Abstract
The dairy industry, notorious by generating wastewater rich in organic and nitrogenous content, necessitates sustainable recycling solutions. Biological treatment emerges as a cost-effective and chemical-free alternative. This study delves into the potential of microbial consortium, a microbial consortium, for recycling dairy effluent, aiming at water reclamation and environmental sustainability. Effluent samples from Madurai's Dairy Industry underwent microbial consortium treatment in a recycling prototype, with treatment efficacy assessed through physicochemical parameters and contaminant removal efficiency.
Guided by a biodegradability index of 4.51, the study showcased EM's impact, revealing a notable decrease in pH levels, fostering an alkaline environment (2.35 ± 0.06 ppt). Dissolved oxygen increased significantly to 4.50 ppm, indicating improved aerobic conditions. EM treatment led to substantial reductions in calcium (53 %), magnesium (95 %), nitrogen (22 %), sulfate (79 %), phosphate (86 %), BOD (78 %), and COD (82 %). In contrast, dairy effluent treated without microbial consortium during the sludge activation process exhibited negligible water quality improvement.
These findings underscore microbial consortium efficacy in advancing biological treatment of dairy effluent, demonstrating a significant reduction in contaminants and showcasing its potential for sustainable water reclamation. Improved alkalinity, dissolved oxygen, and nutrient content further signify positive impacts on ecosystem health. Microbial consortium emerges as a promising avenue for recycling dairy effluent, offering an economically viable and environmentally friendly solution. The study emphasizes the crucial role of microbial treatments in achieving efficient water reclamation, contributing to a cleaner and sustainable environment. Future research and broader implementation of microbial consortium in dairy industry wastewater management are recommended for enhanced environmental benefits.
Keywords: Green bioremediation solutions, Progressive biodegradability assessment, Dynamic sludge activation process, Optimal contaminant elimination, Elevated water quality standards
Graphical abstract

Highlights
-
•
Bioremediation selected for dairy effluent with high biodegradability.
-
•
EM Consortium removes ions in raw dairy wastewater effectively.
-
•
Favourable oxidation and mineralization of inorganic compounds observed.
-
•
EM application efficiently purifies and recycles dairy effluent contaminants.
1. Introduction
A substantial and varying milk and dairy products occur worldwide [1], and India is the leading milk producer, with a production of approximately 221.06 million tons [2]. Millions of people in India depend on the dairy sector for their living, which significantly boosts the nation's economy [3], and it is well evident that the stable growth of the industry to meet out the rising domestic and global demands for dairy products [4]. The dairy and milk processing industries, which consume a significant volume of water [5], are crucial commodities. Additionally, these industries also discharge large volumes of effluents, a byproduct of extensive water consumption from various natural sources, including municipal water supplies. Apart from water usage, dairy industries generate a substantial volume of effluents during cleaning, pasteurization, and packaging processes [6,7]. The volume of effluent generated by dairy processing can be 2–10 times greater than the volume of milk processed to obtain milk products [8].
Dairy processing effluent contains high levels of organic matter, nutrients, and pathogens, making it into a cause for concern [9], as environmental pollution occurs through discharge of the dairy effluents. The presence of high concentration of organic substances, Farizoglu et al. [10] and Kaur [11] would be possibly causing significant water pollution effect through eutrophication. More adversely, elevated water temperature, pH alterations, and increased levels of phosphate and nitrate contents [12] in the dairy effluent and hence the dairy effluents are necessarily subjected into proper treatment methods. Discharging dairy effluents without proper treatment methods has been associated with deleterious environmental impacts [13] and poses potential hazards to human health [[14], [15], [16]]. Implementing advanced technologies for wastewater treatment can help to mitigate the negative effects on the environment caused by these processes [17].
To address these environmental challenges, it is imperative to develop robust principles, guidelines, and technologies for dairy effluent management [11]. This is particularly important in the context of sustainable industry development, where proper environmental protection is a key consideration [18]. The adopting effective dairy effluent recycling practices has emerged as a vital component of industrial pollution management systems, aligning with the broader goals of sustainable industry practices.
While recycling and reusing treated effluents can contribute to sustainability efforts, it is crucial to acknowledge the presence of residual contaminants. These contaminants pose challenges to water quality and hinder compliance with environmental and agricultural water reuse standards [19,20]. Therefore, a comprehensive approach to dairy effluent management must address not only treatment processes but also the mitigation of residual contaminants to ensure that the recycled water meets established standards. Hence the development and implementation of suitable dairy effluent recycling practices are essential for effective industrial pollution management. By integrating advanced principles, guidelines, and technologies, the dairy industry can contribute to environmental preservation and sustainable development while meeting water quality standards for both agricultural and environmental reuse.
Aeration coupled with filtration has proven to be effective in wastewater recycling, as demonstrated by Ravish Singh et al. [21]. A myriad of reports detail various recycling and treatment procedures for dairy effluent. Biological treatments, including microbes [22], bioremediation [[23], [24], [25], [26]], phytoremediation [27,28], plant-based coagulants [29], reverse osmosis [30], electrocoagulation [31,32], nanoabsorbents [33], and anaerobic treatment [34], have been explored. Physiochemical methods, which are expensive and lack efficient COD removal [13], pose potential environmental pollution risks when treated water is utilized in crop irrigation [35]. Cost-effective biological wastewater treatment technologies with significant removal of organic substances and nitrogen from dairy effluent have been identified [13,22,36]. Microbial consortia are often made up of a variety of microorganisms naturally oxidizing the pollutants and complex organic compounds present in the contaminated water. The selection and cultivation of certain microbial populations that are unique to the effluents composition can improve the effectiveness of pollutant removal [37]. Therefore, nutrient-rich dairy effluent is converted into ammonium by the activity of microbial consortium has another benefit of making into ammonia-rich biofertilizer and thereby reducing CO2 emissions [38]. Understanding the composition and biochemical properties of wastewater microbiota, along with the optimal metabolic and physico-chemical conditions are crucial for the adoption and to achieve the efficient biological wastewater treatment [39]. Moreover, the use of microorganisms can have the possibility of thriving under certain environmental conditions prevailing in contaminating pollutants and successfully remediate the site [40].
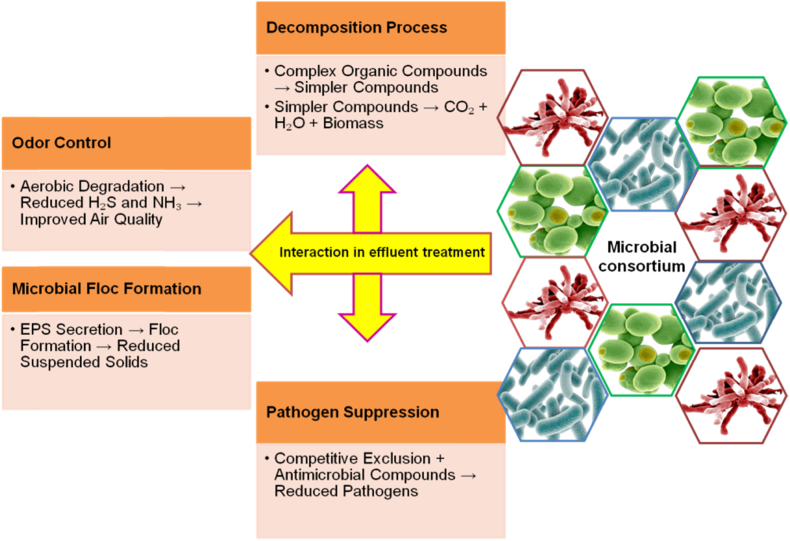
The application of green technologies in wastewater treatment, leading to reduced energy consumption and a minimized ecological footprint, contributes to sustainable water management [17]. Efficient energy transfer within the microbial consortium is facilitated by one group of microbes oxidizing organic matter and producing electron donors, while another group functions as electron acceptors. This process enables more effective degradation of organic matter and transfer of electrons, ultimately resulting in enhanced overall energy production within the consortium. Consequently, the microbes are able to flourish and sustain each other in a symbiotic relationship [41,42]. Hence, commercially available Effective Microorganisms™ (EMs) culture contains beneficial microorganisms including lactic acid bacteria, photosynthetic bacteria, and yeast [43,44]. These microorganisms facilitate the decomposition of complex organic compounds present in food industry effluent into simpler substances. These simpler compounds undergo further degradation into carbon dioxide, water, and biomass, thereby reducing the biological oxygen demand (BOD) and chemical oxygen demand (COD) of the effluent. EM promotes microbial floc formation by secreting extracellular polymeric substances (EPS). These flocs settle more easily, reducing suspended solids in the effluent. EM suppresses pathogenic bacteria in wastewater through competitive exclusion, where beneficial microbes defeat pathogens for resources and produce antimicrobial compounds that inhibit pathogen growth. EM reduce malodorous compounds like hydrogen sulfide and ammonia by promoting aerobic degradation over anaerobic processes, preventing the formation of foul-smelling gases, and improving air quality in wastewater treatment [45] (Plate 1).
Plate 1.
The diagram illustrates the interaction of the microbial consortium with food industry effluent to achieve various treatment objectives.
The application of EMs efficiently performing the environmental remediation [46] at several instances, including the wastewater treatment [47,48], dye removal, and biodegradation of PAHs [49]. EM technology has been demonstrated with encouraging results pertinent to restoring water quality [50,51], treating wastewater [18,52,53], removing pollutants such as phosphorus, total suspended substances, nitrogen, and chemical oxygen demand (COD) [54] and reducing sludge volume [55]. Furthermore, the use of these microbes in domestic and industrial treatments has no adverse effects, making recycled water suitable for safe landfilling and reuses [43,56]. EMs Produce substances that act as antioxidants, inhibit harmful microbes, enhance the growth of beneficial organisms, and detoxify harmful substances [[57], [58], [59]].
Addressing water scarcity and treating wastewater consistently to high quality offers a dependable and easily accessible water supply, particularly in regions facing freshwater shortages [60]. The reuse of recycled greywater, which utilizes biological treatment in cotton crop development, has resulted in significantly better yield and fiber quality [61] and has shown promise in various non-potable uses [62]. Additionally, resource recovery, including nutrient and energy extraction from wastewater, has been successfully implemented [63].
