Abstract
Introduction
Current guidelines for pain treatment recommend a personalized, multimodal and interdisciplinary approach as well as the use of a combination of drug and non-drug therapies. Risk factors for chronification should already be reduced in patients with acute pain, e.g., after surgery or trauma. Auricular vagus nerve stimulation (aVNS) could be an effective non-drug therapy in the multimodal treatment of chronic and acute pain. The aim of this systematic review and meta-analysis is to evaluate the clinical efficacy and safety of aVNS in treating chronic and acute pain conditions.
Methods
A systematic literature search was performed regarding the application of auricular electrical stimulation in chronic and acute pain. Studies were classified according to their level of evidence (Jadad scale), scientific validity and risk of bias (RoB 2 tool) and analyzed regarding indication, method, stimulation parameters, duration of treatment and efficacy and safety. A meta-analysis on (randomized) controlled trials (using different comparators) was performed for chronic and acute pain conditions, respectively, including subgroup analysis for percutaneous (pVNS—needle electrodes) and transcutaneous (tVNS—surface electrodes) aVNS. The visual analog pain scale (VAS) was defined as primary efficacy endpoint.
Results
A total of n = 1496 patients were treated with aVNS in 23 identified and analyzed studies in chronic pain, 12 studies in acute postoperative pain and 7 studies in experimental acute pain. Of these, seven studies for chronic pain and six studies for acute postoperative pain were included in the meta-analysis. In chronic pain conditions, including back pain, migraine and abdominal pain, a statistically significant reduction in VAS pain intensity for active compared to sham aVNS or control treatment with an effect size Hedges’ g/mean difference of − 1.95 (95% confidence interval [CI]: − 3.94 to 0.04, p = 0.008) could be shown and a more favorable effect in pVNS compared to tVNS (− 5.40 [− 8.94; − 1.85] vs. − 1.00 [− 1.55; − 0.44]; p = 0.015). In acute pain conditions, single studies showed significant improvements with aVNS, e.g., in kidney donor surgery or tonsillectomy but, overall, a non-statistically significant reduction in VAS pain intensity for active compared to sham aVNS or control with − 0.70 [− 2.34; 0.93] (p = 0.15) could be observed in the meta-analysis. In acute pain results vary greatly between studies depending especially on co-medication and timepoints of assessment after surgery. A significant reduction in analgesics or opiate intake was documented in most studies evaluating this effect in chronic and acute pain. In 3 of the 12 randomized controlled trials in patients with chronic pain, a sustainable pain reduction over a period of up to 12 months was shown. Overall, aVNS was very well tolerated.
Conclusion
This systematic review and meta-analysis indicate that aVNS can be an effective and safe non-drug treatment in patients with specific chronic and acute postoperative pain conditions. Further research is needed to identify the influence of simulation parameters and find optimal and standardized treatment protocols while considering quality-of-life outcome parameters and prolonged follow-up periods. A more standardized approach and harmonization in study designs would improve comparability and robustness of outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40122-024-00657-8.
Keywords: Neuromodulation, Auricular vagus nerve stimulation, Chronic pain, Acute pain, Postoperative pain
Key Summary Points
| Auricular vagus nerve stimulation is an easy-to-use method with a low side effect profile. |
| The method can be an effective, minimally invasive, non-drug treatment for chronic back pain, abdominal pain and migraine. |
| Results for acute postoperative pain and experimental pain are still inconclusive, although single studies show a significant reduction in post-surgery pain in specific procedures. |
| Further studies should focus on standardization of stimulation parameters and treatment regimens to strengthen clinical evidence. |
Introduction
Chronic pain affects > 30% of people worldwide and often causes an enormous reduction in patients' quality of life as well as high socioeconomic costs [1]. In particular, back pain, headache and musculoskeletal pain are among the diseases with the highest years lost to disability (YLD) [1].
Current guidelines for chronic pain treatment recommend a personalized, multimodal and interdisciplinary approach as well as the use of a combination of drug (e.g., acetaminophen, NSAIDs, opioid analgesics) and non-drug therapies (e.g., multidisciplinary rehabilitation, cognitive behavioral therapy or neuromodulative therapies) [1, 2]. Risk factors for chronification (e.g., based on peripheral or central sensitization) should already be reduced during the treatment of acute pain, e.g., after surgery or trauma [3].
With existing drug therapies, often only minor and/or short-term improvements are achieved. Moreover, side effects and/or interactions with other drug therapies must be considered, and the possibility of addiction in long-term medication/opioid use should be weighed as a risk.
Thus, there is a high need for complementary, effective and safe non-drug treatment options, and in particular neuromodulative approaches, for patients with acute and chronic pain conditions [1, 4, 5].
The Vagus Nerve
The vagus nerve is the tenth cranial nerve and the most important parasympathetic nerve in the autonomic nervous system [6, 7]. It innervates structures and organs of the throat, thorax and abdomen and is responsible for transmitting sensory information to the brain as well as motor and parasympathetic signals to the body. Through its auricular branch, the vagus nerve also innervates the outer ear sensorially [8]. About 80% of vagal fibers are afferent, projecting to the nucleus spinalis nervi trigemini (NSNT) and nucleus tractus solitarii (NTS). The vagus nerve is essential in maintaining autonomic function [6, 7] and is a mediator of anti-inflammatory effects, e.g., via the so-called cholinergic anti-inflammatory pathway [7, 9–11]. The activity of the autonomic nervous system is particularly associated with pain perception and modulation, e.g., pain increases heart rate, blood pressure and skin conductance and decreases vagal tone [12].
Auricular Vagus Nerve Stimulation and its Applications in Pain Therapy
Electrical stimulation of the cervical branch of the vagus nerve (VNS) has been approved since the 1990s for the treatment of therapy-refractory epilepsy and chronic, therapy-resistant depression [7]. Here, a pulse generator is implanted delivering electrical impulses to the, preferably left, cervical vagus nerve via a cuff electrode.
However, there is an increasing research interest in non- or minimally invasive technologies for vagus nerve stimulation to reduce side effects (e.g., hoarseness, cough, pain), complications due to implantation (e.g., nerve injury, infection) [13] and costs. Furthermore, this allows the method to be accessible for a broader range of indications and a larger group of patients [7, 14].
Non- or minimally invasive stimulation can be achieved either via stimulation of the cervical vagus nerve using surface electrodes (transcutaneous stimulation) or by stimulating the auricular branch of the vagus nerve (aVNS) innervating the pinna of the ear using surface or needle electrodes (transcutaneous or percutaneous stimulation) in the cymba concha, the concha and, to a lesser extent, the (crus) antihelix, fossa triangularis, tragus and crus helix (see Fig. 1).
Fig. 1.
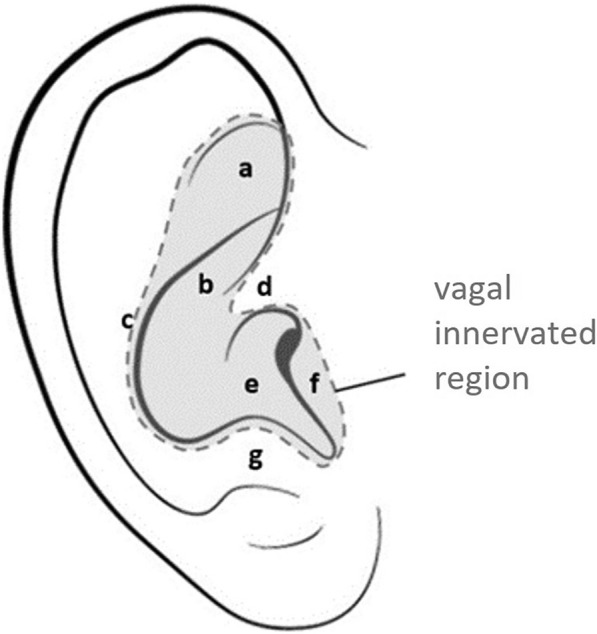
Ear anatomy with marked vagal innervated area and specific anatomical regions [8, 104]. a Triangular fossa, b cymba conchae, c antihelix, d crus helix, e cavum conchae, f tragus, g antitragus. With permission from Likar et al. 2023 [40]
Transcutaneous and percutaneous vagus nerve stimulation is currently studied in a broad range of indications [2, 9, 14–25]. In the following, we will specifically focus on applications of aVNS in chronic and acute pain conditions.
