Summary
Transcranial magnetic stimulation (TMS) to early visual cortex modulates the effect of adaptation and eliminates the effect of exogenous (involuntary) attention on contrast sensitivity. Here, we investigated whether adaptation modulates exogenous attention under TMS to V1/V2. Observers performed an orientation discrimination task while attending to one of two stimuli, with or without adaptation. Following an attentional cue, two stimuli were presented in the stimulated region and its contralateral symmetric region. A response cue indicated the stimulus whose orientation observers had to discriminate. Without adaptation, in the distractor-stimulated condition, contrast sensitivity increased at the attended location and decreased at the unattended location via response gain—but these effects were eliminated in the target-stimulated condition. Critically, after adaptation, exogenous attention altered performance similarly in both distractor-stimulated and target-stimulated conditions. These results reveal that (1) adaptation and attention interact in the early visual cortex, and (2) adaptation shields exogenous attention from TMS effects.
Subject areas: Biological sciences, Neuroscience, Cognitive neuroscience
Graphical abstract

Highlights
-
•
Adaptation and exogenous attention interact in the early visual cortex
-
•
TMS to V1-V2 eliminate the effect of exogenous attention without adaptation
-
•
But after adaptation, TMS to V1-V2 does not eliminate the attentional effect
-
•
Causal evidence that adaptation shields exogenous attention from TMS effects
Biological sciences; Neuroscience; Cognitive neuroscience
Introduction
Due to the brain’s limited metabolic resources and the high energy cost of cortical computation, we are unable to process all the information available in the environment. To maximize perceptual performance, energy must be allocated according to task demands. Both visual adaptation and attention help manage the limited energy, optimizing visual processing, and sensitivity.1,2,3 On the one hand, adaptation reduces the visual system’s response to repetitive stimuli while enhancing sensitivity to non-adapted stimulus features4; e.g., prolonged viewing of a stimulus recenters contrast sensitivity away from the adapter. Adaptation reduces sensitivity via contrast gain—the contrast response function (CRF) shifts rightward: higher stimulus contrast is required for observers to reach the same performance than before adaptation (Figure 1A).5,6 On the other hand, covert spatial attention—the selective processing of information at a specific location without shifting our gaze—enhances contrast sensitivity at the attended location and impairs it at unattended locations, via a “push-pull” mechanism.7,8 Because orientation discriminability is contingent upon contrast sensitivity, we discriminate stimulus orientation better when covert attention is allocated to the stimulus location than elsewhere.2
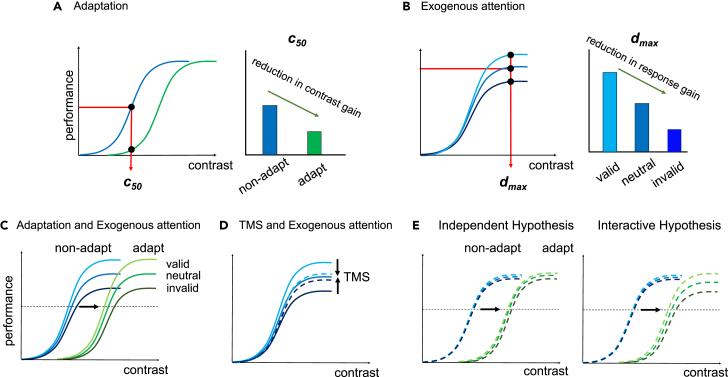
Figure 1.
Effects of adaptation and attention effects on contrast sensitivity
(A) Adaptation reduces contrast gain: The c50 (semi-saturation point) is higher in the adapted than non-adapted condition.5,9,10,11
(B) Exogenous attention modulates performance via response gain: performance at d’ max (asymptote) is highest in the valid, followed by neutral and invalid trials.2,7,9,12,13
(C) Exogenous attention restores contrast sensitivity via response gain even if adaptation depresses overall contrast sensitivity via contrast gain.9
(D) TMS to the target will disrupt the response gain brought by exogenous attention.12
(E) Hypotheses: If the effects of adaptation and attention are independent in the early visual cortex, we should observe that the attentional effect is still eliminated by TMS after adaptation (left panel). Otherwise, the attentional effect will still emerge under the influence of TMS after adaptation (right panel).