Despite significant advancements in the biological treatment of dairy effluent, there remains a lack of comprehensive understanding of the unique group of microbial consortia for enhanced treatment efficiency. Building on the findings of Lavanya and Kannan [18,63], who highlighted the superior performance of EMs in treating municipal and food industrial effluents compared with other recycling methods, this study aimed to elucidate the efficacy of microbial consortium in the biological treatment of dairy effluent compared that with without addition of the microbial consortium during the sludge activation process. A control group was established to evaluate the baseline performance without the addition of microbial consortium. The control samples underwent the same environmental conditions and operational parameters of temperature, pH, and retention time without addition of EM microbial consortium treatment. This allowed us to determine the effects of microbial consortium by comparing the control group to the microbial consortiumtreated samples in terms of BOD, COD, salinity, conductivity, nutrient reduction, removal of TDS, turbidity, and odor control. The quality of treated water in terms of Removal Efficiency (RE), and treatment efficacy of microbial consortium was further analyzed via ANOVA and Principal Component Analysis (PCA) applied in this experiment. The ultimate goal is to establish microbial consortium as a viable alternative for improving the overall quality of treated dairy effluent.
2. Materials and methods
2.1. Description of the study area and sampling
Tamil Nadu state owned Government's Milk Co-Operative Society (Latitude 9°55′34.1″ and Longitude 78°08′33.7″), South India. Effluent samples were collected directly from the outlet point of the Aavin industry, in Madurai during January and February 2019. Dairy effluent samples were collected in new clean plastic bottles, using a standard collection procedure [64] and stored at 40 °C in the laboratory for the water quality analysis and further recycling experiments.
2.2. Function of microbial consortium
Certified Effective MicroorganismTM, culture, containing a consortium of microbes, including Rhodopseudomonas palustris, Lactobacillus casei, and Saccharomyces cerevisiae, was used to activate the culture. Lactobacillus casei involved in the fermentation of organic matter, producing lactic acid as a byproduct and also contributes to the breakdown of complex carbohydrates and proteins into simpler compounds [65,66], facilitating further degradation by other microorganisms in the consortium. Rhodopseudomonas palustris play a vital role in energy cycling by using light as an energy source for the oxidation of organic and inorganic compounds. They participate in nitrogen fixation, the breakdown of toxic substances, and the degradation of organic pollutants [67]. Photosynthetic bacteria also produce bioactive compounds that promote the growth of other beneficial microorganisms. Saccharomyces cerevisiae contribute to the decomposition of organic material, particularly carbohydrates, through fermentation [68]. They produce ethanol and other metabolic byproducts that can be further utilized by other microorganisms in the consortium. Additionally, yeasts enhance the aggregation of suspended solids and floc formation by secreting extracellular polymeric substances (EPS), which help bind particles together [69]. The reduction in nutrient levels in EM-treated wastewater is attributed to the metabolic activities of photosynthetic bacteria and the bioaccumulation of nutrients by various microorganisms in the consortium. Yeasts and lactic acid bacteria secrete extracellular polymeric substances (EPS), which facilitate the aggregation of suspended solids and bacteria into larger flocs. These flocs are more easily settled in the sedimentation process, reducing the concentration of suspended solids in the effluent. The photosynthetic bacteria and lactic acid bacteria favor aerobic metabolic pathways, which produce CO₂ and water instead of foul-smelling gases. Additionally, lactic acid bacteria can convert sulfur-containing compounds into non-volatile forms, further minimizing odors.
2.3. Microbial consortium culture preparation
The microbial consortium culture was prepared by dissolving 250 g of jaggery in 1 L of distilled water, followed by adding of 150 ml of commercial EM culture into the jaggery solution. The mixture was then transferred into clean plastic bottles and incubated for 5 days, under air-tight condition. The culture was properly maintained by releasing excess gas to be removed from the extended microbial culture prepared container bottles avoiding excessive foaming [51]. Using the extended form of the microbial consortium, Bokashi (Jp: fermented) balls were prepared by mixing them with commercial grade sodium aluminium silicate (zeolite) and rolling them in freshly plucked teak leaves. Then the prepared Bokashi balls were stored in an airtight container, for 7–9 days to facilitate fermentation. These Bokashi balls were used in sludge activation during dairy effluent recycling treatment.
2.4. Recycling treatment prototype
A prototype of the Effluent Treatment (ET) system with a 5 L capacity (Fig. 1) was fabricated, comprising an effluent collection tank, flocculation through mechanical aeration, a settling tank, and a final filtration process for the collection of reclaimed water. The dimensions of the standard treatment plant model, as per Samer [70] and Qasim [71], were used to scale this prototype appropriately.
Fig. 1.
Series of strategic steps designed in the recycling process of dairy effluent, utilizing microbial consortium (EM means beneficial microbial consortium).
In the effluent collection tank, the raw effluent sample was mixed with microbial consortium Bokashi balls, and the subsequent processes were conducted as detailed in previous work [18]. Aeration (5mpa) was performed using a mechanical aerator (1.25L/min) for 6 h, followed by a 12h settling period in the settling tank with retention. Afterwards, the cleaned water at the top of the tank runs through a sand filter for further purification. The treated water was then collected. For the dairy industry effluent sample, a similar procedure was employed, excluding the addition of microbial consortium inoculums for sludge activation. Following the treatment process, the recycled water was collected and stored for physico-chemical analysis.
Effluent samples were collected from different phages of the treatment process viz..
-
a.
Aerator tank
-
b.
Settling down tank
-
c.
Filter bed tank
2.5. Water quality analysis
The physical characteristics and water chemistry were analyzed using the standard procedures APHA, 2012. Temperature, pH, Dissolved Oxygen (DO), Salinity, Electrical Conductivity (EC), Total dissolved solids (TDS), and Turbidity were measured with a Water Analysis Kit (Systronics Model No. 371). Wet chemical analysis was applied to analyze Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), acidity, alkalinity, total hardness, calcium, magnesium, nitrogen, sulfate, and phosphate.
After the effluent recycling process was completed, the removal efficiency (RE) of pollutants from the recycled water samples was used to calculate the rate of degradation of organic and inorganic components.
2.6. Statistical analysis
Descriptive statistics were calculated using SPSS software (version 16.0). To analyze treatment effects between the variables, One-way and Two-way ANOVA methods were applied with significance determined at the P ≤ 0.05 level. Principal Component Analysis (PCA) was carried out using PAST software version 4.03 for a comprehensive examination of the relationships between the analyzed variables.
3. Results and discussion
3.1. Physical quality of treated effluent
The raw effluent samples extracted from the dairy industry exhibited a distinct milky white hue coupled with a potent rancid odor, as outlined in Table 1. This observation aligns with the findings from a prior study by Kushwaha et al. [72] and Birwal et al. [73], affirming the prevalence of these characteristics in such samples. These pronounced attributes can be attributed to the heightened concentration of organic molecules including lipids, proteins, and lactose in the dairy effluent [22], substantiating the need for a comprehensive biological treatment process. Anaerobic treatment [74] potentially reduced the organic load of the dairy effluent. A favourable temperature range between 20 and 30 °C is required to promote the best possible biological processes [75] in the wastewater recycling treatment. A similar temperature range was observed in dairy effluent treated using a microbial consortium (Table 1).
Table 1.
Physico-chemical Quality of Dairy Effluent Before and After Application of Microbial Consortium in Water Recycling.
| Parameters | Raw diary effluent | Without microbial consortium treated effluent | With microbial consortium treated effluent |
|---|---|---|---|
| Colour | Milky white | Pale black | Transparent |
| Odor | Strong rancid | Moderate | Less |
| Temperature | 29.7° C | 29.3°C | 28.1° C |
| TDS/Conductivity ratio | 0.53 | 0.56 | 0.68 |
| Bio-degradability Index | 4.51 | 5.21 | 3.63 |
The Biodegradability Index (BI) provides valuable information for determining the extent to which organic matter in wastewater is readily biodegradable by microorganisms [76]. The BI of the effluent sample stood at 4.51 (Table 1), which is comparable to the BI measurements in various industrial wastewater samples [18,77] and the dairy effluent falls within a range conducive to biological treatment processes. The BI rises when oxidizing agents are used because they tend to decrease the amount of organic pollutants that can be broken down [78]. Hence, dairy effluent treated with a microbial consortium effectively utilized organic pollutants, resulting in a Biodegradability Index (BI) of 3.63, compared to a BI of 5.21 for effluent treated without the microbial consortium (Table 1). The pH of dairy effluent, known for its dynamic variations, particularly in relation to production scale [75], and its level is important for every step of treating dairy effluent [79]. The existence or lack of different ionic substances can affect the effluent pH [80] and found to be effectively regulated in this microbial consortium experiment. Optimal degradation rates were achieved at a pH of 6.5, promoting efficient decomposition of organic compounds and minimizing the environmental impact [81]. The initial alkaline pH of the raw dairy effluent, attributed to the use of alkaline cleaning solutions in the dairy industry [82], was effectively mitigated by the microbial consortium treatment. The application of microbial consortium significantly reduced the alkaline pH from 8.18 ± 0.05 to 7.47 ± 0.01 (Table 2), showcasing its efficacy in modulating the pH. This is in line with the alkalinity reduction achieved through the application of rice husks in coagulant form [83]. Moreover, pH of the dairy effluent significantly influences the permeate quality during nanofiltration [84] and a proper pH management can help prevent fouling and maintain high permeate quality in the treatment process. From this view and the experimental result confirm the effectiveness of the microbial consortium in contaminant removal from dairy effluent. Moreover, the pH level of the sedimentation tank sample is more acidic compared to without microbial consortium treated effluent due to the efficient degradation [85] of lactose content in the dairy effluent by the lactic acid bacteria found in the microbial consortium. A similar degradation study was previously reported by Slavov [13]. With the confirmation of this beneficial effect of microbial consortium treatment, it is affirmed that dairy effluent treated with microbial consortium is suitable for safe landfilling in an eco-friendly manner.
Table 2.