The modulation of nociception via vagal afferents was first investigated in systematic studies in the 1980s [26–30]. The peripheral and central systems regulating cardiovascular and autonomic functions, for example, were described as closely linked to the systems involved in the control of nociception [31]. Meanwhile, in the 2010s, it was shown that afferent stimulation of the NTS and NSNT modulates a variety of relevant brain structures in pain processing and perception, such as nucleus dorsalis nervi vagi, locus ceruleus (LC; noradrenergic), raphe nuclei (RN; serotonergic), amygdala, thalamus, periaqueductal gray (PAG), cingulate cortex and prefrontal cortex [6, 32–34]. Thereby—according to current evidence—the analgesic effect of aVNS can be attributed to the following mechanisms: partial activation of descending noradrenergic and serotonergic systems associated with release of enkephalin and a corresponding effect on opioid receptors [6, 32], an effect on the limbic system, as shown, e.g., in patients with migraine [33, 35, 36], a parasympathetic activation and sympatholytic effect [7, 35] and an activation of the vagal mediated cholinergic anti-inflammatory reflex with a positive effect on pain [7, 12, 37–39]. Clinical trials showed beneficial effects of aVNS in different acute and chronic pain conditions, like for acute post-surgery pain in orthopedic surgery, and for chronic back pain, abdominal pain or migraine [40].
Comprehensive reviews and meta-analysis on aVNS in chronic and acute pain conditions are limited [17, 18, 40, 41], although essential to elucidate the clinical efficacy and safety as well as the optimal patient and parameter selection, and this should be elaborated further in this paper.
Materials and Methods
Literature Review and Evaluation
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. A systematic literature search was performed in the databases PubMed, Scopus and Semantic Scholar. The search was narrowed to the period 1 January 2000 to 1 May 2023. The search was based and updated regarding a previously published review in Likar et al. 2023 (published under the creative commons license, which evaluated studies till 1 June 2022) [40]. All databases were searched using predefined, thematically relevant keywords. The following keywords were defined: auric* vagus nerve stimulation, auric* elect* stimulation, auric* elect* vagus nerve stimulation, auricular neurostimulation (+ pain [if > 1,000 results]), VNS and pain.
Listed results were screened for duplicates and removed on a case-by-case basis. The remaining hits were screened based on title, abstract and the following exclusion criteria: year of publication < 2000, no abstract available, language used not English or German, preclinical study/animal study, study protocols, case study, review and no relation to aVNS. For further qualification of the studies, the full text was used to check for compliance with the inclusion criteria: indication (pain) and intervention (aVNS, transcutaneous or percutaneous).
Included studies were scored by two independent reviewers using the Jadad scale (maximum score 5) and according to their scientific validity (maximum score 4) (Supplementary Table 1), and the respective mean values of the evaluations were summed up (possible total score 9) [42]. Studies that could not be assessed according to the Jadad scale (no randomization, no blinding) were evaluated only based on defined scientific validity criteria (maximum 4 points) [43–45]. The risk of bias for individual studies was assessed with the Cochrane Risk of Bias 2 tool (version 22 August 2019) [46] by two independent reviewers. Case series and retrospective analyses were excluded from the risk-of-bias analysis.
Furthermore, full texts of the publications were analyzed and summarized regarding study type and level of evidence [44, 47], indication (classification into: chronic pain, acute postoperative pain, acute experimental pain), method (intervention, stimulation points—see also Fig. 1—and control), stimulation parameters, duration of treatment, primary and secondary outcomes and observed adverse events.
Data Synthesis and Meta-Analysis
A meta-analysis of study data was performed for chronic and acute pain conditions, respectively. Inclusion criteria comprised randomized controlled trials (RCTs) and controlled clinical trials published in peer-reviewed journals. Comparative calculations were performed between treatment (aVNS stimulation) groups vs. control groups or vs. sham stimulation groups for within and inter-group study designs. Moreover, a small subgroup analysis for percutaneous (pVNS—needle electrodes) vs. transcutaneous (tVNS—surface electrodes) aVNS was performed. The primary efficacy endpoint (outcome) for this meta-analysis was defined as acute or chronic pain measured on a visual analog scale (VAS; 0–100 mm). Studies reporting pain on a numeric rating scale (NRS; 0–10) were also included. To standardize scales, we assume VAS from 0 to 100 mm is equivalent to NRS from 0 to 10, by dividing VAS values by ten [48]. In acute pain studies, we used data from or within the first 24 h post-surgery (between 30 min to 24 h). For chronic pain studies, we used data from end of treatment (on average 4.7 weeks, 4 days to 8 weeks). Studies were included if the presented data allowed for derivation of necessary datapoints to calculate effect size.
For between and within-group analysis, a random-effects model meta-analysis was employed to account for heterogeneity between studies. This approach assumes that the true effects vary across studies because of differences in patients’ characteristics, interventions and methodologies.
Subgroup analyses were performed to explore the potential sources of heterogeneity by stratifying the studies based on either the treated conditions or type of aVNS stimulation.
Mean and standard deviation (SD) of each treatment outcome between active stimulation and sham stimulation or a control group, for between-group analysis, were used to calculate effect sizes. Sham stimulation indicates a treatment group with either needle application at the ear at equal stimulation points but without an electrical signal applied or an active stimulation at other stimulation points of the ear (e.g., earlobe). Control groups include standard of care or alternative treatments.
Results are shown as forest plots including groups tested, weight (%), Hedges’ g/mean difference [95% CI—confidence interval] and combined effect size for overall and subgroup analysis.
Differences between groups were tested using a chi-square test. A p value < 0.05 was considered statistically significant.
Results
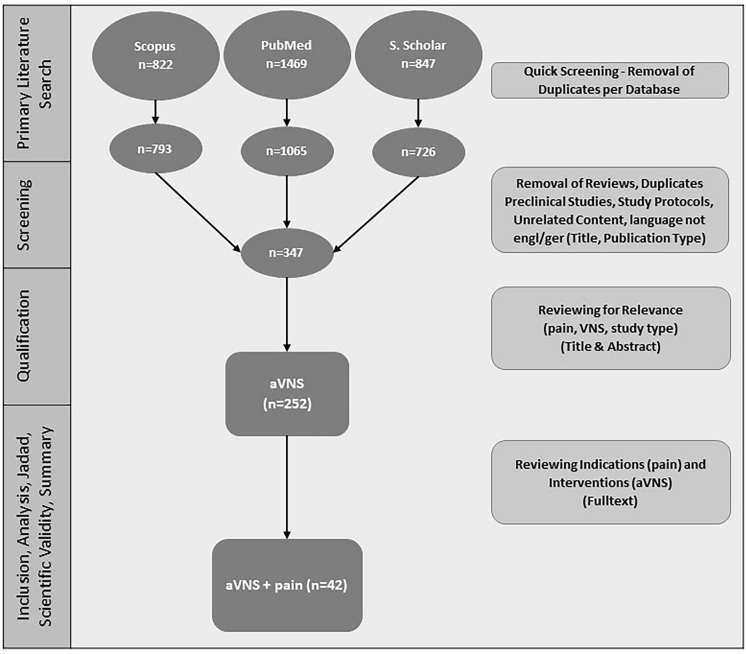
In total, n = 3138 results (Fig. 2) were found in the primary literature search. After screening based on title and abstract, n = 252 studies were qualified. After reviewing the full text, n = 42 studies were evaluated according to the Jadad scale and scientific validity, and studies were comprehensively analyzed. Results of the evaluation can be found in Supplementary Table 2–4. A summary and analysis of all studies are depicted in Table 1 and in more detail in the supplementary material (Supplementary Table 5).
Fig. 2.
Flowchart and results of systematic literature search. Extended and translated from Likar et al. 2023 with permission [40]
Table 1.