There are two types of covert spatial attention: Endogenous attention is voluntary, conceptually driven and sustained; exogenous attention is involuntary, stimulus driven, and transient.2,14 Exogenous attention primarily alters the CRF via response gain: an increase at the attended location and a decrease at the unattended location in the upper asymptote (Figure 1B).7,12 When jointly manipulated, adaptation and attention affect contrast sensitivity: while adaptation decreases contrast sensitivity via contrast gain, exogenous attention still alters sensitivity via response gain (Figure 1C).9
Transcranial magnetic stimulation (TMS) induces a magnetic field that alters the local electric field in the brain,15,16,17 thus enabling inferences regarding the causal role of specific brain areas to perception and cognition.18 Effects of TMS on performance depend on TMS-pulse intensity19 and brain state20: When the initial state of the neuronal population is active, TMS suppresses activity, but when the initial state is suppressed, TMS disinhibits the neuronal population in adaptation,10,21 covert attention,12,22 and presaccadic attention23 studies.
TMS on early visual cortex (V1/V2) extinguishes the benefit and cost of exogenous attention on contrast sensitivity (Figure 1D).12 Observers were instructed to perform an orientation discrimination task while two TMS pulses were applied during stimulus presentation. A response cue indicated the patch whose orientation observers had to discriminate. The response cue either matched—target stimulated—or did not match—distractor stimulated—the stimulated side. When the distractor was stimulated, exogenous attention yielded the typical performance benefit and cost in the valid and invalid cue conditions, respectively, consistent with the exogenous attention effect without TMS.7,9 But when the target was stimulated, all three conditions had similar performance (Figure 1D). By suppressing activity at the attended location and disinhibiting suppressed activity at the unattended location, this study provides evidence for the TMS state-dependent effect.
Adaptation,10,21 exogenous attention,12 and TMS24 each alter activity in early visual cortex. Here, we investigated whether adaptation and attention are independent or interactive by examining if TMS eliminates exogenous attentional effects after adaptation. Were these independent processes, adaptation would not modulate the effect of TMS on exogenous attention—TMS would still extinguish attentional benefits and costs. Were these interactive processes, adaptation would modulate the effect of TMS on exogenous attention (Figure 1E).
Results
We titrated the tilt angle needed to achieve 75% correct discrimination performance and derive the semi-saturation point (c50; Figure 1A) of the CRF for each individual. The asymptote level was set at 80% contrast for all observers (dmax; Figure 1B; see STAR Methods). Thirteen observers discriminated whether a stimulus was tilted counterclockwise or clockwise off vertical at c50 or dmax contrasts. We tested observers’ performance at these two contrasts across attention, adaptation, and TMS conditions to infer contrast gain and response gain mechanisms.
The adaptation and non-adaptation sessions were administered on different days. In the adaptation sessions, observers experienced flickering Gabors (100% contrast) followed by an attentional cue and the stimuli. In the non-adaptation sessions, the procedure was the same but without the flickering Gabors (Figure 2A). Observers received two TMS pulses separated by 50 ms during target presentation (Figure 2B). Many studies have used paired-pulse TMS technique to investigate visual12,22,23,25,26,27,28,29 and motor functions.30,31,32,33 The two pulses are usually separated by 2–200 ms.34,35 The subthreshold pulses can recruit several types of intracortical circuits, including GABAergic (inhibitory) and glutamatergic (excitatory), and whether the pulses are triggering excitatory or inhibitory behavioral responses depends on the current state of neuronal populations (i.e., the state-dependency of TMS). In our task, considering the stimuli duration in an exogenous attention task, we chose 50 ms as the inter-pulse interval, which corresponds to a long interval intracortical inhibition protocol and can effectively interfere with neuronal responses.35,36
Figure 2.
The psychophysics-TMS task
(A) Phosphene mapping: observers were stimulated near the occipital pole before they started the psychophysics-TMS task. They were instructed to draw the perceived phosphene outline using the cursor. This phosphene mapping procedure was repeated at the beginning of every session.
(B) Trial timeline. Two TMS pulses were given during target presentation (separated by 50 ms).
(C) The experimental design: Observers performed the adaptation or non-adaptation blocks in different experimental sessions. In the valid trial, the peripheral cue matched the location of the response cue. In the invalid trial, the peripheral cue mis-matched the location of the response cue. In the neutral trial, the peripheral cues were shown on both sides. In the target-TMS condition (middle panel), the response cue indicated the target in the stimulated region. In the distractor-TMS condition (bottom panel), the response cue indicated the target in the non-stimulated region (and the distractor was stimulated).