One-way Analysis of Variance for Water Quality in Raw Dairy Industry Effluent, Without and With microbial consortium in Sludge Activation. Values are presented as mean ± SE (n = 3); different letters in each row indicate significant differences (p ≤ 0.05) based on the Student Newman-Keuls Test.
| Without microbial consortium treatment |
With microbial consortium treatment |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Tap water | Raw effluent | Aerator tank effluent | sedimentation tank effluent | Filter effluent | Aerator tank effluent | sedimentation tank effluent | Filter effluent |
| pH | 7.11 ± 0.03 | 6.83 ± 0.02a | 8.33 ± 0.02e | 8.39 ± 0.01e | 8.18 ± 0.05b | 7.73 ± 0.09c | 6.86 ± 0.02a | 7.47 ± 0.01d |
| DO(ppm) | 5.40 ± 0.07 | 3.50 ± 0.3d | 2.53 ± 0.08bc | 2.53 ± 0.10bc | 2.05 ± 0.02a | 2.40 ± 0.07b | 2.90±0c | 4.50±0e |
| Salinity(ppt) | 0.13 ± 0 | 2.35 ± 0.06b | 2.67±0d | 2.66±0d | 2.57 ± 0.01c | 3.02 ± 0.01e | 3.16 ± 0.01f | 0.97 ± 0.01a |
| EC(mS cm−1) | 0.18 ± 0 | 3.25±0d | 2.68 ± 0.02c | 2.71 ± 0.01c | 2.38±0b | 3.75 ± 0.01f | 3.35 ± 0.01e | 1.26±0a |
| TDS(ppm) | 0.09 ± 0 | 1.75±0d | 1.65±0c | 1.65±0c | 1.34±0b | 1.85±0e | 1.92 ± 0.01f | 0.66±0a |
| Turbidity(NTU) | 0.00 ± 0 | 84.67 ± 0.4e | 39.33 ± 1.08d | 27.33 ± 0.4c | 10±0b | 87 ± 0.7f | 26.33 ± 0.4c | 2.07 ± 0.04a |
| Acidity(mg/l) | 0.21 ± 0.01 | 1.10 ± 0.15c | 0.43 ± 0.01b | 0.46 ± 0.01b | 1 ±0a | 1.83 ± 0.08e | 1.39 ± 0.08d | 0.5±0a |
| Alkalinity(mg/l) | 1.07 ± 0.08 | 4.13 ± 0.08d | 3.80 ± 0.14c | 3.73 ± 0.08c | 2.60 ± 0.14b | 5.17 ± 0.04f | 4.67 ± 0.08e | 1.40 ± 0.14a |
| Hardness(mg/l) | 10.00 ± 0 | 52.67 ± 0.40f | 37.67 ± 0.4e | 21.67 ± 0.40c | 13.33 ± 0.8b | 32.33 ± 0.81d | 21±0c | 5.33 ± 0.40a |
| Calcium (mg/l) | 2.38 ± 1.63 | 8.68 ± 0.82b | 12.02±0c | 20.16 ± 0.14d | 48.09±0e | 85.50 ± 1.63f | 84.16±0f | 4.08±0a |
| Magnesium(mg/l) | 4.23 ± 0.09 | 50.43 ± 0.76f | 34.74 ± 0.4e | 16.71 ± 0.40d | 2.31±0b | 11.55 ± 0.70c | 0.54±0a | 2.70 ± 0.09b |
| Nitrogen(mg/l) | 14.66 ± 0 | 102.60±0b | 706.66±0f | 130.66±0c | 906.00±0g | 208.89 ± 5.44e | 165.33 ± 1.22d | 80±0a |
| Sulfate(mg/l) | 5.37 ± 0 | 225.37±0f | 8.95±0a | 111.94±0d | 129.85±0e | 102.98±0c | 101.49±0c | 46.71±0b |
| Phosphate(mg/l) | 10.79 ± 1.3 | 529.12 ± 9.05f | 241.30±0d | 163.00±0c | 508.69±0b | 273.91±0e | 280.60 ± 4.09e | 76.46 ± 2.89a |
| BOD(mg/l) | 2.27 ± 0.04 | 64.3 ± 0.26f | 14.7 ± 0.16a | 15.0 ± 0.07a | 20.7 ± 0.04b | 14.0 ± 0.07a | 18.0±0ab | 14.3 ± 0.04a |
| COD(mg/l) | 80.00 ± 0 | 290±0g | 120±0e | 113.33 ± 2.04d | 108±0b | 140±0f | 110±0c | 52±0a |
The oxidation-reduction process, which is crucial for enhanced pollutant degradation, relies heavily on oxygen levels. The rapid depletion of oxygen in water can be achieved by discharging wastes that have a high oxygen demand [86]. Hence, effluent with lower DO levels has a higher organic matter content [87]. The raw effluent with a notably low DO level of 3.50 ppm, which further decreased after sludge activation without microbial consortium treatment (2.05 ppm). Conversely, the application of microbial consortium during the sludge activation process resulted in an improved DO level of 4.50 ppm (Table 2). This positive impact on DO levels aligns with previous research highlighting the beneficial role of microbial consortium in increasing DO [88], thereby controlling the growth of pathogenic microbes and mitigating unpleasant odors in wastewater [89,90]. The release of effluent that requires oxygen can quickly remove oxygen from natural water resources [91] and this environment may lead to the cause of aquatic organisms to die. Therefore, utilizing microbial consortium in the dairy effluent can help restore the balance and health of water bodies, through improved microbial remediation.
The use of salt in cheese production leads to a significant concentration of salt in the resulting dairy effluent [92]. Due to the low economic value of saline effluents [93], it is essential to perform a proper treatment process with cost-effectively as possible. The salinity levels in dairy effluent, which typically range from 1.2 ppm to 1.6 ppm [94], were unexpectedly higher in both the raw dairy effluent and the samples from the sludge activation process, measuring 2.35 ppt and 2.66 ppt, respectively (Table 2). Similarly, the range of salt content generates in cheese-making process [95] which can be harmful when discharged onto arable lands. The application of the microbial consortium significantly decreased the salinity to 0.97 ppt, surpassing the effectiveness of nanofilter technology in salt and mineral removal from effluent [96]. This reduction in ionic strength was consistent with the higher removal of conductivity observed in the aerobic treatment than in the anaerobic treatment [97].
Conductivity is used to determine the presence of impurities in water and to quantify the ionic components dissolved in it [94]. The raw dairy effluent exhibited a higher conductivity value of 3.25 mS cm−1(Table 2), which decreased to 2.38 mS cm−1 during sludge activation without microbial consortium and further reduced to 1.26 mS cm−1 with microbial consortium application. This reduction in conductivity indicates the effectiveness of microbial consortium in treating dairy wastewater. These findings are in line with previous research involving rice husk slurry [98] microbial fuel cells [99] and electro-coagulation [100] for dairy effluent treatment. However, the high cost of these processes may limit its practical application in large-scale dairy wastewater treatment facilities.
The salinity level's dependence on Total Dissolved Solids (TDS) was evident, with minimal TDS removal observed in sludge activation without microbial consortium treatment (1.34 ppm), contrasting with considerable TDS removal in microbial consortium treated effluent (0.66 ppm). This effect is attributed to the separation of most of the bio-solids by sand filtration after the microbial consortium treated the dairy wastewater and such a result aligns with the similar phytoremediation, primary clarification, and sand-carbon filter [101] effects observed for TDS reduction, which improved water quality [28]. Notably, raw dairy effluent displayed remarkably low TDS and EC ratio. However, after the application of microbial consortium treatment, a substantial increase in the ratio to 0.68 was observed, indicating significant enhancement in the sludge activation process (Table 2). This trend mirrors the findings for treated the cheese industrial effluent [102].
Microorganisms consume organic matter and suspended particles for their growth and survival, which contributes to the effluents decreasing the turbidity levels [27]. The disruption of the association between conductivity and TDS is attributed to the physical conditions of contaminants present in the effluents [102]. The turbidity in the raw dairy effluent was notably high at 84.67 NTU and subsequently decreased reduced to 2.07 NTU following microbial consortium treatment than without microbial consortium (10 NTU) treatment (Table 2). This reduction indicates the efficacy of the microbial consortium treatment in removing both TDS and turbidity from dairy effluent, which is consistent with findings from the use of jute waste rice husks, and bioflocculant [5,103]. Hence, the application of microbial consortium in dairy effluent treatment can help improve water quality by promoting the breakdown of organic pollutants and reducing suspended solids, ultimately leading to clearer effluent discharge.
3.2. Chemical quality of the treated dairy effluent
The acidity of raw effluent was 1.10 mg/l, while the acidity of the sludge activated with and without microbial consortium was 0.46 mg/l and 0.5 mg/l, respectively. The alkalinity level of the raw effluent (4.13 mg/l) could not be reduced using sludge activation performed in the prototype (Table 2). However, treatment with the microbial consortium resulted in a notable reduction, in the alkalinity level to 1.40 mg/l. This consistent state achieved through syntrophic metabolism is a critical aspect of effluent treatment, contributing to enhanced water quality in the recycling treatment process. Hence, the application of the microbial consortium was found to be effective in reducing the alkalinity level (Table 2), further confirming the previous work of Nyaki and Njau [88].
A higher hardness level (52.67 mg/l) was found in the raw effluent, and microbial consortium application significantly reduced the hardness in the aerator and settling tanks, with a considerably lower hardness (5.33 mg/l) than that in the untreated effluent (13.33 mg/l) (Table 2). Therefore, microbial consortium treatment could be considered a much more effective technique than the relatively more expensive and less efficient reverse osmosis process, as shown by Sankar et al. [104]. Therefore, these experimental results indicate the effectiveness of the microbial consortium in significantly reducing of alkalinity and hardness of dairy effluents, resulting in improved water quality.
Calcium levels in dairy effluents ranged from 1.4 to 960 mg/L [105]. The application of the microbial consortium to the dairy effluent in the present study resulted in a significant reduction in calcium and magnesium contents, followed by the raw dairy effluent which had higher values of these contents (Table 2). These experimental results are in accordance with those of Singh et al. [106], who reported efficient removal of calcium from wastewater via bacterial degradation. In addition, higher concentrations of calcium and magnesium in the effluent improve precipitation [107] and bioflocculation activity [108], respectively.