Summary and analysis of studies of auricular vagus nerve stimulation (aVNS) in chronic pain, acute post-surgery pain and acute experimental pain (short version, details in Supplementary Table 5)
| Author | Indication | Primary results |
|---|---|---|
| Chronic pain | ||
| Sator-Katzenschlager et al. [60] | Chronic cervical syndrome | VAS↓ pVNS vs. sham |
| Sator-Katzenschlager et al. [58] | Chronic low back pain | VAS↓ pVNS vs. sham |
| Kong and Ng [56] | Spondylosis, migraine | VAS improvement based on single cases reported pVNS vs. baseline |
| Napadow et al. [53] | Chronic pelvic pain/endometriosis | Evoked pain intensity ↔ , anxiety↓ tVNS vs. sham |
| Straube et al. [57] | Chronic migraine | Headache days↓ tVNS (1 Hz) vs. tVNS (25 Hz) |
| Sacco et al. [68] | Chemotherapy-induced peripheral neuropathy | NRS↓ pVNS vs. baseline |
| Kovacic et al. [50] | Chronic abdominal pain (11–18 years) | PFSD↓ pVNS vs. sham |
| Grolaux [49] | IB pain and chronic pain | IBS-SSS ↔ tVNS vs. baseline |
| Krasaelap et al. [51] | IBD pain | Severe abdominal pain↓ pVNS vs. baseline |
| Mion et al. [52] | IBS pain | IBS-SSS↓ tVNS vs. baseline |
| Kutlu et al. [66] | Fibromyalgia | VAS, depression, anxiety, functionality, SF-36 ↔ tVNS + training vs. training |
| Shi et al. [70] | IBS-C, chronic abdominal pain | CSBMs/week↑, VAS↓ tVNS vs. sham |
| Széles et al. [61] | Chronic low back pain | NRS↓ pVNS vs. baseline |
| Aranow et al. [65] | SLE and musculoskeletal pain | Pain↓, fatigue↓ tVNS vs. sham |
| Woodbury et al. [67] | Fibromyalgia | VAS ↔ , sleep↑, activity↑, mood↑ pVNS vs. control |
| Marsal et al. [64] | Rheumatoid arthritis | DAS28-CRP↓ tVNS vs. baseline |
| Zhang et al. [36] | Migraine without aura | Migraine days↓, pain intensity↓, duration↓ tVNS vs. sham |
| Feng et al. [55] | Migraine without aura | VAS↓, frequency↓, duration↓, MSQ↑, SDS↓, SAS↓ tVNS vs. baseline |
| Courties et al. [63] | Osteoarthritis/hand pain | VAS↓ tVNS vs. baseline |
| Santucci et al. [54] | Chronic functional abdominal pain (11–18 years) | VAS↓, nausea↓, anxiety↓ pVNS vs. baseline |
| Li et al. [69] | Depression with chronic pain | VAS↓, depression↓ tVNS + EA vs. baseline, no difference vs. citalopram |
| Ünal et al. [59] | Myofascial pain syndrome (neck) | VAS↓, algometer↑, Jamar↑, SF-36↑ tVNS + IC + exercise vs. baseline, no/low difference vs. exercise + IC |
| Uzlifatin et al. [62] | Chronic low back pain | CRP ↔ tVNS + exercise vs. exercise |
| Acute postoperative pain | ||
| Sator-Katzenschlager et al. [73] | Perioperative (oocyte-aspiration) | VAS↓ pVNS vs. sham |
| Likar et al. [77] | Postoperative (laparoscopic nephrectomy) | VAS at rest↓, VAS on exertion↓ 1 h postoperative pVNS vs. sham |
| Michalek-Sauberer et al. [79] | Postoperative (third molar tooth extraction) | VAS ↔ , analgesic consumption ↔ pVNS vs. sham |
| Kager et al. [78] | Postoperative (tonsillectomy) | VAS↓ 9, 12, 24 h postoperative pVNS vs. sham |
| Holzer et al. [71] | Postoperative (gynecological surgery) | VAS ↔ pVNS vs. sham |
| Tsang et al. [72] | Postoperative (hysterectomy) | VAS↓, PEFR ↔ tVNS vs. baseline/control |
| Chakravarthy et al. [15] | Postoperative (cesarean section) | NRS↓ pVNS vs. control |
| Ahmed et al. [74] | Postoperative (Roux-en-Y gastric bypass) | OME ↔ 24 h post-OP pVNS vs. control |
| Blank et al. [75] | Postoperative (colorectal surgery) | OME ↔ pVNS vs. sham (overall); OME↓ pVNS vs. sham (open surgery) |
| Chelly et al. [76] | Postoperative (kidney donor surgery) | OME↓ 24 h post-OP pVNS vs. control |
| Ilfeld et al. [81] | Postoperative (orthopedic and breast surgery) | Low NRS, low opioid requirement |
| Zhou et al. [80] | Postoperative (rebound pain ropivacaine femoral nerve block for ACLR) | NRS↓ sleep disturbances↓, analgesics↓ pVNS vs. sham (8 h/12 h after surgery) |
| Acute experimental pain in healthy participants | ||
| Busch et al. [82] | Experimental mechanical/heat pain | Pain threshold ↔ mechanical/pressure pain, pain↓ (heat) tVNS vs. sham |
| Laqua et al. [86] | Experimental pain threshold | Pain threshold↑ (n = 15), pain threshold↓(n = 6) tVNS vs. control |
| Frøkjaer et al. [85] | Acute mechanical pain, bowel motility | CPM ↔ , pain threshold bone pain↑ tVNS vs. sham |
| Usichenko et al. [87] | Experimental heat pain | Pain threshold↑ (n = 8), pain threshold↓(n = 12) tVNS vs. sham |
| Janner et al. [84] | Experimental heat pain | VAS↓ tVNS vs. control |
| Farmer et al. [83] | Esophageal pain, hypersensitivity | Prevented/reversed acid-induced esophageal HS tVNS vs. sham |
| Dumoulin et al. [88] | Experimental pain | Somatosensory perception ↔ tVNS vs. sham |
Extended and translated from Likar et al. 2023 with permission [40]. ↔ No significant difference/no change; ↑/↓ significant improvement/change
ACLR anterior cruciate ligament reconstruction, CPM conditioned pain modulation, CSBM complete spontaneous bowel movements, HS hypersensitivity, IBS-SSS Irritable Bowel Syndrome-Severity Scoring System, IC ischemic compression, MSQ Migraine-Specific QoL Questionnaire, NRS numeric rating scale, OME oral morphine equivalents, PEFR peak expiratory flow rate, PFSD pain frequency-severity-duration, SAS/SDS Self-Rating Anxiety Scale/Self-Rating Depression Scale
The risk of bias was assessed in a total of n = 22 studies for chronic and acute pain (Table 2).
Table 2.
Results of the risk-of-bias 2 tool assessment [46]
| Author | Risk-of-bias domains | |||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | Overall | |
| Chronic pain | ||||||
| Sator-Katzenschlager et al. [60] | SC | H | L | SC | L | High |
| Sator-Katzenschlager et al. [58] | SC | H | L | SC | L | High |
| Napadow et al. [53] | L | H | L | SC | L | High |
| Straube et al. [57] | SC | SC | L | SC | L | High |
| Kovacic et al. [50] | L | L | L | L | L | Low |
| Krasaelap et al. [51] | L | H | L | L | L | High |
| Kutlu et al. [66] | H | H | L | SC | L | High |
| Shi et al. [70] | SC | SC | L | SC | L | High |
| Aranow et al. [65] | SC | L | L | L | L | Some concern |
| Woodbury et al. [67] | SC | H | L | SC | L | High |
| Zhang et al. [36] | L | SC | L | L | L | Some concern |
| Li et al. [69] | L | SC | L | L | L | Some concern |
| Ünal et al. [59] | SC | H | L | SC | L | High |
| Uzlifatin et al. [62] | SC | H | L | SC | L | High |
| Acute postoperative pain | ||||||
| Sator-Katzenschlager et al. [73] | SC | SC | L | SC | L | High |
| Likar et al. [77] | L | L | L | L | L | Low |
| Michalek-Sauberer et al. [79] | H | SC | L | SC | L | High |
| Kager et al. [78] | L | L | L | L | L | Low |
| Holzer et al. [71] | L | L | L | L | L | Low |
| Tsang et al. [72] | L | H | L | L | L | High |
| Blank et al. [75] | SC | L | L | L | SC | Some concern |
| Zhou et al. [80] | L | L | L | L | L | Low |
D1 bias arising from the randomization process, D2 bias due to deviation from intended intervention, D3 bias due to missing outcome data, D4 bias in measurement of the outcome, D5 bias in selection of the reported result, H high, SC some concern, L low
Chronic Pain
Twenty-three studies on chronic pain with a total number of n = 696 (n = 725 including dropouts) aVNS-treated patients were included in the analysis. These studies addressed chronic inflammatory bowel disease/abdominal pain (7 studies [49–54, 70], n = 150), migraine (4 studies [36, 55–57], n = 134), back pain (6 studies [56, 58–62], n = 229), rheumatoid arthritis (RA)/osteoarthritis (2 studies [63, 64]; n = 45), fibromyalgia/systemic lupus erythematosus (SLE) (3 studies [65–67], n = 49), chemotherapy-induced peripheral neuropathy (1 study [68]; n = 58), nonspecific chronic pain (1 study [49], n = 3) and chronic pain with depression comorbidity (1 study [69], n = 28). Of these, 12 studies [36, 50, 51, 57–60, 62, 65, 66, 69, 70] were high-quality RCTs with an average score of 7.2 out of 9 (Supplementary Table 2). One study [53] was designed as a randomized cross-over study with a rating of 5 points. Furthermore, seven case series [49, 52, 54–56, 63, 64], two retrospective cohort studies [61, 68] and one case-control study [67] with an average score of 3.5 points were identified.
Pain on a VAS or NRS, psychological well-being and tolerability/safety of stimulation were the most common primary and secondary endpoints. Depending on the indication, (additional) more specific symptom-related and disease-relevant endpoints were chosen.
In most of the studies, an improvement on VAS or NRS pain scale or a more specific disease-related endpoint was observed, with this improvement being statistically significant in 18 studies compared to baseline and/or compared to the chosen control group (see Table 1). In 3 of the 12 RCTs (for chronic back pain and abdominal pain), sustained pain reduction over up to 12 months was documented [50, 58, 60].
In eight studies [52, 55, 57, 58, 60, 61, 63, 68], the demand for pain medication was assessed. In six of these studies (3 for chronic back pain, 1 for chronic migraine, 1 in chemotherapy-induced peripheral neuropathy, 1 in irritable bowel syndrome) [52, 57, 58, 60, 61, 68], pain medication intake was reduced during and/or after aVNS (compared to baseline and/or control group). Secondary outcomes such as psychological well-being, disability scores, anxiety, sleep, fatigue and quality of life also significantly improved in the majority of studies [50, 53–55, 57, 58, 60, 65–67, 69, 70].