We presented the two stimuli in the TMS stimulated region and its symmetric location in the other hemifield; the stimulus was presented for each observer according to their phosphene location (Figure 2C). In half of the trials, the response cue instructed observers to report the orientation of the stimulus at the stimulated region (contralateral to TMS; target-stimulated), and in the other half, the symmetric region (ipsilateral; distractor-stimulated). In each case, the test was either preceded by a valid, invalid, or neutral cue, with equal probability (Figure 2A; see STAR Methods).
Adaptation effects
To examine the adaptation effect on contrast sensitivity, we first assessed performance in the distractor-stimulated, neutral condition (Figure 3)—in which we expected no effect of TMS. A 2 (adaptation, non-adaptation) × 2 (c50, dmax) within-subject analysis of variance (ANOVA) revealed higher performance (d’) in the dmax than c50 conditions [F(1,12) = 95.69, p <0 .001, η2 = 0.89) and an interaction [F(1,12) = 11.81, p = 0.005, η2 = 0.5): Performance was lower at c50 contrast, [t(12) = 4.02, p = 0.002, d = 1.3], but not at the dmax contrast, [t(12) = 2.01, p = 0.068], in the adaptation than non-adaptation conditions. This finding is consistent with adaptation depressing contrast sensitivity primarily via contrast gain.5,9,10,11
Figure 3.
Performance (indexed by d’) in the distractor-stimulated neutral condition
An interaction revealed a lower d’ in the c50 condition but not in the dmax condition. The error bars indicate ±1 SEM. ∗p < 0.01, ∗p < 0.05.
To explore the effects of attention under different adaptation conditions, we conducted a 4-way within-subject ANOVA on attention (valid, neutral, and invalid), adaptation (adapt and non-adapt), TMS (distractor- and target-stimulated), and contrast (c50, dmax). There were main effects of attention [F(2,24) = 10.3, p <0 .001, η2 = 0.46], adaptation [F(1,12) = 23.8, p <0 .001, η2 = 0.66], and contrast [F(1,12) = 184.2, p <0 .001, η2 = 0.94]. There was no 4-way interaction [F(2,24)<1], but there were 3-way interactions among attention, adaptation, and TMS [F(2,24) = 5.9, p = 0.008, η2 = 0.33] and attention, adaptation, and contrast [F(2,24) = 3.96, p = 0.033, η2 = 0.25]. To interpret these 3-way interactions, we assessed the attention effect under TMS for c50 and dmax without and with adaptation.
The attentional effects under TMS without adaptation
Overall, d’ were lower than in the study of Fernández and Carrasco.12 This difference resulted from the tilt and contrast stimulus parameters we used, based on pilot data so that attention and adaptation would have room to increase or decrease performance. We examined the attentional effect without adaptation using within-subjects ANOVAs on attention (valid, neutral, and invalid) and TMS (distractor-TMS and target-TMS). For c50 (Figure 4A), there were no main effects on attention [F(2,24) = 1.91, p = 0.169] or TMS [F(1,12)<1], nor was there an interaction [F(2,24) = 1.75, p = 0.195].
Figure 4.
Performance at each experimental condition
(A–D) Performance as indexed by d’ in the (A) non-adapt c50 condition, (B) non-adapt dmax condition, (C) adapt c50 condition, (D) adapt dmax condition. The error bars within the bar plots depict ±1 SEM (Cousineau corrected) of the condition. The error bars above the bar plots indicate ±1 SEM of the difference between the valid and invalid conditions. ∗∗p < 0.01, ∗p < 0.05.
For dmax (Figure 4B), there was an interaction between attention and TMS [F(2,24) = 4.23, p = 0.027, η2 = 0.26]; there was an attentional effect in the distracter-stimulated condition [t(12) = 3.36, p = 0.006, d = 0.93] but not in the target-stimulated condition [t(12) = 0.18, p = 0.857]. When comparing the target-stimulated condition to the distractor-stimulated condition, the enhancement in the invalid condition (11 out of 13 observers) was more consistent across observers than the suppression in the valid condition (8 out of 13 observers). The finding that TMS eliminated the exogenous attentional effect is consistent with Fernández and Carrasco’s study.12
The attentional effects under TMS with adaptation
For c50 (Figure 4C), there were neither main effects of attention [F(2,24)<1] nor TMS[F(1,12) = 1.21, p = 0.293], nor was there an interaction [F(2,24) = 1.09, p = 0.352]. For dmax (Figure 4D), there was a main effect of attention [F(2,24) = 11.81, p <0 .001, η2 = 0.5], but no effect of TMS [F(1,12) = 2.41, p = 0.146] or its interaction with attention [F(2,24)<1]. This result indicates that after adaptation, TMS did not influence the effect of exogenous attention on contrast sensitivity.