Dairy effluent has largely reduced amounts of nitrogen and phosphate, which are generated from proteinaceous dairy wastes [109], and sanitizers used in post-production cleaning operations [110]. Significantly lower nitrogen content was detected in the dairy effluent samples treated with the microbial consortium (80 mg/L), than in the effluent without microbial consortium (906 mg/L) treatment (Table 2). Nitrogen and phosphorus contents in dairy effluent were effectively reduced by microalgae, especially Chlorella vulgaris [111]. Likewise, the application of aeration and activated sludge processes reduced nitrogen and phosphorus contents due to the utilization of such compounds as food sources for bacterial growth [14]. This indicates that microbial consortium treatment is effective in reducing the nitrogen content of the raw effluent. Therefore, the experimental procedure for the promising technology treatment employed in the recycling of dairy effluent was found to be very effective.
High concentrations of sulfates in wastewater can have laxative effects [112] and pose risks to human health and aquatic ecosystems if released into the environment. The raw effluent had higher sulfate and phosphate contents 225.37 mg/l and 529.12 mg/l, respectively. This value was found to be very high in comparison to the usual range of sulfate levels in dairy effluents [15]. No significant reduction in sulfate and phosphate contents was detected in the effluent treated with sludge activation without microbial consortium (Table 2). Nanotechnology-incorporated bioremediation has been previously reported to effectively reduce phosphate levels in dairy effluent [113]. However, the application of microbial consortium effectively reduced the phosphate content up to 76.46 mg/L (Table 2), which clearly indicates that microbial consortium treatment has the ability to reduce phosphate levels. The Sulfate and phosphate contents in dairy effluent are utilized as food sources for the development of Delftia species [114]. Based on these findings, the use of bioremediation increased the formation of biofilm and decreased the nutrient content, thereby improving the overall treatment effectiveness [115].
The use of bacterial consortia has been shown to reduce the BOD and COD levels of effluent [116]. Similarly, the BOD level in the raw dairy effluent was 64.3 mg/l, and a substantial reduction in the BOD level was found in the microbial consortium treated effluent, compared to that in the effluent without microbial consortium treatment (Table 2). COD depends on organic matter contaminants [117]. The COD reduction was greater in the microbial consortium treated effluent than in the sludge activated without microbial consortium. Likewise, a significant COD reduction was observed when dairy effluent was treated with electro-coagulation [33] and nano–absorbent [31] technologies. However, high electric voltage application requires higher energy inputs; hence, the process has a high cost [118] considering its cost-effectiveness, which would ideally be an effective method for reducing BOD and COD levels (Table 3). Seesuriyachan et al. [119] reported that lactic acid bacteria, a predominately occur in the dairy effluents; significantly contribute to COD reduction levels. Aerobic conditions are employed in this effluent treatment, which has been proven for be more efficient to the degradation of organic contents than anaerobic treatment [120,121].
Table 3.
Two Factor-analysis of water quality parameters in the raw dairy effluent and after the recycling treatment, using with and without the application of microbial consortium (∗∗∗Significant at p ≤ 0.001; ∗∗Significant at p ≤ 0.01 and ∗Significant at p ≤ 0.05 levels).
| Interaction between treatment points |
Raw effluent Vs treated effluent |
Without microbial consortium × With microbial consortium | Error | ||||
|---|---|---|---|---|---|---|---|
| Without microbial consortium | With microbial consortium | RE × without microbial consortium | RE × with microbial consortium | ||||
| Df | F6,21 | F3,12 | F3,12 | F1,6 | F1,6 | F1,6 | F6,21 |
| pH | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | 0.04 |
| DO(ppm) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗ | ∗∗ | ∗∗∗ | 0.03 |
| Salinity(ppt) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗ | ∗∗∗ | ∗∗∗ | 0 |
| Conductivity(mS cm−1) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | 0 |
| TDS(ppm) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | - | ∗∗∗ | 0 |
| Turbidity(NTU) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | 0.62 |
| Acidity(mg/l) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | - | 0.01 |
| Alkalinity(mg/l) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗ | 0.02 |
| Hardness(mg/l) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | 0.57 |
| Calcium (mg/l) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗ | - | 0.96 |
| Magnesium(mg/l) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗ | 0.40 |
| Nitrogen(mg/l) | ∗∗∗ | - | ∗∗∗ | - | ∗ | ∗ | 8.89 |
| Sulfate(mg/l) | ∗ | - | ∗ | - | ∗ | ∗ | 0 |
| Phosphate(mg/l) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗ | ∗∗∗ | ∗∗∗ | 30.62 |
| BOD(mg/l) | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | 0.032 |
| COD(mg/l) | ∗∗∗ | - | ∗ | - | ∗ | ∗ | 1.19 |
3.3. Removal Efficiency (RE) of contaminants from dairy effluent
High calcium and nitrogen contents were detected in the sludge activated without the addition of the microbial consortium (Fig. 2a), and a considerable reduction in nitrogen was detected in the microbial consortium treated samples. The results of this experiment, using microbial consortium containing Lactobacillus, further agreed with the findings of Gil-Pulido et al. [122] and Ahmad et al. [19], who showed that Lactobacillus sp. was effective in significantly removing ammonia from wastewater.
Fig. 2.
Removal Efficiency of Analyzed Physico-Chemical Parameters in Dairy Effluent with and without Microbial Consortium Application.
The application of microbial consortium in dairy effluent treatment resulted in a relatively greater reduction in BOD (78 %) and COD (82 %) than sludge activation without microbial consortium treatment (Fig. 2b). The results of the present study prove that this approach is much more effective than previously reported methods [117]. Similarly, the microbial consortium-treated effluent showed a significant phosphate reduction (86 %) compared with that of sludge activated without the addition of microbial consortium (Fig. 2a and b). A higher rate of sulfate and magnesium removal was noticed in the microbial consortium treated dairy effluent. Similar results were observed in our previous work on wet grinding effluent recycling treatment using an EM consortium [18].
3.4. Factor analysis
Two-way ANOVA interpretation has been extensively applied in water quality assessment studies of freshwater ecosystems [123]. A highly significant difference (P ≤ 0.001) was observed for the microbial consortium treated effluent (Table 3). The treatment efficacy was further confirmed by examining the factors of treatment stage versus all treatment stages, microbial consortium treatment versus stage without microbial consortium treatment, and the interaction between without microbial consortium treated effluent versus microbial consortium treated effluent (Table 3). The analysis can facilitate the recognition of relationships among these factors which might impact the overall water quality.
Nitrogen, sulfate content, and COD were not found to be removed using sludge activation, without microbial consortium application. In contrast, the effluent treated with microbial consortium showed a significant reduction (P ≤ 0.05) (Table 3). These findings underscore the importance of employing microbial consortium treatment for achieving the efficient reduction of nitrogen, sulfate, and COD in wastewater treatment processes. Overall, these results highlight the promising potential of microbial consortium treatment as a sustainable and effective solution for dairy effluent treatment.
3.5. PC analysis of treated water quality
During the experimental period, the recycling of dairy effluent underwent various treatment stages, which were segregated into four principal components (PCs) with total percentages of variance of 35.816 %, 29.017 %, 16.261 %, and 12.99 %. The cumulative percentage of these four PCs ranged from 35.816 % to 94.086 % (Fig. 3).
Fig. 3.
PCA biplots of dairy industry effluent (DE) treated with and without microbial consortium application during sludge activation, accompanied by Varimax with Kaiser Normalization method (EM means beneficial microbial consortium).
The initial principal component captures the most significant variability within the dataset followed by the next component [124]. The five most influential PCs were DO, salinity, conductivity, turbidity, acidity, and hardness. Among them, turbidity and hardness are sensory characteristic indices [125] those are determining the suitability of water for consumption and other uses. Magnesium and COD emerged as the second-strongest loading parameters, with BOD exhibiting the greatest negative influence. The degree of organic pollutants in recycled effluent may be determined using the COD [126]. By maintaining low levels of organic pollutants, reclaimed effluent can be safely utilized for various non-potable applications [127]. Sulfate and nitrogen were identified as the third-strongest parameters, and this pattern of compound segregation aligns with previous findings in water quality assessments [128,129].
Olive mill waste underwent treatment through various biological methods, and the recycling treatment efficiency was assessed using PCA, which is recognized as an effective tool for such analyses [130,131]. The PCA results indicated the presence of a few outliers, suggesting some variability in effluent management (refer to Fig. 3).
These findings imply that dissolved oxygen, salinity, conductivity, turbidity, acidity, and hardness exerted the most significant influence on effluent management treatment efficiency (Fig. 3). Notably, this study revealed that efficient bacteria play a crucial role in mineralizing contaminants and effectively decomposing organic waste.
3.6. Scale and applicability of microbial consortium
The scalability of microbial consortium treatment is promising due to its cost-effective mass production and adaptability to industrial, agricultural, and environmental contexts. However, large-scale application faces challenges such as varying efficacy across different environments, logistical difficulties in distribution and storage, and regulatory hurdles. Additionally, climate, soil types, and local biodiversity can impact performance, requiring region-specific adjustments. To address these challenges, localized customization of the microbial consortium treatment and pilot testing in diverse regions are recommended to optimize performance and ensure environmental compatibility across different geographic areas.
3.7. Long-term efficacy of microbial consortium
The use of microbial consortium is the self-sustaining nature of microbial populations. Over the long term, microbes can adapt to various environmental conditions in wastewater, such as fluctuating nutrient loads, temperature changes, and variations in organic and inorganic pollutants. This adaptability allows EM consortium to maintain their effectiveness even as wastewater composition changes. The economic viability of using microbial consortia, such as EM, in wastewater treatment is based on several key cost-saving advantages. Purchasing EM cultures is relatively affordable compared to the high upfront investments needed for advanced wastewater technologies. These microbial consortia can be integrated into existing systems without significant infrastructure modifications, reducing capital expenditure [132]. One major cost-saving benefit is the reduction in sludge production. Microbial consortia treatment lowers sludge production, reducing the costs associated with transportation and disposal, which can account for up to 50 % of traditional wastewater treatment costs [133] The possible reduction in the need for additional chemical treatments or remediation efforts due to the sustained efficacy of microbial consortium.