Clinically and statistically highly significant results were shown for indications chronic low back pain [58], chronic cervical syndrome [60], myofascial pain syndrome (neck) [59], depression with chronic pain [69], chronic abdominal pain [50, 51, 70] and chronic migraine [36, 55, 57].
In the present studies, aVNS was most used concomitantly to drug therapy. The average treatment period was 5.7 (1–26) weeks. However, duration of treatment as well as stimulation parameters within these applications could differ remarkably.
In three studies [36, 55, 67], fMRI examinations were performed alongside therapy to characterize the influence of aVNS on brain activity. fMRI revealed particularly an increase in brain connectivity in the areas of the executive control network (prefrontal brain regions), thalamus and cerebellum during or shortly after aVNS.
Fourteen studies were included in the risk-of-bias analysis. Only one study was rated low risk of bias [50], three studies with some concern [36, 65, 69] and ten studies with an overall high risk of bias [51, 53, 57–60, 62, 66, 70] (Table 2).
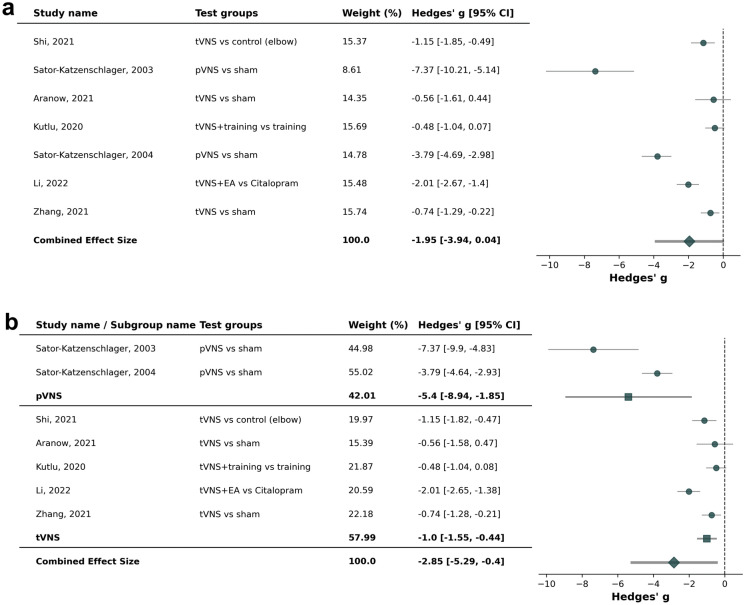
Seven studies [36, 58, 60, 65, 66, 69, 70] were included in the meta-analysis. The combined effect size and mean difference of − 1.95 (95% confidence interval [CI]: − 3.94 to 0.04, p = 0.008) was in favor of active aVNS compared to sham aVNS or control treatments (Fig. 3). Highest effect sizes were seen in chronic cervical syndrome and chronic low back pain [58, 60]. Active pVNS [58, 60] showed a higher effect size compared to active tVNS [36, 65, 66, 69, 70] (− 5.40 [− 8.94; − 1.85] vs. − 1.00 [− 1.55; − 0.44]; p = 0.015).
Fig. 3.
Forest plot for studies in chronic pain conditions (cervical syndrome, low back pain, migraine, abdominal pain, fibromyalgia, musculoskeletal pain; [a]), including subgroup analysis for percutaneous auricular vagus nerve stimulation (pVNS, needle electrodes) and transcutaneous auricular vagus nerves stimulation (tVNS, surface electrodes) (b)
Acute Postoperative Pain
Twelve studies on acute postoperative pain with a total of n = 291 aVNS-treated patients (n = 311 including dropouts) were included in the analysis. Surgery included gynecological interventions (4 studies [15, 71–73], n = 115), abdominal surgery (4 studies [74–77], n = 66), tonsillectomy (1 study [78], n = 16), molar tooth extraction (1 study [79], n = 48), outpatient orthopedic surgery and anterior cruciate ligament reconstruction (1 study each [80, 81], n = 46). Of these, eight studies [71–73, 75, 77–80] were high-quality RCTs with an average score of 7.6 out of 9 (Supplementary Table 3). Two other studies [15, 74] were case-control studies, and two [76, 81] were case series with an average score of 3.6 points.
Eight studies observed significant improvement in pain and/or need for opioids after surgery using aVNS; see Table 1 [15, 72, 73, 75–78, 80]. In one study [74], slight, non-significant improvements were observed. Differences in nausea, fatigue and use of non-opioid analgesics were not significant in any of the studies (if included in endpoint analysis).
aVNS was used either shortly before (perioperatively) or immediately after surgery (postoperatively) for a period of 2–5 days. In one study (hysterectomy), stimulation was performed for only a few minutes [72] and in another study (oocyte aspiration) for only a few hours [73].
Clinically and statistically highly significant results were found for oocyte aspiration for in vitro fertilization [73], laparoscopic nephrectomy [77], open colorectal surgery [75] and rebound pain after femoral nerve block for ACLR [80].
Eight studies were included in the risk-of-bias analysis. Four were rated with overall low risk of bias [71, 77, 78, 80], one with some concern for risk of bias [75] and three with a high risk of bias [72, 73, 79] (Table 2).
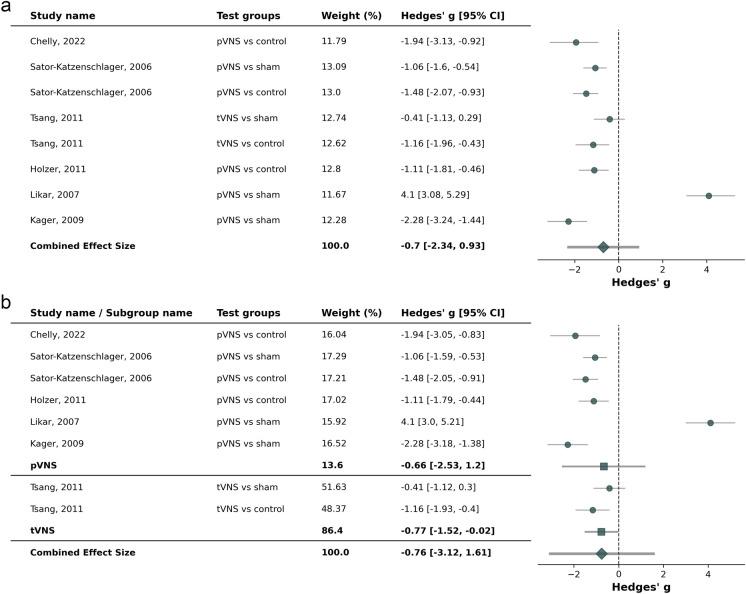
Six studies [71–73, 76–78] were included in the meta-analysis. Two studies allowed for separate evaluation between active aVNS vs. sham aVNS as well as active aVNS vs. control [72, 73]. The combined effect size and mean difference of − 0.70 [− 2.34; 0.93] (p = 0.15) was not in favor of active aVNS compared to sham aVNS or control treatments (Fig. 4). However, outcome strongly depended on the specific study and indication as well as on co-medication and timepoints of assessments. For instance, in Likar et al. 2007, there were statistically significant improvements in rest-pain at 1 h post-surgery but not after 24 h, whereas the benefit was sustainable in exertion [77]. Highest effect sizes in favor of active aVNS was seen in tonsillectomy and kidney donor surgery [76, 78]. The effect size was comparable between pVNS and tVNS (− 0.66 [− 2.53; 1.20] vs − 0.77 [− 1.52; − 0.02]).
Fig. 4.
Forest plot for studies in acute pain conditions (kidney donor surgery, oocyte-aspiration, hysterectomy, gynecological surgery, nephrectomy, tonsillectomy; [a]), including subgroup analysis for percutaneous auricular vagus nerve stimulation (pVNS, needle electrodes) and transcutaneous auricular vagus nerves stimulation (tVNS, surface electrodes) (b)
Acute Experimental Pain
For acute experimental pain, seven randomized cross-over studies with a total of n = 226 healthy volunteers (n = 229 including dropouts) were identified. Six of the studies [82–87] could be evaluated with the Jadad scale and reached an average rating of 5.4 points (Supplementary Table 4). The experimental pain stimuli included heat or heat and pressure (4 studies [82, 84, 87, 88], neurometer (1 study [86]), cold and pressure (3 studies [85, 88, 89]) and acid-induced hypersensitivity (1 study [83]). In six of the seven studies [82–87], differences in pain perception or intensity (lower) and pain threshold (higher) were found during or after stimulation with aVNS, cf. Table 1. The available studies indicate that stimulation during experimental pain in some patients does not result in any change of pain perception (non-responders) or even exhibit a pro-nociceptive effect, reflected in an increased pain perception and lower pain threshold during and directly after stimulation. Current evidence does not provide consistent results or conclusions in experimental pain.
