Abstract
Introduction
Ciprofol is a novel propofol analogue with a characteristic of hemodynamic stability. At present, there is a lack of research comparing the hemodynamic stability of ciprofol and propofol during painless colonoscopy. In this study, we aim to test the hypothesis that ciprofol is superior to propofol in terms of hemodynamic stability for sedation anesthesia in patients undergoing colonoscopy.
Methods
A total of 222 patients were randomized into two groups. Patients in group P (n = 112) and group C (n = 110) received propofol and ciprofol sedation, respectively. Noninvasive blood pressure were monitored starting from induction (T0) to the end of the procedure, at 2-min intervals (T1 to T10). Heart rate variability (HRV), pain injection, Modified Observer's Assessment of Alertness and Sedation (MOAA/S) score, body movement, doses of norepinephrine, modified Aldrete score, drug-related adverse reactions, and patient satisfaction and endoscopist satisfaction were recorded.
Results
In group C, fewer patients experienced a decrease in blood pressure with a higher HRV after induction sedation, the incidence of pain injection was reduced, the amount of norepinephrine dose was decreased, patient satisfaction was increased compared with group P (all P < 0.05). There were no significant differences in induction time, modified Aldrete score, alertness time, drug-related adverse reactions, and endoscopist satisfaction.
Conclusions
Our study indicated intravenous induction with ciprofol was superior, with regard to hemodynamic stability and reduced injection pain, than induction with propofol for anesthesia in patients undergoing painless colonoscopy.
Trial Registration
Chinese Clinical Trial Registry (ChiCTR2200061814).
Keywords: Ciprofol, Propofol, Sedation, Painless colonoscopy, Anesthesia
Key Summary Points
| Ciprofol is a new type of non-barbiturate general intravenous anesthetic. Clinical trials have shown that ciprofol has the advantages of fast onset, fast recovery, and less injection pain. However, there are few studies comparing the hemodynamic stability, injection pain, and adverse reactions of ciprofol and propofol during painless colonoscopy. |
| In this study, we aimed to investigate that ciprofol is superior to propofol in terms of hemodynamic stability and injection pain in patients undergoing painless colonoscopy. |
| Ciprofol sedation was safe and effective for anesthesia induction during colonoscopy procedures, with a decrease in injection pain and a lower blood pressure reduction observed relative to propofol sedation. |
| Intravenous injection of ciprofol has a superior hemodynamic stability with lower incidence of injection pain and higher patient satisfaction compared with propofol for sedation during painless colonoscopy. |
Introduction
Although sedation/anesthesia is widely used in gastrointestinal endoscopy, the choice of anesthetics used is not uniform. Propofol, etomidate, midazolam, and remiazolam are used for painless gastroscopy [1–3]. Propofol-assisted opioid drugs are commonly used sedatives in clinical practice. Propofol has the advantages of a rapid anesthetic effect and short recovery time, and is one of the first-choice drugs for painless gastrointestinal endoscopy. However, it has inhibitory effects on the respiratory and circulatory systems, and its dosage should be strictly controlled for people with cardiopulmonary diseases, especially the elderly [4]. Furthermore, the loading dose of intravenous propofol has been reported to lead to low blood pressure, respiratory depression, and a high incidence of injection pain [5].
Ciprofol (HSK3486; Haisco Pharmaceutical Group, Liaoning, China) is a new short-acting intravenous sedative based on a structural modification of propofol that has been independently developed in China. Ciprofol is reported to be as effective as propofol, but at a dose of only one-quarter to one-fifth that of propofol [6]. Ciprofol has high efficacy, good selectivity, and a low rate of adverse reactions, and exhibits good clinical application potential. A number of ciprofol clinical trials, from phase I to Phase III, have been completed in China [7–9].
The sedative effects of ciprofol in various procedures, including gastroscopy and colonoscopy, have been evaluated in several clinical studies [10, 11]. However, there is a lack of research comparing the hemodynamic stability of ciprofol with that of propofol during painless colonoscopy.
In this study, we aim to test the hypothesis that ciprofol is superior to propofol in terms of hemodynamic stability for anesthesia in patients undergoing colonoscopy and has a safety profile.
Methods
Patients and Study Design
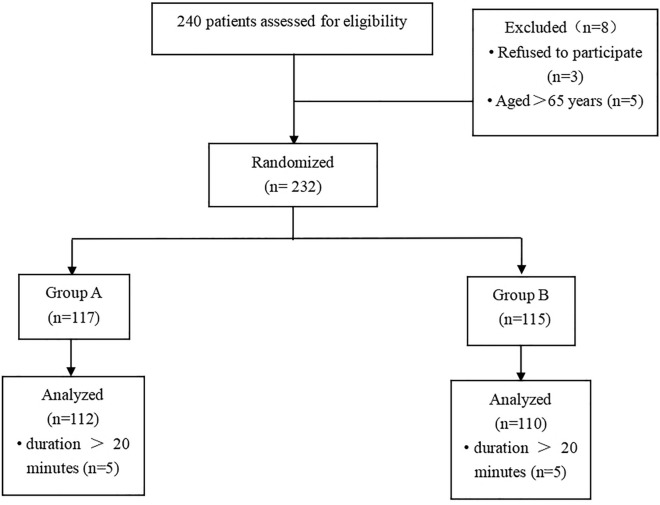
The protocol for this randomized, double-blind controlled study was approved by the Ethics Committee of the First Affiliated Hospital of University of Science and Technology of China (USTC) (2022KY-106) and conducted in accordance with the ethical guidelines of the 1975 revision of the Declaration of Helsinki. It was registered at www.chictr.org.cn (Registration No. ChiCTR2200061814) before patient enrollment, written informed consent was obtained from all subjects, and all patients consented to the publication of the data. The trial was conducted between June and December 2022, and included 222 patients (range 18–65 years) who were scheduled to receive painless colonoscopy at The First Affiliated Hospital of USTC (Anhui Provincial Hospital) (Fig. 1).
Fig. 1.
Patient group assignment and results; reasons for withdrawal are indicated
Patients scheduled for painless colonoscopy from June to December 2022 were screened. Patients with the American Society of Anesthesiologists (ASA) physical status class I–II, aged 18–65 years, were eligible for the study. The exclusion criteria were as follows: (1) arrhythmia; (2) allergies to opioids, propofol, or ciprofol ingredients; (3) history of narcotic abuse; (4) breastfeeding or pregnant status; (5) body mass index (BMI) > 30 kg/m2; (6) participation in any pharmacological clinical trials within the last 3 months; and (7) painless colonoscopy duration of longer than 20 min.
Finally, 240 screened patients were analyzed (Fig. 1). The patients were randomized to one of the two groups at a ratio of 1:1 using a computer-generated randomization schedule. The randomized number was hidden in a sealed opaque kraft paper envelope. Administers of analgesia were unaware of the anesthetic administered, as ciprofol or propofol were extracted in 20-ml syringes which were identical in physical appearance and collected the data perioperatively. Both patients and assessors were blinded to the group assignment. When serious adverse events occurred in patients, emergency unblinding was required. Patients in group P (n = 112) and group C (n = 110) received propofol and ciprofol, respectively, during the colonoscopy procedure.
Intervention and Sedation/Anesthesia Protocol
All the participants fasted for 8 h before surgery, and water intake was forbidden for 2 h after taking intestinal purging drugs. After entering the endoscopy room, 200–300 ml of equilibrium liquid was infused from the upper dorsal vein. Electrocardiographic changes, heart rate, pulse oxygen saturation (SpO2), noninvasive blood pressure (BP), and heart rate variability (HRV) were monitored starting from induction (T0) to the end of the procedure, at 2-min intervals (T1 to T10); i.e., vital signs were recorded for 20 min after induction. Oxygen inhalation of 5 l/min via nasal tube was performed until patients were alert after the endoscopic procedure. Patients received 0.05 μg/kg sufentanil (Renfu Pharmaceutical, Yichang, China) before intravenous infusion of either 0.4 mg/kg ciprofol (Haiske Pharmaceutical, Liaoning, China) or 2 mg/kg propofol (Fresenius Kabi Pharmaceutical, Beijing, China) over 1 min for induction. Painless colonoscopy was performed when the Modified Observer's Assessment of Alertness and Sedation (MOAA/S) score was ≤ 1, which was evaluated by the anesthesiologist every 30 s and 2 min, during sedation induction and the maintenance phase, respectively. A supplemental top-up dose of 1/3 of the initial study dose was injected within 10 s at the appearance of signs of agitation or insufficient sedation, as needed. Sedation was considered unsuccessful if more than five supplementary doses were required within 15 min; in this scenario, propofol was the only alternative sedative allowed in this trial.
The average of the two consecutive systolic blood pressure (BP) was recorded as baseline systolic BP when induction anesthesia started. Hypotension and severe hypotension are defined as systolic BP values lower than the base value of ≥ 20% or ≥ 30%, respectively. If hypotension occurred, norepinephrine was injected at a dose of 4–12 µg; if sinus bradycardia occurred (heart rate < 50 beats/min), atropine was injected at 0.25–0.5 mg; if hypoxemia (SpO2 < 92%) occurred and improvement was not achieved via the jaw-thrust maneuver, pressure support oxygen ventilation was delivered by a face mask or tracheal intubation was necessary in case of severe hypoxemia that could not be ameliorated. Administration of the study drugs was stopped when the colonoscope was removed, and all patients were transferred to the post-anesthesia care unit (PACU). Discharge criteria were defined as a modified Aldrete score of ≥ 9 in the PACU post-recovery, which was assessed at 2-min intervals.
Outcomes
The primary endpoint was the incidence of hypotension events defined as a systolic BP 20% lower than the baseline systolic BP for the colonoscopy procedure in non-operating room settings.
Secondary outcomes included: (1) HRV; (2) incidence of injection pain; (3) induction time (time of MOAA/S score ≤ 1); (4) body movement during the procedure; (5) doses of norepinephrine used; (6) duration of colonoscopy procedure; (7) alert time (modified Aldrete score ≥ 9); (8) drug-related adverse reactions including sinus bradycardia, dizziness, hypoxemia, postoperative nausea and vomiting; and (9) patient satisfaction and endoscopist satisfaction.
Statistical Analysis
The incidence of hypotension was the primary outcome. The calculation of the minimum sample size was based on our pre-experimental results that induction with ciprofol could reduce this incidence to 20% compared to 40% with propofol in colonoscopy procedures.
The sample size was calculated by PASS 21.0.0 (NCSS, Kaysville, USA), and indicated that 106 patients per group should be recruited based on expected clinically relevant proportions that induction with ciprofol could reduce the incidence of hypotension events to 20%, a type I error rate of 0.05, and power of 90%. Based on an potential dropout rate of 10%, we enrolled 240 patients in our study.
Numeric data were assessed for normality of distribution and equal variance, and presented as means and standard deviations (SDs). Unpaired t test involved independent samples for a difference in mean for continuous values: age, height, weight, BMI, HRV, duration of colonoscopy procedure, induction time of MOAA/S score ≤ 1, and alert time. Chi-square tests of association were used to examine gender, ASA classification, and rates of hypotension, hypoxemia, injection pain, and body movement. Non-parametric tests were used to compare patient satisfaction and endoscopist satisfaction. SPSS 21.0 software (IBM, Armonk, NY, USA) was used for statistical analysis. Two-sided P values < 0.05 were considered to indicate statistically significant results.
Results
Basic Characteristics
A total of 240 participants were enrolled for eligibility and 232 were randomized to each of the group because 3 patients refused to participate and 5 patients were aged over 65. Finally, 222 patients were incorporated into the study after excluding 10 patients on account of the operation time more than 20 min, 112 in group P and 110 in group C (Fig. 1).
There were no differences between the groups in terms of age, gender, height, weight, BMI, ASA classification, and history of chronic diseases (Table 1).
Table 1.
Basic demographic and medical information of patients
| Group A (n = 112) | Group B (n = 110) | P value | |
|---|---|---|---|
| Age (years) | 49.0 ± 9.7 | 48.0 ± 11.2 | 0.469 |
| Gender (M/F) | 46/66 | 54/56 | 0.281 |
| Height (cm) | 165.2 ± 7.5 | 166.2 ± 9.0 | 0.372 |
| Weight (kg) | 65.1 ± 10.3 | 65.9 ± 12.0 | 0.590 |
| BMI (kg/m2) | 23.8 ± 2.7 | 23.7 ± 2.9 | 0.821 |
| ASA (I/II) | 36/76 | 29/81 | 0.378 |
| Hypotension history, n (%) | 16 (14.29%) | 17 (15.45%) | 0.852 |
| Diabetes history, n (%) | 5 (4.46%) | 5 (4.55%) | 1.000 |
| Coronary heart disease history, n (%) | 2 (1.79%) | 1 (0.91%) | 1.000 |
Data are expressed as means ± SDs or numbers (%) of patients, as appropriate
ASA American Society of Anesthesiologists, BMI body mass index,
Primary Endpoint
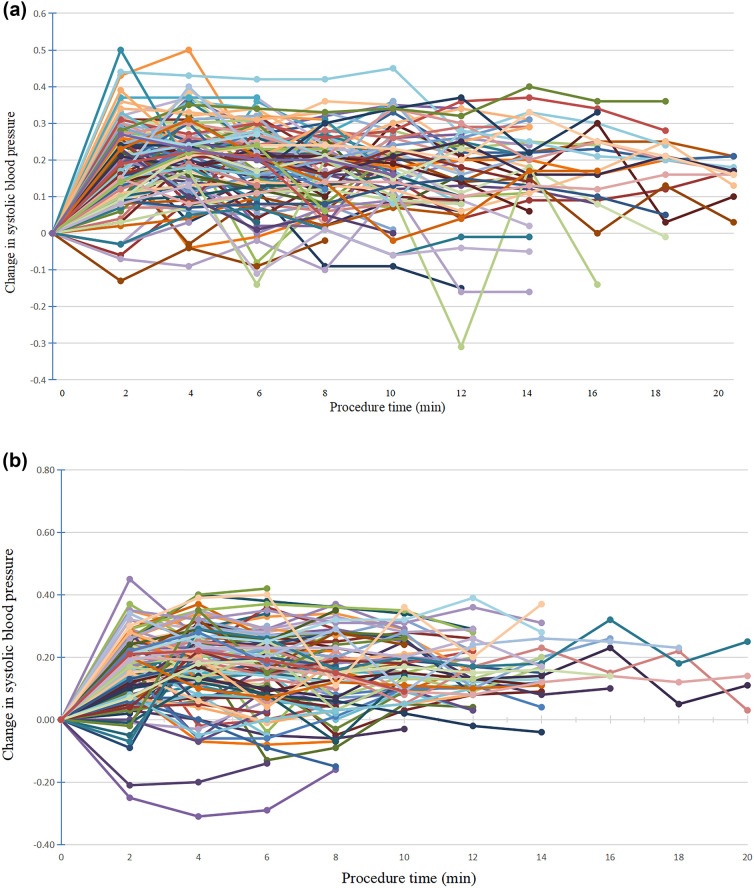
Most patients experienced hypotension throughout the painless colonoscopy. Hypotension and severe hypotension occurred in more patients with intravenous injection of propofol and the dose of norepinephrine treatment was 3.7 μg higher in group P compared with group C. The incidence of hypotension was significantly higher in group P than in group C at T1, T2 and T3 (50.89% vs. 36.36%, P = 0.031; 62.50% vs. 47.27%, P = 0.031; 55.05% vs. 39.62%, P = 0.029; respectively) (Table 2). In addition, the rates of severe hypotension was significantly increased in group P compared with group C at T1 and T2 (36.61% vs. 7.27%, P = 0.039; 23.21% vs. 11.82%, P = 0.034), respectively (Table 3). Changes in systolic blood pressure in group P ranged from 18.18 to 62.50% while systolic blood pressure reduction in group C ranged from 25.00 to 50.00% during the procedure (Fig. 2A, B). 86 (76.79%) and 71 (64.55%) patients experienced no less than one episode of hypotension in group P and group C, respectively.
Table 2.
Patients with hypotension at different time points during the painless colonoscopy procedure
| Group A (%) | Group B (%) | P value | |
|---|---|---|---|
| T1 | 57/112 (50.89) | 40/110 (36.36) | 0.031 |
| T2 | 70/112 (62.50) | 52/110 (47.27) | 0.031 |
| T3 | 60/109 (55.05) | 42/106 (39.62) | 0.029 |
| T4 | 48/97 (49.48) | 32/84 (38.01) | 0.136 |
| T5 | 35/70 (50.00) | 22/61 (36.07) | 0.116 |
| T6 | 26/56 (46.43) | 13/40 (32.50) | 0.208 |
| T7 | 19/38 (50.00) | 7/23 (30.43) | 0.184 |
| T8 | 11/22 (50.00) | 4/8 (50.00) | 1.000 |
| T9 | 11/17 (64.71) | 2/5 (40.00) | 0.274 |
| T10 | 2/11 (18.18) | 1/4 (25.00) | 1.000 |
Proportion of hypotensive patients undergoing painless colonoscopy at different time points
Table 3.
Patients with severe hypotension at different time points during the painless colonoscopy procedure
| Group A (%) | Group B (%) | P value | |
|---|---|---|---|
| T1 | 19/112 (36.61) | 8/110 (7.27) | 0.039 |
| T2 | 26/112 (23.21) | 13/110 (11.82) | 0.034 |
| T3 | 16/109 (14.68) | 9/106 (8.49) | 0.202 |
| T4 | 11/97 (11.34) | 9/84 (10.71) | 1.000 |
| T5 | 11/70 (15.71) | 6/61 (9.84) | 0.436 |
| T6 | 6/56 (10.71) | 2/40 (5.00) | 0.462 |
| T7 | 6/38 (15.79) | 2/23 (8.70) | 0.695 |
| T8 | 5/22 (22.73) | 1/8 (12.50) | 1.000 |
| T9 | 1/17 (5.89) | 0/5 (0.00) | 1.000 |
| T10 | 0/11 (0.00) | 0/4 (0.00) | 1.000 |
Proportion of severe hypotensive patients undergoing painless colonoscopy at different time points
Fig. 2.
A Individual percentage change from baseline in systolic blood pressure for patients in group P during colonoscopy procedure. B Individual percentage change from baseline in systolic blood pressure for patients in group C during colonoscopy procedure
Secondary Endpoints
HRV was analyzed from T1 to T3 on account of the significant difference of hypotension occurred in both groups at T1, T2 and T3. HRV analysis was assessed by root mean square of the successive differences (RMSSD) which was calculated by the successive differences being neighboring RR intervals of the electrocardiogram. RMSSD in both groups decreased from T0, to T2 and slightly increased at T3 as the integral changes in the autonomic nervous system (Fig. 3). The RMSSDs were significantly higher in group C compared with group P at T1 to T3 (38.5 ± 7.2 vs. 36.9 ± 6.3 s, P = 0.090, 35.2 ± 6.9 vs. 32.5 ± 5.9 s, P = 0.002, and 36.2 ± 6.5 vs. 33.9 ± 5.5 s, P = 0.012, respectively).
Fig. 3.
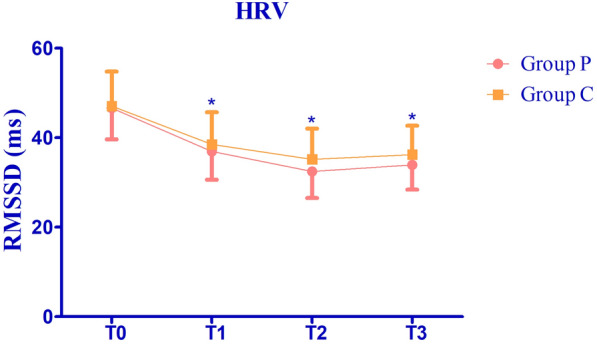
Time domain and RMSSD at different time points of group P and group C. Values are expressed as mean ± SD. *P < 0.05
More patients induced with propofol suffered from injection pain and severe injection pain than patients induced with ciprofol (33.04% vs. 1.82%, P < 0.001, and 18.75% vs. 0.91%, P < 0.001, respectively) (Table 4).
Table 4.
Secondary outcomes
| Group A (n = 112) | Group B (n = 110) | P value | |
|---|---|---|---|
| Injection pain (Y/N) | 37/75 (33.04%) | 2/108 (1.82%) | < 0.001 |
| Severe injection pain (Y/N) | 21/91 (18.75%) | 1/109 (0.91%) | < 0.001 |
| Induction time of MOAA/S ≤ 1 (s) | 58.63 ± 5.59 | 57.43 ± 5.57 | 0.109 |
| Bradycardia (Y/N) | 9/103 (8.04%) | 7/103 (6.36%) | 0.796 |
| Body movement (Y/N) | 25/87 (22.32%) | 17/93 (15.45%) | 0.394 |
| Norepinephrine dose (μg) | 11.66 ± 11.51 | 7.95 ± 8.93 | 0.008 |
| Modified Aldrete score | 9.0 (9.0, 10.0) | 9.0 (9.0, 10.0) | 0.138 |
| Alertness time (min) | 7.16 ± 1.61 | 7.38 ± 1.87 | 0.347 |
| Dizziness (Y/N) | 26/86 (23.21%) | 31/79 (28.18%) | 0.444 |
| Hypoxemia (Y/N) | 4/108 (3.57%) | 2/108 (1.82%) | 0.683 |
| Nausea and vomiting (Y/N) | 5/107 (4.46%) | 7/103 (6.36%) | 0.568 |
| Duration of colonoscopy procedure (min) | 11.61 ± 4.33 | 10.72 ± 3.65 | 0.100 |
| Patient satisfaction | 10.00 (9.00, 10.00) | 10.00 (9.50, 10.00) | < 0.001 |
| Endoscopist satisfaction | 10.00 (9.50, 10.00) | 10.00 (9.88, 10.00) | 0.587 |
Data are expressed as means ± SDs, numbers (%), or median (range interquartile) of patients as appropriate
Induction with propofol or ciprofol showed no significant difference in induction time, defined as time at which MOAA/S score ≤ 1 was achieved) (58.63 ± 5.59 vs. 57.43 ± 5.57 s, P = 0.109) (Table 4).
The rate of body movement was comparable between the groups during the colonoscopy procedure (22.32% vs. 15.45%, P = 0.394) (Table 4).
The dose of norepinephrine needed was higher in group P than in group C because of the higher incidence of hypotension (11.66 ± 11.52 vs. 7.95 ± 8.93 μg, P = 0.008) (Table 4).
The duration of colonoscopy procedure was not significantly different in both groups (11.61 ± 4.33 vs. 10.72 ± 3.65 min, P = 0.100). Modified Aldrete scores when patients were fully alert and time to alertness were comparable for the groups P and C [9.0 (9.0, 10.0) vs. 9.0 (9.0, 10.0), P = 0.138, and 7.16 ± 1.61 vs. 7.38 ± 1.87, P = 0.347, respectively] (Table 4).
Patient satisfaction and endoscopist satisfaction were evaluated when patients left the PACU. While there was no obvious between-group difference in endoscopist satisfaction, there was a higher satisfaction in patient satisfaction [10.00 (9.50, 10.00) vs. 10.00 (9.88, 10.00), P = 0.587, and 10.00 (9.00, 10.00)) vs. 10.00 (9.50, 10.00), P < 0.001, respectively] (Table 4).
No significant differences were observed in drug-related adverse reactions such as bradycardia, hypoxemia, dizziness, nausea, and vomiting (8.04% vs. 6.36%, P = 0.796, 3.57% vs. 1.82%, P = 0.683, 23.21% vs. 28.18%, P = 0.444, and 4.46% vs. 6.36%, P = 0.568, respectively) (Table 4).
Discussion
According to a recent national survey, sedatives are used for gastrointestinal endoscopy at a rate of about 50% in China [3]. However, there is no recommended optimal sedation regimen for painless colonoscopic procedures. Propofol is widely used worldwide for sedation/anesthesia [12]. However, propofol can cause dose-dependent respiratory depression and hemodynamic instability. In addition, the incidence of injection pain caused by propofol sedation is 25–74% [13].
Ciprofol is a new 2,6-dissubstituted phenol derivative and a close analog of propofol [14]. Ciprofol is considered superior to propofol because of the following advantages: (1) higher affinity for γ-aminobutyric acid-A receptor-4–5 times that of propofol; (2) favorable respiratory profile and maintenance of stable cardiac function; and (3) reduced injection pain [8, 9, 15]. Therefore, we compared intravenous induction by 0.4 mg/kg ciprofol with, in terms of hemodynamic stability, induction by 2 mg/kg propofol for sedation in patients undergoing painless colonoscopy.
HRV is a noninvasive indicator that reflects the dynamic balance of nervous system regulation of the heart and blood vessels and indirectly reflects hemodynamic stabilization. Intravenous anesthesia drugs affect the balance of autonomic nerves by acting on the central and autonomic nervous systems of patients, resulting in changes in HRV [16].
Propofol reduces systemic vascular resistance and is associated with perioperative hypotension. Additionally, HRV dynamics changed through propofol sedation and propofol induction was followed by an overall reduction of HRV [17]. HRV decreased further with the further reduction of blood pressure by propofol sedation [18]. Time of RMSSD was used to analyze the depth of anesthesia as well as the reduction of blood pressure.
Up to 46% of patients experienced at least one episode of hypotension during the painless colonoscopy by propofol sedation in an analysis of 380 patients [1]. In our study, almost 51% of patients experienced at least one episode of hypotension after induction by propofol which was similar with previous studies. Although both ciprofol and propofol groups exhibited a decrease in blood pressure, significantly fewer subjects experienced reduced blood pressure in the ciprofol group. In addition, rates of severe hypotension in patients induced with ciprofol were significantly lower than in patients induced with propofol, despite norepinephrine being given at different time points when systolic BP decreased by 20% more than the baseline.
During induction, 6 or 8 mg/kg/h of ciprofol was superior in hemodynamic stability to 40 mg/kg/h of propofol [19]. Similar to our study, intravenous induction with 0.4 mg/kg ciprofol was superior, in terms of hemodynamic stability, to induction with 2 mg/kg propofol for sedation in patients undergoing painless colonoscopy. In addition, time of RMSSD was correspondingly higher in patients induced by ciprofol as the less reduction of blood pressure after induction. However, in Li’s study [10], hypotension just occured in 7.7% and 13.2% of the patients in the colonoscopy in the propofol and ciprofol groups, respectively, as patients received 300–500 ml of sterile 0.9% sodium chloride solution before sedation, and were given 1.5 mg/kg propofol which offered more equilibrium liquid to increase preload and less dose of propofol to inhibit the decrease of peripheral vascular resistance in order to raise blood pressure than patients in our study.
In Zeng’s study, induction and maintenance anesthesia in elective surgical patients by ciprofol produced less drug-related hypotension, indicating that ciprofol was superior in terms of hemodynamic stability than propofol; the results of our study are consistent with these findings [20]. In our study, both ciprofol and propofol groups experienced a significant drop in mean blood pressure after induction anesthesia. However, compared with the ciprofol group, more patients in the propofol group received norepinephrine and experienced longer episodes of low blood pressure, which reduced further to severe low mean blood pressure in a higher proportion of cases after induction sedation. These results indicated that the incidence of out-of-range low blood pressure was significantly attributed to long-term preoperative fasting and fluid deficiency; 200–300 ml of equilibrium fluid was insufficient to improve preoperative fluid loss before sedation. Our research showed that a larger proportion of patients in the propofol group (18.18–64.71%) but no more than 50% patients (25.00–50.00%) in the ciprofol group suffered hypotension during the painless colonoscopy procedure. This was attributed to insufficient liquid capacity, because most patients experienced a long duration of fasting (more than 12 h) and withheld fluids (2 h). Painless colonoscopy was less likely to irritate to the oropharynx and cause reflux aspiration or hypoxemia. Gratifyingly, quite a few patients in both groups needed airway intervention to ensure oxygenation.
Propofol is commonly associated with pain at the injection site, which occurs in as high as 50–70% of cases [10, 21]. Aqueous propofol solutions directly stimulate nerve endings in blood vessel walls or produce substances that lead to injection pain [22]. According to our study, more propofol-induced patients experienced severe pain; ciprofol and propofol both caused injection pain, at a rate of 1.82% and 33.04%, respectively. This difference may be related to emulsion modification, in that ciprofol has a lower free drug concentration in the aqueous phase under the same conditions than propofol.
All patients maintained MOAA/S scores of ≤ 1 during the procedure. Ciprofol exhibited a rapid onset of action and maintenance-similar to that of propofol. The present trial showed a comparable induction time (58.63 ± 5.59 s vs. 57.43 ± 5.57 s, P = 0.109) and time to full alertness (7.16 ± 1.61 min vs. 7.38 ± 1.87 min, P = 0.347) for ciprofol and propofol sedation. These findings are consistent with those of a phase II clinical trial evaluating the efficacy and safety of ciprofol for the induction and maintenance of general anesthesia in patients undergoing elective surgery [20]. However, time to full alertness in the phase III clinical trial for patients undergoing colonoscopy was longer for ciprofol-induced patients [23]. A possible reason for this is that the average time during painless colonoscopy sedation in our study was shorter than their average time for sedation. In regard to the most common drug-related adverse reactions, such as bradycardia, dizziness, nausea, and vomiting, a reduction in the total amount of sedative required as well as in adverse events was observed when 0.05 μg/kg of sufentanil was combined with either ciprofol or propofol sedation, which was similar to the findings of previous studies [3, 24].
When evaluating anesthetics for painless colonoscopies, it is essential to provide a comfortable experience for patients to ensure their compliance and to reduce adverse effects. In previous trials, propofol sedation was associated with high patient satisfaction because of its rapid onset and short duration. To evaluate patient satisfaction and comfort levels after painless colonoscopy with ciprofol, patients were asked to rate their pain perception, comfort, and drug-related adverse reactions using a satisfaction scale from 1 to 10, with higher scores indicating higher satisfaction. A similar satisfaction scale was devised for the endoscopists fluent in the procedure in order to evaluate the effectiveness of the sedatives. In the completed study, the endoscopist perceived no significant difference in satisfaction for both groups as the vast majority of the patients successfully completed the painless colonoscopy procedure without experiencing body movement, which was the most annoying problem. Nevertheless, patients in the ciprofol group reported higher satisfaction scores, mainly resulting from less pain during injection. Modified Aldrete scores were used to assess whether patients could be discharged after the procedure when they were alert; there was no significant difference in modified Aldrete scores and recovery time between the two groups, as both propofol and ciprofol have rapid onset and short duration of action.
Limitations
Two limitations should be noticed in this study. Firstly, considering the confounding factor of patients' history, like hypertension, stratification should be analyzed to further clarify the hemodynamic stability of ciprofol with regards to hypertensive and normotensive populations undergoing painless colonoscopy procedures. In addition, most patients experienced a long time of preoperative fasting and fluid deficiency for more than 8 h before the surgery in fear of anesthesia-related complications, and 200–300 ml of equilibrium liquid was not enough to supplement preoperative fluid loss. So most patients in both groups experienced varying degrees of blood pressure reduction during the procedure. To further verify the effect of ciprofol versus propofol on hemodynamics in patients undergoing painless colonoscopy, fasting time should be shortened for future studies.
Conclusion
In this trial, ciprofol sedation was found to be safe and effective for anesthesia induction during colonoscopy procedures, with a decrease in injection pain and superior hemodynamic stability observed relative to propofol sedation.
Acknowledgements
We are grateful to all the patients participated in the study and the endoscopist involved in the research.
Medical Writing and Editorial Assistance
Charlesworth Author Services provided medical writing for polishing the language of the manuscript and the source of funding for this assistance was provided by the corresponding author.
Author Contributions
Ke Qiang He: Writing—original draft. Ting Ting Huang: Writing-Review & Editing. Meng Yuan Tan: Data Curation. Chen Gao: Formal analysis, Conceptualization. Sheng Wang: Project administration, Funding acquisition.
Funding
The journal’s Rapid Service Fee was funded by the authors. Beijing Kangmeng philanthropic foundations (S063). Xiaoping Science and Technology Development Foundation (CXPJJH1200000-07-113). Health research project of Anhui Province (AHWJ2023BAc20089).
Data Availability Statement
The data associated with the article are not publicly available but are available from the corresponding author, upon reasonable request.
Declarations
Conflict of interest
Ke Qiang He, Ting Ting Huang, Meng Yuan Tan, Chen Gao and Sheng Wang declared no conflicts of interest in this work.
Ethical approval
The study was approved by the Ethics Committee of the First Affiliated Hospital of University of Science and Technology of China (USTC) (2022KY-106) and conducted in accordance with the ethical guidelines of the 1975 revision of the Declaration of Helsinki. It was registered at www.chictr.org.cn (Registration No. ChiCTR2200061814) before patient enrollment.
Footnotes
Ke Qiang He and Ting Ting Huang contributed equally to this work. Chen Gao and Sheng Wang are co-corresponding authors.
Contributor Information
Chen Gao, Email: gaochen8805@126.com.
Sheng Wang, Email: iamsheng2020@ustc.edu.cn.
References
- 1.Sneyd JR, Absalom AR, Barends CRM, et al. Hypotension during propofol sedation for colonoscopy: a retrospective exploratory analysis and meta-analysis. Br J Anaesth. 2022;128(4):610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doi M, Morita K, Takeda J, et al. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–53. [DOI] [PubMed] [Google Scholar]
- 3.Zhou S, Zhu Z, Dai W, et al. National survey on sedation for gastrointestinal endoscopy in 2758 Chinese hospitals. Br J Anaesth. 2021;127(1):56–64. [DOI] [PubMed] [Google Scholar]
- 4.Li DN, Zhao GQ, Su ZB. Propofol target-controlled infusion in anesthesia induction during painless gastroscopy. J Coll Physicians Surg Pak. 2019;29(7):604–7. [DOI] [PubMed] [Google Scholar]
- 5.Desousa KA. Pain on propofol injection: causes and remedies. Indian J Pharmacol. 2016;48(6):617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei Y, Qiu G, Lei B, et al. Oral delivery of propofol with methoxymethylphosphonic acid as the delivery vehicle. J Med Chem. 2017;60(20):8580–90. [DOI] [PubMed] [Google Scholar]
- 7.Luo Z, Tu H, Zhang X, et al. Efficacy and safety of HSK3486 for anesthesia/sedation in patients undergoing fiberoptic bronchoscopy: a multicenter, double-blind, propofol-controlled, randomized, phase 3 study. CNS Drugs. 2022;36(3):301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng Y, Ou M, Wang X, et al. Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: phase IIa and IIb multi-center clinical trials. Eur J Pharm Sci. 2021;164: 105904. [DOI] [PubMed] [Google Scholar]
- 9.Hu C, Ou X, Teng Y, et al. Sedation effects produced by a ciprofol initial infusion or bolus dose followed by continuous maintenance infusion in healthy subjects: a phase 1 trial. Adv Ther. 2021;38(11):5484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Wang X, Liu J, et al. Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: a multi-centre, non-inferiority, randomized, controlled phase 3 clinical trial. Basic Clin Pharmacol Toxicol. 2022;131(2):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin L, Ren L, Wan S, et al. Design, synthesis, and evaluation of novel 2,6-disubstituted phenol derivatives as general anesthetics. J Med Chem. 2017;60(9):3606–17. [DOI] [PubMed] [Google Scholar]
- 12.Walsh CT. Propofol: Milk of Amnesia [published correction appears in Cell. 2022 Dec 8;185(25):4861]. Cell. 2018;175(1):10–13. [DOI] [PubMed]
- 13.Abad-Santos F, Gálvez-Múgica MA, Santos MA, et al. Pharmacokinetics and pharmacodynamics of a single bolus of propofol 2% in healthy volunteers. J Clin Pharmacol. 2003;43(4):397–405. [DOI] [PubMed] [Google Scholar]
- 14.Bian Y, Zhang H, Ma S, et al. Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol. 2021;87(1):93–105. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Yang D, Li Q, et al. Safety, pharmacokinetics, and pharmacodynamics of a single bolus of the γ-aminobutyric acid (GABA) receptor potentiator HSK3486 in Healthy Chinese Elderly and Non-elderly. Front Pharmacol. 2021;12: 735700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson TA, Segaran JR, Toda C, et al. High-frequency heart rate variability index: a prospective, observational trial assessing utility as a marker for the balance between analgesia and nociception under general anesthesia. Anesth Analg. 2020;130(4):1045–53. [DOI] [PubMed] [Google Scholar]
- 17.Mäenpää M, Penttilä J, Laitio T, et al. The effects of surgical levels of sevoflurane and propofol anaesthesia on heart rate variability. Eur J Anaesthesiol. 2007;24(7):626–33. [DOI] [PubMed] [Google Scholar]
- 18.Kanaya N, Hirata N, Kurosawa S, et al. Differential effects of propofol and sevoflurane on heart rate variability. Anesthesiology. 2003;98(1):34–40. [DOI] [PubMed] [Google Scholar]
- 19.Zhong J, Zhang J, Fan Y, et al. Efficacy and safety of Ciprofol for procedural sedation and anesthesia in non-operating room settings. J Clin Anesth. 2023;85: 111047. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Y, Wang DX, Lin ZM, et al. Efficacy and safety of HSK3486 for the induction and maintenance of general anesthesia in elective surgical patients: a multicenter, randomized, open-label, propofol-controlled phase 2 clinical trial. Eur Rev Med Pharmacol Sci. 2022;26(4):1114–24. [DOI] [PubMed] [Google Scholar]
- 21.Jalota L, Kalira V, George E, et al. Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ. 2011;342: d1110. [DOI] [PubMed] [Google Scholar]
- 22.Lu M, Liu J, Wu X, Zhang Z. Ciprofol: a novel alternative to propofol in clinical intravenous anesthesia? Biomed Res Int. 2023;2023:7443226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Wang X, Liu J, et al. Effects of ciprofol for the induction of general anesthesia in patients scheduled for elective surgery compared to propofol: a phase 3, multicenter, randomized, double-blind, comparative study. Eur Rev Med Pharmacol Sci. 2022;26(5):1607–17. [DOI] [PubMed] [Google Scholar]
- 24.Dossa F, Medeiros B, Keng C, et al. Propofol versus midazolam with or without short-acting opioids for sedation in colonoscopy: a systematic review and meta-analysis of safety, satisfaction, and efficiency outcomes. Gastrointest Endosc. 2020;91(5):1015–26 (e7). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with the article are not publicly available but are available from the corresponding author, upon reasonable request.