This study primarily focuses on the environmental benefits and the effectiveness of microbial consortium in improving water quality, and reducing pollution, conserving water, and promoting sustainability. Cleaner water supports a diverse range of aquatic organisms, including those sensitive to pollution, reducing pollutant stress, and preventing the spread of invasive species. It also minimizes nutrient overload, improves oxygen levels, and preserves ecosystem health. The microbes in the water contribute to nutrient cycling in the soil, enhancing plant growth and resilience. By promoting the reuse of microbial consortium treated water, supports a circular water economy, where water is reused in closed-loop systems instead of being wasted. This leads to more sustainable water management and reduces the environmental footprint of industries and urban areas. It also enhances recreational activities, reduces the cost of treating water for human consumption, and reduces the prevalence of diseases like cholera, dysentery, and typhoid. Improved water quality also boosts economic activities like tourism, fishing, and agriculture, supporting livelihoods that depend on natural resources.
EM consortium also minimizes the need for chemical disinfection treatments, such as chlorination or UV radiation, reducing costs associated with chemicals and energy consumption. Overall, the application of microbial consortium reduced operational cost, energy requirements, and minimal infrastructure needs contribute to more efficient wastewater treatment, enhancing effluent quality without the need for expensive equipment or complex processing. Application of microbial consortium in effluent treatment often highlight its simplicity, low cost, and environmental benefits, making it a viable long-term solution for sustainable water management.
4. Conclusion and future prospects
Our research highlights the effectiveness of the biological treatment method utilizing the microbial consortium as a sustainable and environmentally sound system in the recycling of the dairy effluent. The study demonstrates significant removal of contaminants, including reductions in nitrogen, sulfate, phosphate, BOD, and COD, affirming the efficacy of the microbial consortium treatment over traditional sludge activation processes.
Key findings indicate that the microbial consortium-treated effluent exhibited substantial improvements in water quality parameters. Notably, the microbial consortium treatment resulted in a significant reduction of phosphate and sulfate contents, which are typically challenging to mitigate using conventional methods. Additionally, the microbial consortium treatment improved the DO levels, which is crucial for enhancing the degradation of organic matter and maintaining aerobic conditions favourable for microbial activity.
These outcomes underscore the potential of microbial consortium technology not only to achieve high removal efficiencies for various pollutants but also to offer a cost-effective alternative to more energy-intensive and expensive physico-chemical methods. The bioremediation approach with microbial consortium proves to be a promising solution for the dairy industry, particularly in regions with stringent environmental regulations and sustainability goals.
Future research should concentrate on optimizing EM formulations for various types of wastewater, such as industrial, agricultural, and household wastewater. The application of EM in a range of industries may be expanded for increasing overall efficacy. This may be increased by changing the microbial composition to target specific pollutants or waste streams, such as heavy metals, pharmaceuticals, or emerging contaminants. The adoption of microbial consortium based bioremediation methods presents a viable path forward for sustainable industrial practices. It highlights the importance of continuous scientific inquiry and technological innovation to refine these processes, ensuring their effectiveness and environmental benefits are maximized. As industries strive towards greener practices, the insights gained from this study serve as a beacon for embracing eco-friendly solutions that preserve natural resources and promote a health-conscious environment.
CRediT authorship contribution statement
Lavanya Velmurugan: Writing – original draft, Formal analysis, Data curation. Kannan Dorai Pandian: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Conceptualization.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Ethics approval and consent to participate
Not applicable.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors express their gratitude to the management of Thiagarajar College, Madurai, India, for providing laboratory facilities essential for conducting the experiment and data analysis. Special thanks are extended to the Manager of Aavin Dairy Industry, Madurai, India, for granting permission to collect the effluent samples.
References
- 1.Agrawal V., Achari M., Kharade S., Vadaliya K., Karkar M., Sarode D. Understanding the operations of the Indian Dairy Industry-A case study. Bhartiya Krishi Anusandhan Patrika. 2023;38(1):84–90. doi: 10.18805/BKAP619. [DOI] [Google Scholar]
- 2.Anonymous, Ministry of fisheries, Animal Husbandry, and Dairy Milk Production Report (2022–2023). Chapter 4, pp. 55–70.
- 3.Kumar T.N., Das S., Gulati A. Dairy value chain. Agricultural Value Chains in India. 2022;195 doi: 10.1007/978-981-33-4268-2_6. [DOI] [Google Scholar]
- 4.Bojovic M., McGregor A. A review of megatrends in the global dairy sector: what are the socioecological implications? Agric. Hum. Val. 2023;40:373–394. doi: 10.1007/s10460-022-10338-x. [DOI] [Google Scholar]
- 5.Pathak U., Das P., Banerjee P., Datta S. Treatment of wastewater from a dairy industry using rice husk as adsorbent: treatment efficiency, isotherm, thermodynamics, and kinetics modelling. Journal of Thermodynamics. 2016. 2016 p.7. [DOI]
- 6.Bella K., Rao P.V. Anaerobic digestion of dairy wastewater: effect of different parameters and co-digestion options–a review. Biomass Convers. Biorefin. 2021;13:2527–2552. doi: 10.1007/s13399-020-01247-2. [DOI] [Google Scholar]
- 7.Ganta A., Bashir Y., Das S. Dairy wastewater as a potential feedstock for valuable production with concurrent wastewater treatment through microbial electrochemical technologies. Energies. 2022;15(23):9084. doi: 10.3390/en15239084. [DOI] [Google Scholar]
- 8.Mehrotra R., Trivedi A., Mazumdar S.K. Study on characterisation of Indian dairy wastewater. Int J Eng Appl Sci Technol. 2016;1(11):77–88. https://www.ijeast.com/papers/77-88,Tesma111,IJEAST.pdf [Google Scholar]
- 9.Gaikwad G.L., Wate S.R., Ramteke D.S., Roychoudhury Kunal, K. R Development of microbial consortia for the effective treatment of complex wastewater.5(4)227. 2014. [DOI]
- 10.Farizoglu B., Keskinler B., Yildiz E., Nuhoglu A. Simultaneous removal of C, N, P from cheese whey by jet loop membrane bioreactor (JLMBR) J Hazard Mater.146. 2007:399–407. doi: 10.1016/j.jhazmat.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 11.Kaur N. Different treatment techniques of dairy wastewater. Groundwater for Sustainable Development. 2021;14(100640) doi: 10.1016/j.jwpe.2022.102622. [DOI] [Google Scholar]
- 12.Carvalho F., Prazeres A.R., Rivas J. Cheese whey wastewater: characterization and treatment. Sci. Total Environ. 2013;445–446:385–396. doi: 10.1016/j.scitotenv.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Slavov A.K. Dairy wastewaters–general characteristics and treatment possibilities–a review. Food Technol. Biotechnol. 2017;55(1):14. doi: 10.17113/ftb.55.01.17.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghunath B.V., Punnagaiarasi A., Rajarajan G., Irshad A., Elango A., Mahesh Kumar G. Impact of dairy effluent on environment—a review. Integrated Waste Management in India: Status and Future Prospects for Environmental Sustainability. 2016:239–249. doi: 10.1007/978-3-319-27228-3_22. [DOI] [Google Scholar]
- 15.Nabbou N., Benyagoub E., Mellouk A., et al. Risk assessment for chemical pollution of dairy effluents from a milk processing plant located in Bechar (Southwest of Algeria) Appl. Water Sci. 2020;10(229) doi: 10.1007/s13201-020-01309-w. [DOI] [Google Scholar]
- 16.Stanchev P., Vasilaki V., Egas D., Colon J., Ponsá S., Katsou E. Multilevelm environmental assessment of the anaerobic treatment of dairy processing effluents in the context of circular economy. J. Clean. Prod. 2020;261 doi: 10.1016/j.jclepro.2020.121139. [DOI] [Google Scholar]
- 17.Obaideen K., Shehata N., Sayed E.T., Abdelkareem M.A., Mahmoud M.S., Olabi A.G. The role of wastewater treatment in achieving sustainable development goals (SDGs) and sustainability guideline. Energy Nexus. 2022;7(100112) doi: 10.1016/j.nexus.2022.100112. [DOI] [Google Scholar]
- 18.Lavanya V., Kannan D. Recycling of wet grinding industry effluent using effective microorganismstm (EM) Heliyon. 2023;9(2) doi: 10.1016/j.heliyon.2023.e13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad T., Aadil R.M., Ahmed H., Soares B.C.V., Souza S.L.Q., Pimentel T.C., Scudino H., Guimarães J.T., Esmerino E.A., Freitas M.Q., Almada R.B., Vendramel S.M.R., Silva M.C., Cruz A.G., Rahman U.ur. Treatment and utilization of dairy industrial waste: a review. Trends Food Sci. Technol. 2019;88:361–372. doi: 10.1016/j.tifs.2019.04.003. [DOI] [Google Scholar]
- 20.Kesari K.K., Soni R., Jamal Q.M.S., et al. Wastewater treatment and reuse: a review of its applications and health implications. Water Air Soil Pollut. 2021;232(208) doi: 10.1007/s11270-021-05154-8. [DOI] [Google Scholar]
- 21.Ravish Singh R., Singh M., Singh S.K. Dairy effluent biodegradation by endogenous fungal isolates in the integrated wastewater treatment system. J. Water Chem. Technol. 2022;44:48–55. doi: 10.3103/S1063455X22010076. [DOI] [Google Scholar]
- 22.Porwal H.J., Mane A.V., Velhal S.G. Biodegradation of dairy effluent by using microbial isolates obtained from activated sludge. Water Resour. Ind. 2015;9:1–15. doi: 10.1016/j.wri.2014.11.002. [DOI] [Google Scholar]
- 23.Djelal H., Amrane A. Biodegradation by bioaugmentation of dairy wastewater by fungal consortium on a bioreactor lab-scale and on a pilot-scale. J. Environ. Sci. 2013;25(9):1906–1912. doi: 10.1016/S1001-0742(12)60239-3. [DOI] [PubMed] [Google Scholar]
- 24.Darshini P.P., Sharpudin J. Bioremediation of industrial andmunicipal waste water using bacterial isolates international. Journal ofEngineering Sciences & Research Technology. 2016;5:173–177. doi: 10.5281/zenodo.51015. [DOI] [Google Scholar]
- 25.Punnagaiarasi A., Elango A., Rajarajan G., Prakash S. Bioremediation—a ecosafe approach for dairy effluent treatment. Bioremediation and Sustainable Technologies for Cleaner Environment. 2017:45–50. doi: 10.1007/978-3-319-48439-6_4. [DOI] [Google Scholar]
- 26.Cardoso N.L.L., Silva F.F., Silva A.K.M., Ribeiro J.A.T., Valinhas R., Penido W.D., Gonçalves D.B. Bioremediation of dairy wastewater using bacteria: a panoramic review. Research. Society and Development. 2022;11(7) doi: 10.33448/rsd-v11i7.29830. [DOI] [Google Scholar]
- 27.Schierano M.C., Maine M.A., Panigatti M.C. Dairy farm wastewater treatment using horizontal subsurface flow wetlands with Typha domingensis and different substrates. Environ. Technol. 2017;38(2):192–198. doi: 10.1080/09593330.2016.1231228. [DOI] [PubMed] [Google Scholar]
- 28.Goala M., Yadav K.K., Alam J., Adelodun B., Choi K.S., Cabral-Pinto M.M., Shukla A.K. Phytoremediation of dairy wastewater using Azolla pinnata: application of image processing technique for leaflet growth simulation. J. Water Proc. Eng. 2021;42(102152) doi: 10.1016/j.jwpe.2021.102152. [DOI] [Google Scholar]
- 29.Justina M.D., Muniz B.R.B., Bröring M.M., Costa V.J., Skoronski E. Using vegetable tannin and polyaluminium chloride as coagulants for dairy wastewater treatment: a comparative study, J. Water Proc. Eng. 2018;25:173–181. doi: 10.1016/j.jwpe.2018.08.001. [DOI] [Google Scholar]
- 30.Vourch M., Balannec B., Chaufer B., Dorange G. Treatment of dairy industry wastewater by reverse osmosis for water reuse. Desalination. 2008;219(1–3):190–202. doi: 10.1016/j.desal.2007.05.013. [DOI] [Google Scholar]
- 31.Tchamango S., Nanseu-Njiki C.P., Ngameni E., Hadjiev D., Darchen A. Treatment of dairy effluents by electrocoagulation using aluminium electrodes. Sci. Total Environ. 2010;408(4):947–952. doi: 10.1016/j.scitotenv.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Ankoliya D., Mudgal A., Sinha M.K., Patel V., Patel J. Application of electrocoagulation process for the treatment of dairy wastewater: a mini review. Materials Today: Proceedings. 2023;77:117–124. doi: 10.1016/j.matpr.2022.10.254. [DOI] [Google Scholar]
- 33.Falahati F., Baghdadi M., Aminzadeh B. Treatment of dairy wastewater by graphene oxide nanoadsorbent and sludge separation, using in Situ Sludge Magnetic Impregnation (ISSMI) Pollution. 2018;4(1):29–41. doi: 10.22059/poll.2017.233196.276. [DOI] [Google Scholar]
- 34.Ye M., Sun B., Zhu A., Song L., Ha J., Qin Y., Li Y.Y. Characterization of trace metal impact on organic acid metabolism and functional microbial community in treating dairy processing wastewater with thermophilic anaerobic membrane bioreactor. Bioresour. Technol. 2022;359(127495) doi: 10.1016/j.biortech.2022.127495. [DOI] [PubMed] [Google Scholar]
- 35.Gharbi-Khelifi H., Jmii H., Mosbahi M., Hamdi S., Hamdi R., Brahmi J., et al. Rodríguez E.Á. Microbiological and physicochemical quality enhancement of treated wastewater using raw and chemically modified clays from Sidi Bouzid region, Tunisia. Environ. Res. 2023;239 doi: 10.1016/j.envres.2023.117391. [DOI] [PubMed] [Google Scholar]
- 36.Al-saned A.J.O., Kitafa B.A., Badday A.S. Microbial fuel cells (MFC) in the treatment of dairy wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2021;1067(1) [Google Scholar]
- 37.Kaya C., Ugurlar F., Ashraf M., Hou D., Kirkham M.B., Bolan N. Microbial consortia-mediated arsenic bioremediation in agricultural soils: current status, challenges, and solutions. Sci. Total Environ. 2024;170297 doi: 10.1016/j.scitotenv.2024.170297. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhuri S.R. Green gold from dairy industry: a self-sustained eco-friendly effluent treatment plant. In new advances in the dairy industry. IntechOpen. 2021 doi: 10.5772/intechopen.101254. [DOI] [Google Scholar]
- 39.Janczukowicz W., Zielinski M., Debowski M. Biodegradability evaluation of dairy effluents originated in selected sections of dairy production. Bioresour. Technol. 2008;99(10):4199–4205. doi: 10.1016/j.biortech.2007.08.077. [DOI] [PubMed] [Google Scholar]
- 40.Faria A.F., Liu C., Xie M., Perreault F., Nghiem L.D., Ma J., Elimelech M. Thin-film composite forward osmosis membranes functionalized with graphene oxide–silver nanocomposites for biofouling control. J. Membr. Sci. 2017;525:146–156. doi: 10.1016/j.memsci.2016.10.040. [DOI] [Google Scholar]
- 41.Cavaliere M., Feng S., Soyer O.S., Jiménez J.I. Cooperation in microbial communities and their biotechnological applications. Environ. Microbiol. 2017 Aug;19(8):2949–2963. doi: 10.1111/1462-2920.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu D., Wang W., Yao Y., Li H., Wang Q., Niu B. Microbial interactions within beneficial consortia promote soil health. Sci. Total Environ. 2023;165801 doi: 10.1016/j.scitotenv.2023.165801. [DOI] [PubMed] [Google Scholar]
- 43.Johan S., Jesper M. Antifungal lactic acid bacteria as bio preservatives, Trends Food Sci. Technol. 2005;16(1–3):70–78. doi: 10.1016/j.tifs.2004.02.014. 1–3. [DOI] [Google Scholar]
- 44.Olle M., Williams I.H. Effective microorganisms and their influence on vegetable production — a review. J. Hortic. Sci. Biotechnol. 2015;88(4):380–386. doi: 10.1080/14620316.2013.11512979. [DOI] [Google Scholar]
- 45.Monica S., Karthik L., Mythili S., Sathiavelu A. Formulation of effective microbial consortia and its application for sewage treatment. J. Microb. Biochem. Technol. 2011;3:51–55. doi: 10.4172/1948-5948.1000051. [DOI] [Google Scholar]
- 46.Wdowczyk A., Szymanska-Pulikowska A. Effect of substrates on the potential of Phragmites australis to accumulate and translocate selected contaminants from landfill leachate. Water Resour. Ind. 2023;29(100203) doi: 10.1016/j.wri.2023.100203. [DOI] [Google Scholar]
- 47.Kaur B., Choudhary R., Sharma G., Brar L.K. Sustainable and Effective Microorganisms method for wastewater treatment. Desalination Water Treat. 2024 [Google Scholar]
- 48.Namsivayam S.K.R., Narendrakumar G., Kumar J.A. Evaluation of Effective Microorganism (EM) for treatment of domestic sewage. J. Exp. Sci. 2011;2(7) [Google Scholar]
- 49.Anwar Z.R., Ariffin M., Hassan A., Mahmood I., Khamis A.K. Treatment of rubber processing wastewater by effective microorganisms using anaerobic sequencing batch reactor. J Agrobiotechnology. 2013;4:1–15. https://journal.unisza.edu.my/agrobiotechnology/index.php/agrobiotechnology/article/view/40 [Google Scholar]
- 50.Carles L., Wullschleger S., Joss A., Eggen Rik I.L., Schirmer K., Schuwirth N., Stamm C., Tlili A. Wastewater microorganisms impact microbial diversity and important ecological functions of stream periphyton. Water Res. 2022;225:119119. doi: 10.1016/j.watres.2022.119119. [DOI] [PubMed] [Google Scholar]
- 51.Sindhu Vaishnavi K., Kannan D. Effective microorganisms used in domestic effluent treatment system BALWOIS - ohrid. Republic of Macedonia Europe. 2012:2–9. [Google Scholar]
- 52.Lavanya V., Kannan D. Grey water treatment using effective microorganisms and its impact on water qualities. J. Appl. Sci. 2019;19:188–198. doi: 10.3923/jas.2019.188.198. [DOI] [Google Scholar]
- 53.Kannan D., Lavanya V. Bioremediation of Pickle Industry Effluent Using Effective Microorganisms™ and Method Thereof. The Patent Office Journal No. 38/2022. 2024. https://scholar.google.com/citations?view_op=view_citation&hl=en&user=esDUXdQAAAAJ&citation_for_view=esDUXdQAAAAJ:Y0pCki6q_DkC [Google Scholar]
- 54.Ji M., Zhu J., Zhu R., Chen J., Wang C. Experiments on the effects of dissolved oxygen on the free and immobilization effective microorganisms (EM) in treating polluted river water. Proc Int Conf Mater Environ Eng. 2014;57:5–7. doi: 10.2991/icmaee-14.2014.38. (ICMAEE 2014) [DOI] [Google Scholar]
- 55.Safwat S.M., Matta M.E. Environmental applications of Effective Microorganisms: a review of current knowledge and recommendations for future directions. J. Eng. Appl. Sci. 2021;68(1):48. doi: 10.1186/s44147-021-00049-1. [DOI] [Google Scholar]
- 56.Sharip Z., Razak S.B.A., Noordin N., Yusoff F.M. Application of an effective microorganism product as a cyanobacterial control and water quality improvement measure in Putrajaya Lake, Malaysia. Earth Syst Environ. 2020;4:213–223. doi: 10.1007/s41748-019-00139-4. [DOI] [Google Scholar]
- 57.Kulig A., Barczak R. “Effective microorganisms” (EM) in reducing noxiousness of selected odorant. Environ Prot Eng. 2010;36:13–24. [Google Scholar]
- 58.Mayer J., Scheid S., Widmer F., Fließbach A., Oberholzer H.R. How effective are “Effective microorganisms® (EM)”? Results from a field study in temperate climate. Appl. Soil Ecol. 2010;46:230–239. [Google Scholar]
- 59.Joshi H., Somduttand C.P., Mundra S.L. Role of effective microorganisms (EM) in sustainable agriculture. International Journal of Current Microbiology and Applied Sciences. 2019;8(3):172–181. doi: 10.20546/ijcmas.2019.803.024. [DOI] [Google Scholar]
- 60.Morovati R., Rajabi S., Ghaneian M.T., Dehghani M. Efficiency of Ag3PO4/TiO2 as a heterogeneous catalyst under solar and visible light for humic acid removal from aqueous solution. Heliyon. 2023;9(5) doi: 10.1016/j.heliyon.2023.e15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lavanya V., Rathinabala K., Kannan D. Determination of fiber yield and quality in cotton cultivars, applied with biologically reclaimed greywater. Ind. Crop. Prod. 2023;201:116921. [Google Scholar]
- 62.Bauer S., Wagner M. Possibilities and challenges of wastewater reuse—planning aspects and realized examples. Water. 2022;14(10):1619. doi: 10.3390/w14101619. [DOI] [Google Scholar]
- 63.Hashem M.S., Qi X. Treated wastewater irrigation—a review. Water. 2021;13(11):1527. doi: 10.3390/w13111527. [DOI] [Google Scholar]
- 64.Epa Operating procedure, waste water sampling, Science and Ecosystem support Divission Athens. Georgia. 2013:1–24. [Google Scholar]
- 65.Abedi E., Hashemi S.M.B. Lactic acid production - producing microorganisms and substrates sources-state of art. Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e04974. Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Wu J., Lv M., Shao Z., Hungwe M., Wang J., Bai X., Xie J., Wang Y., Geng W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021 May 12;9:612285. doi: 10.3389/fbioe.2021.612285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phongjarus N., Suvaphat C., Srichai N., Ritchie R.J. Photoheterotrophy of photosynthetic bacteria (Rhodopseudomonas palustris) growing on oil palm and soybean cooking oils. Environ. Technol. Innovat. 2018;10:290–304. doi: 10.1016/j.eti.2018.03.002. [DOI] [Google Scholar]
- 68.Nicula N.O., Lungulescu E.M., Rîmbu G.A., Marinescu V., Corbu V.M., Csutak O. Bioremediation of wastewater using yeast strains: an assessment of contaminant removal efficiency. Int. J. Environ. Res. Publ. Health. 2023;20(6):4795. doi: 10.3390/ijerph20064795. Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang M., Zheng S. Pollutant removal-oriented yeast biomass production from high-organic-strength industrial wastewater: a review. Biomass Bioenergy. 2014;64:356–362. doi: 10.1016/j.biombioe.2014.03.020. [DOI] [Google Scholar]
- 70.Samer M. 2015. Wastewater Treatment Engineering. BoD–Books on Demand. [Google Scholar]
- 71.Qasim S.R. Routledge; 2017. Wastewater Treatment Plants: Planning, Design, and Operation. [Google Scholar]
- 72.Kushwaha J.P., Srivastava V.C., Mall I.D. Organics removal from dairy wastewater by electrochemical treatment and residue disposal. Separ. Purif. Technol. 2010;76(2):198–205. doi: 10.1016/j.seppur.2010.10.008. [DOI] [Google Scholar]
- 73.Birwal P., Deshmukh G., Priyanka S.S., Saurabh S.P. Advanced technologies for dairy effluent treatment. J Food Nutr Popul Health. 2017;1(1):7. https://www.primescholars.com/articles/advanced-technologies-for-dairy-effluent-treatment-94042.html [Google Scholar]
- 74.Demirel B., Yenigun O., Onay T.T. Anaerobic treatment of dairy wastewaters: a review. Process Biochem. 2005;40(8):2583–2595. doi: 10.1016/j.procbio.2004.12.015. [DOI] [Google Scholar]
- 75.Vasina A.I., Basamykina A.N. Local wastewater treatment plant for dairy production: challenges and solutions. IOP Conf. Ser. Earth Environ. Sci. 2022;988(3) doi: 10.1088/1755-1315/988/3/032077. [DOI] [Google Scholar]
- 76.Saravanathamizhan R., Perarasu V.T. Improvement of biodegradability index of industrial wastewater using different pretreatment techniques. Wastewater treatment. 2021:103–136. doi: 10.1016/B978-0-12-821881-5.00006-4. [DOI] [Google Scholar]
- 77.Abdalla K.Z.G., Hammam G. Correlation between biochemical oxygen demand and chemical oxygen demand for various wastewater treatment plants in Egypt to obtain the biodegradability indices. Int. J. Sci. Basic Appl. Res. 2014;13:42–48. https://www.gssrr.org/index.php/JournalOfBasicAndApplied/article/view/1382 [Google Scholar]
- 78.Dahamsheh A., Wedyan M. Evaluation and assessment of performance of Al-Hussein bin Talal University (AHU) wastewater treatment plants. International Journal of Advanced and Applied Sciences. 2017;4(1):84–89. doi: 10.21833/ijaas.2017.01.012. [DOI] [Google Scholar]
- 79.Asha A., Keerthi, Muthukrishnaraj A., et al. Improvement of biodegradability index through electrocoagulation and advanced oxidation process. Int J Ind Chem. 2014;5:4. doi: 10.1007/s40090-014-0004-x. [DOI] [Google Scholar]
- 80.Dinesha B.L., Hiregoudar S., Nidoni U., Ramappa K.T., Dandekar A., Ravi M.V., Sankalpa K.B. Physical properties of influent and effluent samples collected from dairy industry effluent treatment plant. J. Pharmacogn. Phytochem. 2020;9(5):1765–1771. doi: 10.22271/phyto.2020.v9.i5y.12593. [DOI] [Google Scholar]
- 81.Yu H.Q., Fang H.H.P. Acidogenesis of dairy wastewater at various pH levels. Water Sci. Technol. 2002;45(10):201–206. https://pubmed.ncbi.nlm.nih.gov/12188545/ [PubMed] [Google Scholar]
- 82.Verma A., Singh A. Physico-chemical analysis of dairy industrial effluent. International Journal of current microbiology and applied sciences. 2017;6(7):1769–1775. doi: 10.20546/ijcmas.2017.602.213. [DOI] [Google Scholar]
- 83.Foo K.Y., Hameed B.H. A short review of activated carbon assisted electrosorption process: an overview, current stage and future prospects. J. Hazard Mater. 2009;170(2–3):552–559. doi: 10.1016/j.jhazmat.2009.05.057. [DOI] [PubMed] [Google Scholar]
- 84.Luo J., Ding L. Influence of pH on treatment of dairy wastewater by nanofiltration using shear-enhanced filtration system. Desalination. 2011;278(1–3):150–156. doi: 10.1016/j.desal.2011.05.025. [DOI] [Google Scholar]
- 85.Álvarez-Mateos M.P., Pereda Marín J., Carta Escobar F.D.L.Á., Durán-Barrantes M.D.L.M., Guillén Jiménez E. Influence of inoculum and initial pH on dairy effluent biodegradation and mineralization. Chem. Biochem. Eng. Q. 2000;14(3):101–106. http://silverstripe.fkit.hr/cabeq/past-issues/article/685 [Google Scholar]
- 86.Osama A., Patil S.S., Salve K.S. Characterization of dairy wastewater and its effects on environment. World J. Pharmaceut. Res. 2015;4(7) [Google Scholar]
- 87.Tikariha A., Sahu O. Study of characteristics and treatments of dairy industry waste water. Journal of applied & environmental microbiology. 2014;2(1):16–22. doi: 10.12691/jaem-2-1-4. [DOI] [Google Scholar]
- 88.Nyaki A., Njau K. Assessment of dairy wastewater treatment and its potential for biogas production at Tanga fresh limited. Tanzan. J. Sci. 2016;42(1):120–133. https://www.ajol.info/index.php/tjs/article/view/153264 [Google Scholar]
- 89.Headley T., Nivala J., Kassa K., Olsson L., Wallace S., Brix H., et al. Müller R. Escherichia coli removal and internal dynamics in subsurface flow ecotechnologies: effects of design and plants. Ecol. Eng. 2013;61:564–574. doi: 10.1016/j.ecoleng.2013.07.062. [DOI] [Google Scholar]
- 90.Wang F., Chen J., Zhang C., Gao B. Bioresource technology resourceful treatment of cane sugar industry wastewater by Tribonema minus towards the production of valuable biomass. Bioresour. Technol. 2020;316:123902. doi: 10.1016/j.biortech.2020.123902. [DOI] [PubMed] [Google Scholar]
- 91.Kolhe A.S., Pawar V.P. Physico-chemical analysis of effluents from dairy industry. Recent Res. Sci. Technol. 2011;3(5) https://updatepublishing.com/journal/index.php/rrst/article/view/685 [Google Scholar]
- 92.Guinee T.P. Salting and the role of salt in cheese. Int. J. Dairy Technol. 2004;57(2–3):99–109. doi: 10.1111/j.1471-0307.2004.00145.x. [DOI] [Google Scholar]
- 93.Chen G.Q., Gras S.L., Kentish S.E. Eutectic freeze crystallization of saline dairy effluent. Desalination. 2020;480(114349) doi: 10.1016/j.desal.2020.114349. [DOI] [Google Scholar]
- 94.Dhanasekar K., Dinesh R., Naveen K., Sanjay Krsihna S., Ragavendar K. Assessment of Physico-Chemical Characteristics of Wastewater from Dairy industry,” International Journal of Emerging Trends in Engineering Research. 2021;9(5):570–575. doi: 10.30534/ijeter/2021/06952021. [DOI] [Google Scholar]
- 95.Cheng H., Tian G., Liu J. Enhancement of biomass productivity and nutrients removal from pretreated piggery wastewater by mixotrophic cultivation of Desmodesmus sp. CHX1. Desalination Water Treat. 2013;51:7004–7011. doi: 10.1080/19443994.2013.769917. [DOI] [Google Scholar]
- 96.Cheng H., Tian G., Liu J. Enhancement of biomass productivity and nutrients removal from pretreated piggery wastewater by mixotrophic cultivation of Desmodesmus sp. CHX1. Desalination Water Treat. 2013;51:7004–7011. doi: 10.1080/19443994.2013.769917. [DOI] [Google Scholar]