Design of Studies
Of the 42 studies evaluated, 19 were conducted with pVNS devices [15, 50, 51, 54, 56, 58, 60, 61, 67, 68, 71, 73–79, 81] (needle electrodes) and 23 studies used tVNS devices [36, 49, 52, 53, 55, 57, 59, 62–66, 69, 70, 72, 80, 82–88] (surface electrodes) (see Supplementary Table 5). Stimulation electrodes were placed in vagal innervated but partly also in non-vagal innervated areas of the auricle. This was done depending on the device used and the experience of the study authors, with the conchae being the most used stimulation region. The most frequently used control group was a sham aVNS group using an inactive device with the electrodes at the same position as in the intervention group (15 studies [50, 51, 58, 60, 65, 71, 73, 75, 77–79, 82, 84, 86, 87]), followed by active controls of sham aVNS with an active device at other non-vagal innervated ear regions such as the earlobe (7 studies [36, 53, 72, 80, 83, 84, 88]) or other (standard) therapies as controls (pro- and retrospective, 7 studies [15, 59, 62, 67, 72, 74, 76]). One study [57] used an active sham aVNS control at the same points but with different stimulation pattern; one study used electrical stimulation control at the elbow [70]. In three studies [59, 62, 66], aVNS was performed with physical training; in one study [53], stimulation was synchronized with slow and deep breathing; in another study, stimulation was combined with electroacupuncture at acupoints located in the parietal and frontal skin and compared with pharmacotherapy (citalopram) [69].
Stimulation Parameters
Devices available on the market were used for aVNS with device-specific preconfigured stimulation parameters (see Supplementary Table 5). The devices evaluated use monophasic or biphasic square waves with a pulse width of 0.2–1 ms and repetition or alternating frequencies between 1 and 100 Hz. The current or voltage amplitude was either set constantly or was individually adapted to the perception of the patients (from below sensory threshold to clearly perceptible or below pain/discomfort threshold). The most common configuration was biphasic rectangular pulses of 1 ms and 1 Hz and an amplitude that produced a clear, non-painful perception.
Tolerability of aVNS
In most treated patients or subjects, no adverse events associated with aVNS were observed (see Supplementary Table 5). Documented adverse events were mostly mild side effects at the stimulation site, such as skin irritation, pain and slight bleeding at the insertion points of needle electrodes. Dizziness, nausea or fatigue were reported less frequently. One patient collapsed because of needle phobia.
The tolerability of the devices (if surveyed) was rated as good to excellent by a significant majority of patients (> 75%).
Discussion
Chronic pain significantly affects health and individual quality of life of patients [47]. Further development of additional effective therapies for the treatment of these patients as well as approaches to avoid chronification of pain, e.g., after surgery, is of high clinical relevance. This present systematic review and meta-analysis indicates that aVNS is a complementary, non-drug-based, effective treatment for specific chronic pain and acute postoperative pain conditions.
The results prove a consistent pain-reducing effect as well as improvement in patients' quality of life with chronic back pain, abdominal pain and migraine, with a very low side effect profile (see Table 1), even in long-term use [52, 57]. In chronic pain conditions, a higher combined effect size in pVNS compared to tVNS studies was observed, which may be based on the prolonged stimulation durations/increased daily dose of stimulation or a more effective stimulation due to close placement of needle electrodes directly at the nerve (compared to surface electrodes) [90] (see Table 1). However, subgroup analysis comparing pVNS and tVNS has limited power due to the low number of studies per group.
The meta-analysis for acute pain conditions was not in favor of active aVNS compared to sham aVNS or control treatments (Fig. 4). Outcome of acute pain studies was more difficult to interpret and strongly depended on the study design, especially the co-medication and timepoints of pain assessment after surgery, and condition during pain assessment (e.g., rest, huff, exertion). For instance, an overall analysis in Blank et al. [75] was not able to show any effect in the entire cohort, but a significant effect was achieved for open colorectal interventions when performing subgroup analyses. Generally, a better effect was observed in more severe procedures with greater trauma and inflammation compared to minimally invasive procedures. Outcomes of studies on acute experimental pain in healthy volunteers also provided inconsistent results. More detailed considerations can be found in [2, 24, 91].
In chronic pain conditions, several studies documented a sustained therapeutic effect (see Table 1). This sustained effect lasted for weeks to months after the end of stimulation (follow-up between 2 weeks and a maximum of 12 months) [50, 52, 54, 58, 60, 65, 68]. Long-lasting effects of aVNS have already been presented in other indications, such as epilepsy or depression [21, 24, 92]. Possible mechanisms of action are the activation of neuroplastic effects in brain and spinal cord structures involved in pain processing (effect on central sensitization) as well as anti-inflammatory effects, e.g., on neuroinflammatory processes [7, 83]. The meta-analysis was in favor of active aVNS compared to sham or control treatments, although high heterogeneity was observable. Moreover, due to a highly conservative approach, most studies were prone to high bias because blinding and sham stimulations are easily identified and not ideal. A sustainable therapeutic improvement, combined with the good tolerability of the treatment, is of high socioeconomic relevance [93]. Recent health technology assessments have acknowledged the positive value of non-implanted vagus nerve stimulation for pain indications [94–96].
The tolerability of the treatment can be rated as very good based on the available literature review. Interactions with concomitant drug therapies could not be detected in the evaluated studies. The side effect profile of aVNS has already been rated as very good in other studies and indications [24, 83, 97]. A retrospective analysis by Roberts et al. [98] in 1207 applications of pVNS found only 24 (1.98%) documented adverse events of mild bleeding at the insertion sites of needle electrodes, local dermatitis and pain at the insertion sites. No systemic side effects or infections were observed. Studies on possible cardiovascular side effects of aVNS also revealed no increased risks [99]. In user studies, satisfaction of patients with pVNS during several weeks of treatment was surveyed. Eighty percent of patients rated their treatment with pVNS as very satisfactory regarding their subjective perception of quality of life [100].
Despite a high number of well-conducted studies on the effect of aVNS in chronic and acute post-surgery pain, the studies often do not directly compare. For the meta-analysis, a variety of primary endpoints in different studies as well as missing absolute data in publications resulted in exclusion of several studies. Since we only included studies reporting VAS pain intensity, the meta-analysis does not account for, e.g., effects on frequency in migraine attacks, opioid use or more disease-specific outcomes and questionnaires. Difficulties in blinding, due to the immanent procedure and suprathreshold active electrical stimulation, led to higher risk of bias. Differences in control groups, location of stimulation electrodes, stimulation parameters and duration of treatment are vast, cf. Table 1. For instance, due to the dense innervation of the auricle, not only by the vagus nerve but also by the auriculotemporal nerve (branch of the trigeminal nerve), great auricular nerve and minor occipital nerve, a co-stimulation of non-vagal fibers can be assumed in the studies summarized and analyzed here [14, 31, 34]. This is particularly of relevance considering the choice of control groups. In sham controls with active stimulation, e.g., at the earlobe, it cannot be assumed that this stimulation is physiologically inert. There is evidence that stimulation of the great auricular nerve can have a therapeutic effect in migraine or cluster headache [101]. Additionally, in fMRI studies, a corresponding modulation of specific brain regions during earlobe stimulation was shown [34].
To enable superior and comparable evidence in the future, a recent consensus review by Farmer et al. has highlighted the importance of minimal reporting criteria in studies on aVNS. A recent systematic review by Wang et al. [102] also evaluated the significance of differences in used nomenclature for aVNS, which also requires standardization to enable a consistent clinical evaluation. In addition, research and definition of criteria determining the individual treatment success of patients with pain are of high relevance [7]. Some evidence indicates differences in the effectiveness of aVNS in neuropathic and somatic pain [58] as well as in influencing affective components [35]. The inclusion of different physiological parameters and measurements of individual patients before start of therapy could be of enormous importance. There are attempts, e.g., based on the heart rate variability or the autonomic status of a patient, to predict the probability of a therapeutic response of individual patients, thereby simplifying patient selection regarding a positive therapy prognosis [7, 103]. Corresponding guidelines for patient selection will be essential for the clinical use of the method in chronic and acute pain.
Conclusion for Practice
Auricular vagus nerve stimulation is an easy-to-use method with a low side effect profile. The use of auricular vagus nerve stimulation can be an effective supplement to multimodal pain management for chronic back pain, abdominal pain and migraine or for specific operative procedures to reduce post-surgery pain. Results for acute experimental pain are not conclusive. Further studies should focus on optimal and standardized treatment protocols.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
All authors contributed to the study conception. The authors Irina T. Duff, Rudolf Likar and Alaa Abd-Elsayed designed and conducted the study, including data collection and data analysis. Literature research was supported by the authors Caroline Stremnitzer and Stefan Kampusch. Irina Duff prepared the manuscript draft with important intellectual input from Rudolf Likar, Christophe Perruchoud, Stefan Kampusch, Markus Köstenberger, Sabine Sator, Caroline Stremnitzer, Andreas Wolf, Stefan Neuwersch-Sommeregger and Alaa Abd-Elsayed. Statistical analysis was performed by the authors Irina T. Duff and Alaa Abd-Elsayed. All authors had complete access to the study data. All authors read and approved the final manuscript.
