genestra MA, Antich-Rosselló M, Ráez-Meseguer C, et al. Intra-articular injection of platelet lysate-derived extracellular vesicles recovers from knee osteoarthritis in an in vivo rat model. J Orthop Translat. 2024;8(45):1–9. 10.1016/j.jot.2023.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Ajlouni J, Awidi A, Samara O, et al. Safety and efficacy of autologous intra-articular platelet lysates in early and intermediate knee osteoarthrosis in humans: a prospective open-label study. Clin J Sport Med. 2015;25(6):524–8. 10.1097/JSM.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 24.Jeyaraman M, Ramesh R, Prajwal GS. The efficacy of autologous platelet rich plasma vs homologous platelet lysate in patients with early knee osteoarthritis. IP Int J Orthop Rheumatol. 2018;4(2):47–53. 10.18231/2455-6777.2018.0012. [Google Scholar]
- 25.Hosseini S, Soltani-Zangbar MS, Zamani M. Comparative evaluation of autologous platelet-rich plasma and platelet lysate in patients with knee osteoarthritis. Growth Factors. 2023;41(3):165–77. 10.1080/08977194.2023.2227273. [DOI] [PubMed] [Google Scholar]
- 26.Qiao X, Yan L, Feng Y, et al. Efficacy and safety of corticosteroids, hyaluronic acid, and PRP and combination therapy for knee osteoarthritis: a systematic review and network meta-analysis. BMC Musculoskelet Disord. 2023;24(1):926. 10.1186/s12891-023-06925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blair P, Flaumenhaft R. Platelet a-granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177–89. 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altaie A, Owston H, Jones E. Use of platelet lysate for bone regeneration- are we ready for clinical translation? World J Stem Cells. 2016;8(2):47–55. 10.4252/wjsc.v8.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edelstein LC, Mckenzie SE, Shaw C, Holinstat MA, Kunapuli SP, Bray PF. MicroRNAs in platelet production and activation. J Thromb Haemost. 2013;11(Suppl 1):340–50. 10.1111/jth.12214. [DOI] [PubMed] [Google Scholar]
- 30.Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015;7(4 Suppl):S53–9. 10.1016/j.pmrj.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Giusti I, Rughetti A, D’Ascenzo S, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49(4):771–8. 10.1111/j.1537-2995.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 32.Gupta A, Maffulli N, Jain VK. Red blood cells in platelet-rich plasma: avoid if at all possible. Biomedicines. 2023;11(9):2425. 10.3390/biomedicines11092425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khosbin A, Leroux T, Wasserstein D, et al. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy. 2013;29(12):2037–48. 10.1016/j.arthro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Ríos DL, López C, Carmona JU. Evaluation of the anti-inflammatory effects of two platelet-rich gel supernatants in an in vitro system of cartilage inflammation. Cytokine. 2015;76(2):505–13. 10.1016/j.cyto.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Ríos DL, López C, Álvarez ME, Samudio IJ, Carmona JU. Effects over time of two platelet gel supernatants on growth factor, cytokine and hyaluronan concentrations in normal synovial membrane explants challenged with lipopolysaccharide. BMC Musculoskelet Disord. 2015;20(16):153. 10.1186/s12891-015-0605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Civinini R, Nistri L, Martini C, Redl B, Ristori G, Innocenti M. Growth factors in the treatment of early osteoarthritis. Clin Cases Miner Bone Metab. 2013;10(1):26–9. 10.11138/ccmbm/2013.10.1.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laver L, Filardo G, Sanchez M, et al. The use of injectable orthobiologics for knee osteoarthritis: a European ESSKA-ORBIT consensus. Part 1-Blood-derived products (platelet-rich plasma). Knee Surg Sports Traumatol Arthrosc. 2024. 10.1002/ksa.12077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.