- 97.Arora P. vol. 2017. Central University of Punjab; 2018. Physical, chemical and biological characteristics of water (e content module) pp. 1–16. [Google Scholar]
- 98.Sadon F.N., Ibrahem A.S., Ismail K.N. An overview of rice husk applications and modification techniques in wastewater treatment. J Purity Utility Reaction Environ. 2012;1:308–334. ISSN: 2232-1179. [Google Scholar]
- 99.Banerjee S., Dastidar M.G. Use of jute processing wastes for treatment of wastewater contaminated with dye and other organics. Bioresour. Technol. 2005;96(17):1919–1928. doi: 10.1016/j.biortech.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 100.Qasim W., Mane A.V. Characterization and treatment of selected food industrial effluents by coagulation and adsorption techniques. Water Resour. Ind. 2013;4:1–12. doi: 10.1016/j.wri.2013.09.005. [DOI] [Google Scholar]
- 101.Mittal A. 2011. Biological Wastewater Treatment. Book Chapter, Water Today. [Google Scholar]
- 102.Rusydi A.F. Correlation between conductivity and total dissolved solid in various type of water: Rev. Environ. Earth Sci. 2018;118 doi: 10.1088/1755-1315/118/1/012019. [DOI] [Google Scholar]
- 103.Cosa S., Okoh A. Bioflocculant production by a consortium of two bacterial species and its potential application in industrial wastewater and river water treatment. Pol. J. Environ. Stud. 2014;23(3) [Google Scholar]
- 104.Sarkar B., Chakrabarti P.P., Vijaykumar A., Kale V. Wastewater treatment in dairy industries—possibility of reuse. Desalination. 2006;195(1–3):141–152. doi: 10.1016/j.desal.2005.11.015. [DOI] [Google Scholar]
- 105.Ye M., Li Y.Y. Methanogenic treatment of dairy wastewater: a review of current obstacles and new technological perspectives, Sci. Total Environ. 2023;866(161447) doi: 10.1016/j.scitotenv.2023.161447. [DOI] [PubMed] [Google Scholar]
- 106.Singh S.K., Bansal A., Jha M.K., Dey A. An integrated approach to remove Cr (VI) using immobilized Chlorella minutissima grown in nutrient rich sewage wastewater. Bioresour. Technol. 2012;104:257–265. doi: 10.1016/j.biortech.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 107.Sari Erkan H., Onkal Engin G. A comparative study of waste activated sludge disintegration by electrochemical pretreatment process combined with hydroxyl andsulfate radical based oxidants. J. Environ. Chem. Eng. 2020;8(4):103918. doi: 10.1016/j.jece:2020.103918. [DOI] [Google Scholar]
- 108.Liu M., Lu J., Wei L., Wang K., Zhao J. Magnesium hydroxide coagulation performance and floc properties in treating high pH reactive orange wastewater. Water Sci. Technol. 2015;71(9):1310–1316. doi: 10.2166/wst.2015.083. [DOI] [PubMed] [Google Scholar]
- 109.Kiani H., Azimi Y., Li Y., Mousavi M., Cara F., Mulcahy S., et al. Halim R. Nitrogen and phosphate removal from dairy processing side-streams by monocultures or consortium of microalgae. J. Biotechnol. 2022 doi: 10.1016/j.jbiotec.2022.11.011. 361-1-11. [DOI] [PubMed] [Google Scholar]
- 110.Matta G., Nayak Kumar A., et al. Water quality and planktonic composition of river henwal (India) using comprehensive pollution index and biotic-indices, Trans Indian Natl. Acad. Eng. 2020;5:541–553. doi: 10.1007/s41403-020-00094-x. [DOI] [Google Scholar]
- 111.Choi H.J. Dairy wastewater treatment using microalgae for potential biodiesel application. Environmental Engineering Research. 2016;21(4):393–400. doi: 10.4491/eer.2015.151. [DOI] [Google Scholar]
- 112.Stromberg P.E., Cumpston K.L. Sulfates. 2014. [DOI]
- 113.Salama A.M., Behaery M.S., Elaal A.E.A., Abdelaal A. Influence of cerium oxide nanoparticles on dairy effluent nitrate and phosphate bioremediation. Environ. Monit. Assess. 2022;194(5):326. doi: 10.1007/s10661-022-10003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yarza P., Yilmaz P., Pruesse E., Glöckner F.O., Ludwig W., Schleifer K.H., Whitman W.B., Euzéby J., Amann R., Rosselló-Móra R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014;12:635–645. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- 115.Gupta K., Hazarika S.N., Saikia D., Namsa N.D., Mandal M. One step green synthesis and anti-microbial and anti-biofilm properties of Psidium guajava L. leaf extract-mediated silver nanoparticles. Mater. Lett. 2014;125:67–70. doi: 10.1016/j.matlet.2014.03.134. [DOI] [Google Scholar]
- 116.Gavlak G., Vidal C.M.D.S., Souza K.V.D. Enhancing membrane bioreactors for dairy effluent treatment with a mixed mobile bed application. Water Sci. Technol. 2024;89(11):3035–3046. doi: 10.2166/wst.2024.177. [DOI] [PubMed] [Google Scholar]
- 117.Custodio M., Peñaloza R., Espinoza C., Espinoza W., Mezarina J. Treatment of dairy industry wastewater using bacterial biomass isolated from eutrophic lake sediments for the production of agricultural water. Bioresour. Technol. Rep. 2022;17(100891) doi: 10.1016/j.biteb.2021.100891. [DOI] [Google Scholar]
- 118.Bazrafshan E., Moein H., Kord Mostafapour F., Nakhaie S. Application of electrocoagulation process for dairy wastewater treatment. J. Chem. 2013;2013 doi: 10.1155/2013/640139. [DOI] [Google Scholar]
- 119.Seesuriyachan P., Kuntiya A., Sasaki K., Techapun C. Biocoagulation of dairy wastewater by Lactobacillus casei TISTR 1500 for protein recovery using micro-aerobic sequencing batch reactor (micro-aerobic SBR) Process Biochem. 2009;44:406–411. doi: 10.1016/j.procbio.2008.12.006. [DOI] [Google Scholar]
- 120.Biel-Maeso M., González-González C., Lara-Martín P.A., Corada-Fernández C. Sorption and degradation of contaminants of emerging concern in soils under aerobic and anaerobic conditions. Sci. Total Environ. 2019;666:662–671. doi: 10.1016/j.scitotenv.2019.02.279. [DOI] [PubMed] [Google Scholar]
- 121.Azaroff A., Monperrus M., Miossec C., Gassie C., Guyoneaud R. Microbial degradation of hydrophobic emerging contaminants from marine sediment slurries (Capbreton Canyon) to pure bacterial strain. J. Hazard Mater. 2021;402(123477) doi: 10.1016/j.jhazmat.2020.123477. [DOI] [PubMed] [Google Scholar]
- 122.Gil-Pulido B., Tarpey E., Almeida E.L., Finnegan W., Zhan X., Dobson A.D.W., O'Leary N. Evaluation of dairy processing wastewater biotreatment in an IASBR system: aeration rate impacts on performance and microbial ecology. Biotechnol. Rep. 2018;19 doi: 10.1016/j.btre.2018.e00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deb D., Deshpande V.N., Das K.Ch. Assessment of water quality around surface coal mines using principal component analysis and fuzzy reasoning techniques. Mine Water Environ. 2008;27(3):183–193. doi: 10.1007/s10230-008-0030-z. [DOI] [Google Scholar]
- 124.Hao R.X., Li S.M., Li J.B., Zhang Q.K., Liu F. Water quality assessment for wastewater reclamation using principal component analysis. Journal of Environmental Informatics. 2013;21(1):45–54. doi: 10.3808/jei.201300231. [DOI] [Google Scholar]
- 125.Khan W., Nam J.Y., Woo H., Ryu H., Kim S., Maeng S.K., Kim H.C. A proof of concept study for wastewater reuse using bioelectrochemical processes combined with complementary post-treatment technologies. Environmental science: water research & technology. 2019;5(8):1489–1498. doi: 10.1039/C9EW00358D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deng S., Yan X., Zhu Q., Liao C. The utilization of reclaimed water: possible risks arising from waterborne contaminants. Environ. Pollut. 2019;254(113020) doi: 10.1016/j.envpol.2019.113020. [DOI] [PubMed] [Google Scholar]
- 127.Pujar P.M., Kenchannavar H.H., Kulkarni R.M. Real-time water quality monitoring through Internet of Things and ANOVA-based analysis: a case study on river Krishna. Appl. Water Sci. 2020;10(22) doi: 10.1007/s13201-019-1111-9. [DOI] [Google Scholar]
- 128.Hao R.X., Li S.M., Li J.B., Zhang Q.K., Liu F. Water quality assessment for wastewater reclamation using principal component analysis. Journal of Environmental Informatics. 2013;21(1) doi: 10.3808/jei.201300231. [DOI] [Google Scholar]
- 129.Yang W., Zhao Y., Wang D., Wu H., Lin A., He L. Using principal components analysis and IDW interpolation to determine spatial and temporal changes of surface water quality of Xin’anjiang river in Huangshan, China. Int. J. Environ. Res. Publ. Health. 2020;17(8):2942. doi: 10.3390/ijerph17082942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sahnoun H., Serbaji M., Karray B., Medhioub K. Olive mill waste water management study by using principal component analysis. Int. J. Geosci. 2013;4(2):444–453. doi: 10.4236/ijg.2013.42041. [DOI] [Google Scholar]
- 131.Praus P. Evaluation of biological wastewater treatment process using Mahalanobis distances in original and principal component space: a case study. Appl. Water Sci. 2018;8:167. [Google Scholar]
- 132.Hassaan M.A., El Nemr A. Advanced oxidation processes for textile wastewater treatment. International Journal of Photochemistry and Photobiology. 2017;2(3):85–93. doi: 10.11648/j.ijpp.20170203.13. [DOI] [Google Scholar]
- 133.Cheah W.Y., Show P.L., Yap Y.J., Zaid Mohd, H. F, Lam M.K., Lim J.W., Tao Y. Enhancing microalga Chlorella sorokiniana CY-1 biomass and lipid production in palm oil mill effluent (POME) using novel-designed photobioreactor. Bioengineered. 2019;11(SI2):61–69. doi: 10.1080/21655979.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.