Funding
AURIMOD provided funding for the Rapid Service Fee of Pain and Therapy.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Irina T. Duff is an unpaid NANS education committee member. Rudolf Likar has received honoraria for presentations and consultancy and has received research funding from AURIMOD. Christophe Perruchoud has received research funding from AURIMOD and has received honoraria and consulting fees as member of the advisory board of Medtronic, he is president of the Swiss Neuromodulation Society. Stefan Kampusch is CEO and shareholder of AURIMOD, and inventor of several patent applications related to auricular vagus nerve stimulation. Caroline Stremnitzer is employee of AURIMOD. Andreas Wolf has received honoraria for presentations from AURIMOD. Alaa Abd-Elsayed has received consulting fees from AURIMOD, Medtronic, Curonix, Avanos and Averitas, he is a Director of NANS. Rudolf Likar and Alaa Abd-Elsayed are Editorial Board members of Pain and Therapy. Rudolf Likar and Alaa Abd-Elsayed were not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. All other authors do not declare any conflicts of interest.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–97. 10.1016/S0140-6736(21)00393-7. [DOI] [PubMed] [Google Scholar]
- 2.Farmer AD, Strzelczyk A, Finisguerra A, et al. International Consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14: 568051. 10.3389/fnhum.2020.568051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerbershagen HJ. Chronifizierung postoperativer Schmerzen: Physiologie. Risikofaktoren und Prävention Schmerz. 2013;27(1):81–96. 10.1007/s00482-012-1287-5. [DOI] [PubMed] [Google Scholar]
- 4.Cascella M. Editorial to the Special Issue: “Recent advances in the management of chronic pain.” Int J Environ Res Public Health. 2023;20(19):6875. 10.3390/ijerph20196875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois MY, Gallagher RM, Lippe PM. Pain medicine position paper. Pain Med. 2009;10(6):972–1000. 10.1111/j.1526-4637.2009.00696.x. [DOI] [PubMed] [Google Scholar]
- 6.Komisaruk BR, Frangos E. Vagus nerve afferent stimulation: projection into the brain, reflexive physiological, perceptual, and behavioral responses, and clinical relevance. Auton Neurosci. 2022;237: 102908. 10.1016/j.autneu.2021.102908. [DOI] [PubMed] [Google Scholar]
- 7.Kaniusas E, Kampusch S, Tittgemeyer M, et al. Current directions in the auricular vagus nerve stimulation I—a physiological perspective. Front Neurosci. 2019;13:854. 10.3389/fnins.2019.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butt MF, Albusoda A, Farmer AD, Aziz Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat. 2020;236(4):588–611. 10.1111/joa.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. 2016;594(20):5781–90. 10.1113/JP271539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falvey A. Vagus nerve stimulation and inflammation: expanding the scope beyond cytokines. Bioelectron Med. 2022;8(1):19. 10.1186/s42234-022-00100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tynan A, Brines M, Chavan SS. Control of inflammation using non-invasive neuromodulation: past, present and promise. Int Immunol. 2022;34(2):119–28. 10.1093/intimm/dxab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyle BN, McNeil DW. Autonomic arousal and experimentally induced pain: a critical review of the literature. Pain Res Manag. 2014;19(3):159–67. 10.1155/2014/536859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg I, Wang D. Complications of spinal cord stimulator trials and implants: a review. Curr Pain Headache Rep. 2023;27(12):837–42. 10.1007/s11916-023-01190-7. [DOI] [PubMed] [Google Scholar]
- 14.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part II. Headache J Head Face Pain. 2016;56(2):259–66. 10.1111/head.12650. [DOI] [PubMed] [Google Scholar]
- 15.Chakravarthy M, Prashanth A, George A. Evaluation of percutaneous electrical nerve stimulation of the auricle for relief of postoperative pain following cesarean section. Med Acupunct. 2019;31(5):281–8. 10.1089/acu.2019.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miranda A, Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: a multisite, retrospective assessment. Am J Drug Alcohol Abuse. 2018;44(1):56–63. 10.1080/00952990.2017.1295459. [DOI] [PubMed] [Google Scholar]
- 17.Shao P, Li H, Jiang J, Guan Y, Chen X, Wang Y. Role of vagus nerve stimulation in the treatment of chronic pain. NeuroImmunoModulation. 2023. 10.1159/000531626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song D, Li P, Wang Y, Cao J. Noninvasive vagus nerve stimulation for migraine: a systematic review and meta-analysis of randomized controlled trials. Front Neurol. 2023;14:1190062. 10.3389/fneur.2023.1190062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tirado CF, Washburn SN, Covalin A, et al. Delivering transcutaneous auricular neurostimulation (tAN) to improve symptoms associated with opioid withdrawal: results from a prospective clinical trial. Bioelectron Med. 2022;8(1):12. 10.1186/s42234-022-00095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H, Shi W, Fan J, et al. Transcutaneous auricular vagus nerve stimulation (ta-VNS) for treatment of drug-resistant epilepsy: a randomized, double-blind clinical trial. Neurotherapeutics. 2023;20(3):870–80. 10.1007/s13311-023-01353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345–55. 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 22.Guerriero G, Wartenberg C, Bernhardsson S, et al. Efficacy of transcutaneous vagus nerve stimulation as treatment for depression: a systematic review. J Affect Disord Rep. 2021;6: 100233. 10.1016/j.jadr.2021.100233. [Google Scholar]
- 23.Badran BW, Peng X, Baker-Vogel B, et al. Motor activated auricular vagus nerve stimulation as a potential neuromodulation approach for post-stroke motor rehabilitation: a pilot study. Neurorehabil Neural Repair. 2023;37(6):374–83. 10.1177/15459683231173357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yap JYY, Keatch C, Lambert E, Woods W, Stoddart PR, Kameneva T. Critical review of transcutaneous vagus nerve stimulation: challenges for translation to clinical practice. Front Neurosci. 2020;14:284. 10.3389/fnins.2020.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stegeman I, Velde HM, Robe PAJT, Stokroos RJ, Smit AL. Tinnitus treatment by vagus nerve stimulation: a systematic review. PLoS ONE. 2021;16(3): e0247221. 10.1371/journal.pone.0247221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ammons WS, Blair RW, Foreman RD. Vagal afferent inhibition of primate thoracic spinothalamic neurons. J Neurophysiol. 1983;50(4):926–40. 10.1152/jn.1983.50.4.926. [DOI] [PubMed] [Google Scholar]
- 27.Ammons WS, Blair RW, Foreman RD. Vagal afferent inhibition of spinothalamic cell responses to sympathetic afferents and bradykinin in the monkey. Circ Res. 1983;53(5):603–12. 10.1161/01.RES.53.5.603. [DOI] [PubMed] [Google Scholar]
- 28.Randich A, Gebhart GF. Vagal afferent modulation of nociception. Brain Res Rev. 1992;17(2):77–99. 10.1016/0165-0173(92)90009-B. [DOI] [PubMed] [Google Scholar]
- 29.Thies R, Foreman RD. Descending inhibition of spinal neurons in the cardiopulmonary region by electrical stimulation of vagal afferent nerves. Brain Res. 1981;207(1):178–83. 10.1016/0006-8993(81)90690-9. [DOI] [PubMed] [Google Scholar]
- 30.Thies R, Foreman RD. Inhibition and excitation of thoracic spinoreticular neurons by electrical stimulation of vagal afferent nerves. Exp Neurol. 1983;82(1):1–16. 10.1016/0014-4886(83)90238-8. [DOI] [PubMed] [Google Scholar]
- 31.Randich A, Maixner W. Interactions between cardiovascular and pain regulatory systems. Neurosci Biobehav Rev. 1984;8(3):343–67. 10.1016/0149-7634(84)90057-5. [DOI] [PubMed] [Google Scholar]
- 32.Sclocco R, Garcia RG, Kettner NW, et al. Stimulus frequency modulates brainstem response to respiratory-gated transcutaneous auricular vagus nerve stimulation. Brain Stimulat. 2020;13(4):970–8. 10.1016/j.brs.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao J, Zhang Y, Li H, et al. Different modulation effects of 1 Hz and 20 Hz transcutaneous auricular vagus nerve stimulation on the functional connectivity of the periaqueductal gray in patients with migraine. J Transl Med. 2021;19(1):354. 10.1186/s12967-021-03024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimulat. 2015;8(3):624–36. 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frangos E, Richards EA, Bushnell MC. Do the psychological effects of vagus nerve stimulation partially mediate vagal pain modulation? Neurobiol Pain. 2017;1:37–45. 10.1016/j.ynpai.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Huang Y, Li H, et al. Transcutaneous auricular vagus nerve stimulation (taVNS) for migraine: an fMRI study. Reg Anesth Pain Med. 2021;46(2):145–50. 10.1136/rapm-2020-102088. [DOI] [PubMed] [Google Scholar]