Comparing the attentional effect with and without adaptation
To quantify the overall attentional effects, we calculated the difference in the valid d’ and invalid d’ values. Figure 5 shows the comparison between the attentional effect with adaptation (y axis) and without (x axis) adaptation. In the distractor-stimulated condition, for c50 (Figure 5A), a two-way ANOVA on adaptation and attention revealed a main effect of adaptation [F(1,12) = 25.92, p <0 .001, η2 = 0.68] but not of attention [F(1,12) = 3.08, p = 0.105] nor an interaction [F(1,12) = 1.95, p = 0.187]. For dmax (Figure 5B), there was a main effect of attention [F(1,12) = 14, p = 0.003, η2 = 0.54] but neither an effect of adaptation nor an interaction [both F(1,12)<1]. Most individual data points in the scatterplot are along the diagonal.
Figure 5.
Comparison of the attention effect between adapted and non-adapted conditions
(A–D) The attentional effect in the adapted (y axis) and the non-adapted (x axis) conditions for each observer in (A) distractor-TMS) c50, (B) distractor-TMS dmax, (C) target-TMS c50, (D) target-TMS dmax. The red circle indicates the average across observers and the error bars indicate ±1 SEM of the attentional effect.
In the target-stimulated condition, for c50 (Figure 5C), the two-way ANOVA showed a main effect of adaptation [F(1,12) = 60, p <0 .001, η2 = 0.83] but neither a main effect of attention [F(1,12) = 1.25, p = 0.285] nor an interaction with adaptation [F(1,12)<1]. For dmax (Figure 5D), the two-way ANOVA revealed an interaction [F(1,12) = 9.63, p = 0.009, η2 = 0.45]; there was an attentional effect in the adapted condition [t(12) = 4.73, p <0 .001, d = 1.32] but not in the non-adapted condition [t(12) = 0.18, p = 0.857]. Note that in the scatterplot, most points lie in the upper left diagonal. The scatterplot here provides supporting evidence across observers that after adaptation, TMS did not eliminate the effect of attention on performance.
Discussion
Both adaptation and attention induce relatively short-term plastic changes, prioritizing relevant over irrelevant information, whether related to sensory history or behavioral relevance. These changes facilitate the visual system to manage limited resources and adjust to current environmental demands. In this study, we capitalized on previous findings showing that TMS,24,37 exogenous covert attention,12 and adaptation,10,21 each alter brain state in early visual cortex. Here, to investigate the relation between adaptation and attention in the early visual cortex, we manipulated brain state through visual adaptation and attention in a psychophysical experiment while applying TMS.
In the distractor-stimulated condition, in which we expected no effect of TMS, we demonstrated (1) a contrast gain effect of adaptation (Figure 3) and (2) a response gain effect of exogenous attention (Figure 4B). These findings are consistent with those of an adaptation and attention study without neurostimulation.9 The phosphene mapping procedure we used is like that in previous studies.12,22,23,27,28,38,39,40 TMS-induced phosphenes are confined to the contralateral visual hemifield. Thus, the distractor-stimulated condition was an ideal control condition (see STAR Methods), and the target-stimulated condition was the only one in which we expected TMS to disrupt target processing.
In the target-stimulated condition: (1) TMS to early visual cortex eliminated the exogenous attentional effect on contrast sensitivity (Figure 4B), replicating the study of Fernández and Carrasco12; and (2) adaptation eliminated the effect of TMS on attention (Figure 4D). These findings reveal that adaptation and attention interacted in the early visual cortex: by altering the brain state, adaptation enabled exogenous attention to exert its effects on performance and prevented it from being eliminated by TMS.
In the distractor-stimulated conditions, we observed typical adaptation and attentional effects, further indicating that it served as an ideal control condition. Specifically, adaptation shifted the CRF toward the 100%-contrast adapter via contrast gain5,9,10,11,41,42 (Figure 1A). Additionally, exogenous attention multiplicatively enhanced neuronal firing rate as a function of contrast via response gain7,9,13,43,44 (Figure 1B). Specifically, in the distractor-stimulated condition, higher dmax was observed in the valid than invalid trials2,7,9,12,13,45 in both non-adapted and adapted conditions (Figures 4B and 4D). The fact that attention can modify perception after adaptation indicates that adaptation is not merely a by-product of neuronal fatigue; instead, attention can reset the system in a dynamic fashion.9,46,47
TMS induces a magnetic field to alter the neuronal activity15,16,17,48,49,50,51,52 and has been widely used to examine the causal or critical role of different brain regions for distinct perceptual and cognitive processes, such as motion perception,29,53 face perception,54,55 visual awareness,56,57 multisensory information,58,59 working memory,60 and cognitive control.61,62 The effects of TMS on human cortex are state dependent: TMS suppresses the excitatory activity, leading to a performance decrement, and the inhibitory activity (i.e., disinhibition), leading to a performance enhancement.10,12,20,21,22,23,37,63,64,65
In the current study, when attention was deployed to the target location, neural processing was enhanced at that location and depressed elsewhere. Thus, TMS eliminated the benefits in the valid condition while restoring the cost in the invalid condition, specifically, in the target-stimulated condition without adaptation (Figure 4B). Thus, we replicated the extinction of exogenous attention’s effects on performance reported in Fernández and Carrasco,12 confirming that the early visual cortex plays a causal role in the effect of exogenous attention on contrast sensitivity.
In the target-stimulated condition after adaptation, however, TMS did not eliminate the effect of exogenous attention. TMS neither decreased the performance in the valid trials nor did it improve performance in the invalid trials (Figure 4D). We see two possible, non-mutually exclusive ways in which adaptation may have shielded attention from the effect of TMS.
The first one relates to noise. On the one hand, TMS inserts noise into neuronal response and interrupts processing.66,67,68 On the other hand, adaptation could reduce noise in perceptual processing,69,70,71 as it improves coding efficiency by diminishing redundancy in sensory signals, possibly by decorrelating neuronal responses.72,73,74 Thus, we speculate that the enhancement and noise reduction brought by TMS and adaptation, respectively, could cancel each other out while stimuli are being processed, thereby preserving the attentional effect.
The second explanation relates to normalization. Normalization is a “canonical” computation in which a neuron’s response is modulated (normalized) by the pooled activity of other neurons. The normalization model was developed to explain responses in V1 and also operates in other regions of the visual system.75 According to a prominent normalization model of attention,43,76,77 which has received empirical support,43,76,77,78 attention enhances the gain of neuronal responses before normalization. Observed changes in sensory processes and attentional modulation10,12,20,21,22,23,24,27,28,37,38,39,40,79,80 provide indirect evidence that TMS can influence neural circuits by altering gain control and normalization mechanisms. Adaptation, which decreases the gain of neuronal responses, also modulates the normalization process.47,81 Based on the proposal that when the adaptation precedes the stimulus, adaptation weakens the normalization process,81 we speculate that a weakened normalization process could have protected the effect of attention from being abolished by TMS. Future experimental and modeling work can explore this possibility. By simulating the dynamics of neural circuits, including excitatory and inhibitory interactions that underpin normalization, computational models can help elucidate the potential mechanisms by which TMS might alter normalization processes in the brain.
We note that our findings are not consistent with an alternative noise induction account in combination with stochastic resonance, which predicts that TMS affects responses as a function of TMS and stimulus intensity.82,83 According to this proposal, if TMS effects became increasingly smaller as stimulus signal strength increases, one would expect stronger facilitation in the representation for threshold-level stimuli than for aforementioned threshold stimuli. Had a stochastic resonance effect influenced neuronal population, we should have observed a behavioral pattern akin to a low-level noise induction, which would have led to an improvement in the representations of stimuli just below threshold (c50) but a reduction in the representations of stimuli above threshold (dmax)10 in the non-adapted condition. Instead, we observed suppression and excitation in the valid and invalid conditions, respectively, for the response gain (dmax) in the non-adapted exogenous attention condition but not in the adapted condition. We thus conclude that it is unlikely that the effects observed in the present study are due to a stochastic resonance effect of TMS.
Adaptation reduced contrast gain (i.e., the performance at c505,9,10,11,41; Figure 3); however, adaptation can also suppress neural activity47,84,85 and behavior86 at higher contrasts. By using adaptation, attention, and TMS simultaneously to alter the brain state, we provide evidence that adaptation and attention interact in the early visual cortex. This interaction could indicate that similar neuronal populations or similar mechanisms within different neural populations in early visual cortex underlie each effect.
By altering neural activity in V1/V2 with TMS, we reveal that the effect of exogenous attention, otherwise eliminated by TMS, was preserved by adaptation. This interaction between adaptation and exogenous attention provides a possible neural correlate for the psychophysical interaction in texture segmentation, where observers’ adaptation to high spatial frequencies eliminated the effect of exogenous attention at central locations,87 as this task is also supported by the early visual cortex.14,88,89,90,91 But adaptation and attention do not always interact behaviorally; they have independent effects on contrast sensitivity—observers’ adaptation to a Gabor did not modulate the beneficial effect of exogenous attention at the attended location and its cost at the unattended location9–and perceived speed—the effect of attention on perceived speed did not vary with adapter speed.46
To conclude, we used a psychophysics-TMS protocol to investigate how adaptation modulates the effect of exogenous attention in the early visual cortex. We replicated both the typical contrast gain of adaptation and response gain of exogenous attention. Importantly, the extinction of exogenous attention effects on contrast sensitivity occurred when the target was disrupted by the TMS in the non-adaptation condition but not in the adaptation condition. Thus, adaptation shielded the attentional effect from disruption by TMS. This finding indicates that these two mechanisms, crucial for managing limited resources, interact at the initial level of cortical processing of visual information, possibly through similar neuronal populations.
Limitation of the study
It has been reported that TMS impairs performance without adaptation but restores performance after adaptation.10,21,65 The following non-mutually exclusive factors may underlie why we did not observe these effects (Figure 4C): first, performance after adaptation at the c50 contrast was low. We titrated performance at ∼75% accuracy for c50 in the non-adapted, neutral condition and tested it in the adapted condition, where the accuracy for neutral trials was 51.15% in distractor-stimulated and 56.25% in target-stimulated condition, in line with 53% accuracy for the same condition in Perini et al.10 It is likely that neural activity may have reached a floor level and could not be reactivated. Second, the protocols differed: We stimulated one of the hemispheres during the task with two pulses and the intensity ranged from 49% to 65%. Perini et al.10 gave a single pulse at the center of the occipital pole with an intensity around 70%–80%, and the intensity of the TMS pulses can also influence the state-dependency of the TMS effect.19 Third, instead of the no-TMS condition in Perini et al.,10 we used a distractor-stimulated condition. Future studies could systematically examine how the protocol may influence the effects of TMS on cortical excitability and adaptation’s perceptual consequences.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Hsing-Hao Lee (hsinghaolee@nyu.edu).
Materials availability
This study did not generate new specimens or materials.
Data and code availability
-
•
The behavioral data have been uploaded the OSF database and are publicly available.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
This research was supported by NIH NEI R01-EY01 9693 to MC, NIH NINDS Grant F99-NS-120705 to AF, and the Ministry of Education in Taiwan to HHL. We thank current and former Carrasco Lab members, especially Ian Donovan, Laura Dugué, Nina Hanning and Shutian Xue, for their helpful comments.
Author contributions
H.H.L., A.F., and M.C. designed research and interpreted the data; H.H.L. performed research, analyzed data, and drafted the paper; A.F. and M.C. guided and supervised the project; M.C. conceptualized the study, supervised and edited the writing of the paper, and provided funding.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Behavioral data of the 14 observers | This study | https://osf.io/39ey6/ |
| Software and algorithms | ||
| R | Team, R.D.C.92 | https://www.r-project.org/; RRID:SCR_001905 |
| MATLAB R2019a | Mathworks, Natick, MA, USA | http://www.mathworks.com/products/matlab/; RRID:SCR_001622 |
| Psychophysics toolbox | Brainard,93 Pelli94 | http://psychtoolbox.org/; RRID:SCR_002881 |
| Palamedes toolbox | Prins and Kingdom95 | https://www.palamedestoolbox.org/; RRID:SCR_006521 |
| Other | ||
| Eyelink 1000 eye tracker | SR Research Ltd., Ontario, Canada | http://www.sr-research.com; RRID:SCR_009602 |
| Brainsight | Rogue Research | https://www.rogue-research.com/tms/brainsight-tms/; RRID: SCR_009539 |
| