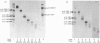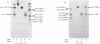Abstract
The CNBr peptides of type I collagen from bone of a patient with lethal osteogenesis imperfecta and age-matched controls were isolated by molecular-sieve chromatography and their amino acid compositions were determined. No differences were found between the compositions of the peptides from the patient and those from the controls, except for an increase in the degree of hydroxylation of lysine in all peptides from the patient. Type I collagen CNBr peptides from chick-embryo skin [Barnes, Constable Morton & Kodicek (1971) Biochem. J. 125, 925--928] and guinea-pig scar tissue [Shuttleworth, Forrest & Jackson (1975) Biochim. Biophys. Acta 379, 207--216] also have an increased degree of hydroxylation of lysine with an otherwise normal amino acid composition, and it was believed that this could be an embryonic form of collagen. As a similar collagen was present in the bones of the patient studied, it seems possible that the same 'embryonic' collagen is synthesized during development, in repair process and also in genetic disorders of collagen metabolism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttinen H., Orava S., Ryhänen L., Kivirikko K. I. Assay of protocollagen lysyl hydroxylase activity in the skin of human subjects and changes in the activity with age. Clin Chim Acta. 1973 Aug 30;47(2):289–294. doi: 10.1016/0009-8981(73)90326-4. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Kodicek E. Bone collagen metabolism in vitamin D deficiency. Biochem J. 1973 Jan;132(1):113–115. doi: 10.1042/bj1320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Royce P. M. Age-related variations in hydroxylation of lysine and proline in collagen. Biochem J. 1974 May;139(2):461–468. doi: 10.1042/bj1390461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Byers P. H. Reduced secretion of structurally abnormal type I procollagen in a form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5142–5146. doi: 10.1073/pnas.78.8.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckmann H., Kresse H., Wollensak J., Buddecke E. Glykosaminoglykan- und Kollagenanalysen bei Osteogenesis imperfecta. Z Kinderheilkd. 1971;110(1):74–84. [PubMed] [Google Scholar]
- Click E. M., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha 1 and alpha 2 chains of human skin collagen. Biochemistry. 1970 Nov 24;9(24):4699–4706. doi: 10.1021/bi00826a012. [DOI] [PubMed] [Google Scholar]
- Eastoe J. E., Martens P., Thomas N. R. The amino-acid composition of human hard tissue collagens in osteogenesis imperfecta and dentinogenesis imperfecta. Calcif Tissue Res. 1973 May 9;12(2):91–100. doi: 10.1007/BF02013724. [DOI] [PubMed] [Google Scholar]
- Glimcher M. J., Shapiro F., Ellis R. D., Eyre D. R. Changes in tissue morphology and collagen composition during the repair of cortical bone in the adult chicken. J Bone Joint Surg Am. 1980 Sep;62(6):964–973. [PubMed] [Google Scholar]
- Heathcote J. G., Dent C. E., Al-Alawi S. J., Heathcote J. G. Letter: Collagen studies in osteogenesis imperfecta. Lancet. 1976 Apr 3;1(7962):756–757. doi: 10.1016/s0140-6736(76)93146-9. [DOI] [PubMed] [Google Scholar]
- Kirsch E., Krieg T., Remberger K., Fendel H., Bruckner P., Müller P. K. Disorder of collagen metabolism in a patient with osteogenesis imperfecta (lethal type): increased degree of hydroxylation of lysine in collagen types I and III. Eur J Clin Invest. 1981 Feb;11(1):39–47. doi: 10.1111/j.1365-2362.1981.tb01763.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Peltonen L., Palotie A., Hayashi T., Prockop D. J. Thermal stability of type I and type III procollagens from normal human fibroblasts and from a patient with osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1980 Jan;77(1):162–166. doi: 10.1073/pnas.77.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen L., Palotie A., Prockop D. J. A defect in the structure of type I procollagen in a patient who had osteogenesis imperfecta: excess mannose in the COOH-terminal propeptide. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6179–6183. doi: 10.1073/pnas.77.10.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth C. A., Forrest L., Jackson D. S. Comparison of the cyanogen bromide peptides of insoluble guinea-pig skin and scar collagen. Biochim Biophys Acta. 1975 Jan 30;379(1):207–216. doi: 10.1016/0005-2795(75)90024-0. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Rubin D., Gross J. Osteogenesis imperfecta congenita: evidence for a generalized molecular disorder of collagen. Lab Invest. 1977 May;36(5):501–508. [PubMed] [Google Scholar]