- 37.Ramos-Martínez IE, Rodríguez MC, Cerbón M, Ramos-Martínez JC, Ramos-Martínez EG. Role of the cholinergic anti-inflammatory reflex in central nervous system diseases. Int J Mol Sci. 2021;22(24):13427. 10.3390/ijms222413427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xanthos DN, Sandkühler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15(1):43–53. 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 39.Bantel C, Trapp S. The role of the autonomic nervous system in acute surgical pain processing—what do we know?: editorial. Anaesthesia. 2011;66(7):541–4. 10.1111/j.1365-2044.2011.06791.x. [DOI] [PubMed] [Google Scholar]
- 40.Likar R, Perruchoud C, Kampusch S, et al. Klinische Wirksamkeit der aurikulären Vagusnervstimulation in der Behandlung chronischer und akuter Schmerzen: Eine systematische Übersichtsarbeit. Schmerz. 2023. 10.1007/s00482-022-00686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilz MJ. Transcutaneous vagus nerve stimulation—a brief introduction and overview. Auton Neurosci. 2022;243: 103038. 10.1016/j.autneu.2022.103038. [DOI] [PubMed] [Google Scholar]
- 42.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17(1):1–12. 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 43.Albert DA. Deciding whether the conclusions of studies are justified: a review. Med Decis Mak. 1981;1(3):265–75. 10.1177/0272989X8100100306. [DOI] [PubMed] [Google Scholar]
- 44.Greenhalgh T. How to read a paper: getting your bearings (deciding what the paper is about). BMJ. 1997;315(7102):243–6. 10.1136/bmj.315.7102.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prel JBD, Röhrig B, Blettner M. Critical appraisal of scientific articles. Dtsch Ärztebl Int. 2009. 10.3238/arztebl.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cochrane. RoB 2: a revised cochrane risk-of-bias tool for randomized trials. Cochrane Methods Bias. 2020. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials. Accessed January 24, 2024
- 47.Blümle A, Meerpohl JJ, Wolff R, Antes G. Evidenzbasierte Medizin und systematische Übersichtsarbeiten: Die Rolle der Cochrane Collaboration. MKG-Chir. 2009;2(2):86–92. 10.1007/s12285-009-0081-6. [Google Scholar]
- 48.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–26. 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 49.Grolaux P. Transcutaneous vagus nerve stimulation in private healthcare center: a small-scale investigation targeting anxiety, irritable bowel syndrome and chronic pain. J Neurol Neuromedicine. 2019;4(5):7–22. 10.29245/2572.942X/2019/5.1251. [Google Scholar]
- 50.Kovacic K, Hainsworth K, Sood M, et al. Neurostimulation for abdominal pain-related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. Lancet Gastroenterol Hepatol. 2017;2(10):727–37. 10.1016/S2468-1253(17)30253-4. [DOI] [PubMed] [Google Scholar]
- 51.Krasaelap A, Sood MR, Li BUK, et al. Efficacy of auricular neurostimulation in adolescents with irritable bowel syndrome in a randomized, double-blind trial. Clin Gastroenterol Hepatol. 2020;18(9):1987-1994.e2. 10.1016/j.cgh.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Mion F, Pellissier S, Garros A, Damon H, Roman S, Bonaz B. Transcutaneous auricular vagus nerve stimulation for the treatment of irritable bowel syndrome: a pilot, open-label study. Bioelectron Med. 2020;3(1):5–12. 10.2217/bem-2020-0004. [Google Scholar]
- 53.Napadow V, Edwards RR, Cahalan CM, et al. Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Med. 2012;13(6):777–89. 10.1111/j.1526-4637.2012.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santucci NR, King C, El-Chammas KI, et al. Effect of percutaneous electrical nerve field stimulation on mechanosensitivity, sleep, and psychological comorbidities in adolescents with functional abdominal pain disorders. Neurogastroenterol Motil. 2022;34(8): e14358. 10.1111/nmo.14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng M, Zhang Y, Wen Z, et al. Early fractional amplitude of low frequency fluctuation can predict the efficacy of transcutaneous auricular vagus nerve stimulation treatment for migraine without aura. Front Mol Neurosci. 2022;15: 778139. 10.3389/fnmol.2022.778139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kong KH, Ng WW. Treatment of chronic pain with an auricular acupuncture device (P-Stim) in Singapore. Acupunct Med. 2009;27(4):187–8. 10.1136/aim.2009.001388. [DOI] [PubMed] [Google Scholar]
- 57.Straube A, Ellrich J, Eren O, Blum B, Ruscheweyh R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J Headache Pain. 2015;16(1):63. 10.1186/s10194-015-0543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sator-Katzenschlager SM, Scharbert G, Kozek-Langenecker SA, et al. The short- and long-term benefit in chronic low back pain through adjuvant electrical versus manual auricular acupuncture. Anesth Analg. 2004. 10.1213/01.ANE.0000107941.16173.F7. [DOI] [PubMed] [Google Scholar]
- 59.Ünal S, Karagözoğlu Coşkunsu D, Hatik SH, Özden AV. Short-term effectiveness of auricular vagus nerve stimulation in patients with myofascial pain syndrome. Eur Res J. 2022;8(5):573–82. 10.18621/eurj.1005161. [Google Scholar]
- 60.Sator-Katzenschlager SM, Szeles JC, Scharbert G, et al. Electrical stimulation of auricular acupuncture points is more effective than conventional manual auricular acupuncture in chronic cervical pain: a pilot study. Anesth Analg. 2003. 10.1213/01.ANE.0000082246.67897.0B. [DOI] [PubMed] [Google Scholar]
- 61.Szeles JC, Kampusch S, Le VH, Enajat DP, Kaniusas E, Neumayer C. Clinical effectiveness of percutaneous auricular vagus nerve stimulation in chronic back pain patients—a single-centre retrospective analysis. Ann Pain Med. 2021;3(1):1009. [Google Scholar]
- 62.Uzlifatin Y, Arfianti L, Wardhani IL, Hidayati HB, Melaniani S. Effect of transcutaneous auricular vagus nerve stimulation addition on disability in chronic low back pain patients: a randomized controlled study. Anaesth Pain Intensive Care. 2023;27(1):73–81. 10.35975/apic.v27i1.2084. [Google Scholar]
- 63.Courties A, Deprouw C, Maheu E, et al. Effect of transcutaneous vagus nerve stimulation in erosive hand osteoarthritis: results from a pilot trial. J Clin Med. 2022;11(4):1087. 10.3390/jcm11041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marsal S, Corominas H, De Agustín JJ, et al. Non-invasive vagus nerve stimulation for rheumatoid arthritis: a proof-of-concept study. Lancet Rheumatol. 2021;3(4):e262–9. 10.1016/S2665-9913(20)30425-2. [DOI] [PubMed] [Google Scholar]
- 65.Aranow C, Atish-Fregoso Y, Lesser M, et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann Rheum Dis. 2021;80(2):203–8. 10.1136/annrheumdis-2020-217872. [DOI] [PubMed] [Google Scholar]
- 66.Kutlu N, Özden AV, Alptekin HK, Alptekin JÖ. The impact of auricular vagus nerve stimulation on pain and life quality in patients with fibromyalgia syndrome. BioMed Res Int. 2020;2020:1–10. 10.1155/2020/8656218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woodbury A, Krishnamurthy V, Gebre M, et al. Feasibility of auricular field stimulation in fibromyalgia: evaluation by functional magnetic resonance imaging, randomized trial. Pain Med. 2021;22(3):715–26. 10.1093/pm/pnaa317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sacco J, Baas W, Barnes MA, Luberto C, Talat R, Cotton S. The efficacy of percutaneous auricular neurostimulation for chemotherapy-induced peripheral neuropathy: a retrospective chart review. Med Acupunct. 2016;28(3):131–6. 10.1089/acu.2016.1170. [Google Scholar]
- 69.Li S, Zhang Z, Jiao Y, et al. An assessor-blinded, randomized comparative trial of transcutaneous auricular vagus nerve stimulation (taVNS) combined with cranial electroacupuncture vs. citalopram for depression with chronic pain. Front Psychiatry. 2022;13: 902450. 10.3389/fpsyt.2022.902450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi X, Hu Y, Zhang B, Li W, Chen JD, Liu F. Ameliorating effects and mechanisms of transcutaneous auricular vagal nerve stimulation on abdominal pain and constipation. JCI Insight. 2021;6(14): e150052. 10.1172/jci.insight.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holzer A, Leitgeb U, Spacek A, Wenzl R, Herkner H, Kettner S. Auricular acupuncture for postoperative pain after gynecological surgery: a randomized controlled trail. Minerva Anestesiol. 2011;77(3):298–304. [PubMed] [Google Scholar]
- 72.Tsang HC, Lam CS, Chu PW, Yap J, Fung TY, Cheing GLY. A randomized controlled trial of auricular transcutaneous electrical nerve stimulation for managing posthysterectomy pain. Evid Based Complement Alternat Med. 2011;2011:1–9. 10.1155/2011/276769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sator-Katzenschlager SM, Wölfler MM, Kozek-Langenecker SA, et al. Auricular electro-acupuncture as an additional perioperative analgesic method during oocyte aspiration in IVF treatment. Hum Reprod. 2006;21(8):2114–20. 10.1093/humrep/del110. [DOI] [PubMed] [Google Scholar]
- 74.Ahmed BH, Courcoulas AP, Monroe AL, Gourash WF, Chelly JE. Auricular nerve stimulation using the NSS-2 BRIDGE device to reduce opioid requirement following laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2021;17(12):2040–6. 10.1016/j.soard.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Blank JJ, Liu Y, Yin Z, et al. Impact of auricular neurostimulation in patients undergoing colorectal surgery with an enhanced recovery protocol: a pilot randomized, controlled trial. Dis Colon Rectum. 2021;64(2):225–33. 10.1097/DCR.0000000000001752. [DOI] [PubMed] [Google Scholar]
- 76.Chelly JE, Monroe AL, Planinsic RM, Tevar A, Norton BE. Auricular field nerve stimulation using the NSS-2 BRIDGE ® device as an alternative to opioids following kidney donor surgery. J Complement Integr Med. 2022;19(2):449–54. 10.1515/jcim-2021-0208. [DOI] [PubMed] [Google Scholar]
- 77.Likar R, Jabarzadeh H, Kager I, Trampitsch E, Breschan C, Szeles J. Elektrische Punktualstimulation (P-STIM) mittels Ohrakupunktur: Eine randomisierte, doppelblinde, kontrollierte Pilotstudie bei laparoskopischen Nephrektomien. Schmerz. 2007;21(2):154–9. 10.1007/s00482-006-0519-y. [DOI] [PubMed] [Google Scholar]
- 78.Kager H, Likar R, Jabarzadeh H, Sittl R, Breschan C, Szeles J. Electrical punctual stimulation (P-STIM) with ear acupuncture following tonsillectomy, a randomised, controlled pilot study. Acute Pain. 2009;11(3–4):101–6. 10.1016/j.acpain.2009.10.001. [Google Scholar]
- 79.Michalek-Sauberer A, Heinzl H, Sator-Katzenschlager SM, Monov G, Knolle E, Kress HG. Perioperative auricular electroacupuncture has no effect on pain and analgesic consumption after third molar tooth extraction. Anesth Analg. 2007;104(3):542–7. 10.1213/01.ane.0000253233.51490.dd. [DOI] [PubMed] [Google Scholar]
- 80.Zhou Q, Yu L, Yin C, et al. Effect of transauricular vagus nerve stimulation on rebound pain after ropivacaine single injection femoral nerve block for anterior cruciate ligament reconstruction: a randomized controlled trial. J Pain Res. 2022;15:1949–58. 10.2147/JPR.S370589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ilfeld BM, Finneran JJ, Dalstrom D, Wallace AM, Abdullah B, Said ET. Percutaneous auricular nerve stimulation (neuromodulation) for the treatment of pain following outpatient surgery: a proof-of-concept case series. Reg Anesth Pain Med. 2022;47(9):581–5. 10.1136/rapm-2022-103777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P. The effect of transcutaneous vagus nerve stimulation on pain perception—an experimental study. Brain Stimulat. 2013;6(2):202–9. 10.1016/j.brs.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 83.Farmer AD, Albusoda A, Amarasinghe G, et al. Transcutaneous vagus nerve stimulation prevents the development of, and reverses, established oesophageal pain hypersensitivity. Aliment Pharmacol Ther. 2020;52(6):988–96. 10.1111/apt.15869. [DOI] [PubMed] [Google Scholar]
- 84.Janner H, Klausenitz C, Gürtler N, Hahnenkamp K, Usichenko TI. Effects of electrical transcutaneous vagus nerve stimulation on the perceived intensity of repetitive painful heat stimuli: a blinded placebo- and sham-controlled randomized crossover investigation. Anesth Analg. 2018;126(6):2085–92. 10.1213/ANE.0000000000002820. [DOI] [PubMed] [Google Scholar]
- 85.Frøkjær JB, Bergmann S, Brock C, et al. Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. Neurogastroenterol Motil. 2016;28(4):592–8. 10.1111/nmo.12760. [DOI] [PubMed] [Google Scholar]
- 86.Laqua R, Leutzow B, Wendt M, Usichenko T. Transcutaneous vagal nerve stimulation may elicit anti- and pro-nociceptive effects under experimentally-induced pain—a crossover placebo-controlled investigation. Auton Neurosci. 2014;185:120–2. 10.1016/j.autneu.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Usichenko T, Laqua R, Leutzow B, Lotze M. Preliminary findings of cerebral responses on transcutaneous vagal nerve stimulation on experimental heat pain. Brain Imaging Behav. 2017;11(1):30–7. 10.1007/s11682-015-9502-5. [DOI] [PubMed] [Google Scholar]
- 88.Dumoulin M, Liberati G, Mouraux A, Santos SF, El Tahry R. Transcutaneous auricular VNS applied to experimental pain: a paired behavioral and EEG study using thermonociceptive CO2 laser. PLoS ONE. 2021;16(7): e0254480. 10.1371/journal.pone.0254480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rapalis A, Piartli P, Jankauskaitė L, Marozas V, Kaniusas E. Induced pain affects auricular and body biosignals: from cold stressor to deep breathing. Front Physiol. 2023;14:1090696. 10.3389/fphys.2023.1090696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dabiri B, Kampusch S, Geyer SH, et al. High-resolution episcopic imaging for visualization of dermal arteries and nerves of the auricular cymba conchae in humans. Front Neuroanat. 2020;14:22. 10.3389/fnana.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verma N, Mudge JD, Kasole M, et al. Auricular vagus neuromodulation—a systematic review on quality of evidence and clinical effects. Front Neurosci. 2021;15: 664740. 10.3389/fnins.2021.664740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. 2018;11:203–13. 10.2147/JIR.S163248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mayer S, Spickschen J, Stein KV, Crevenna R, Dorner TE, Simon J. The societal costs of chronic pain and its determinants: the case of Austria. PLoS ONE. 2019;14(3): e0213889. 10.1371/journal.pone.0213889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morris J, Straube A, Diener HC, et al. Cost-effectiveness analysis of non-invasive vagus nerve stimulation for the treatment of chronic cluster headache. J Headache Pain. 2016;17(1):43. 10.1186/s10194-016-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mwamburi M, Liebler EJ, Tenaglia AT. Review of non-invasive vagus nerve stimulation (gammaCore): efficacy, safety, potential impact on comorbidities, and economic burden for episodic and chronic cluster headache. Am J Manag Care. 2017;23(17 Suppl):S317–25. [PubMed] [Google Scholar]
- 96.Scott A, Hofer V, Al Froukh R, Ma N, Goetz G. Electrical auricular vagus nerve stimulation for pain. Vienna Austrian Inst Health Technol Assess GmbH. 2023. AIHTA Decision Support Documents No. 138.
- 97.Redgrave J, Day D, Leung H, et al. Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review. Brain Stimulat. 2018;11(6):1225–38. 10.1016/j.brs.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 98.Roberts A, Sithole A, Sedghi M, Walker C, Quinn T. Minimal adverse effects profile following implantation of periauricular percutaneous electrical nerve field stimulators: a retrospective cohort study. Med Devices Evid Res. 2016;9:389–93. 10.2147/MDER.S107426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kreuzer PM, Landgrebe M, Husser O, et al. Transcutaneous vagus nerve stimulation: retrospective assessment of cardiac safety in a pilot study. Front Psychiatry. 2012. 10.3389/fpsyt.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kampusch S, Kaniusas E, Thürk F, Felten D, Hofmann I, Széles JC. Device development guided by user satisfaction survey on auricular vagus nerve stimulation. Curr Dir Biomed Eng. 2016;2(1):593–7. 10.1515/cdbme-2016-0131. [Google Scholar]
- 101.Elahi F, Reddy C, Bellinger A, Manolitsis N. Neuromodulation of the great auricular nerve: a case report. Neuromodul Technol Neural Interface. 2014;17(8):784–7. 10.1111/ner.12114. [DOI] [PubMed] [Google Scholar]
- 102.Wang L, Wang Y, Wang Y, et al. Transcutaneous auricular vagus nerve stimulators: a review of past, present, and future devices. Expert Rev Med Devices. 2022;19(1):43–61. 10.1080/17434440.2022.2020095. [DOI] [PubMed] [Google Scholar]
- 103.Bretherton B, Atkinson L, Murray A, Clancy J, Deuchars S, Deuchars J. Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: potential benefits of daily stimulation. Aging. 2019;11(14):4836–57. 10.18632/aging.102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15(1):35–7. 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.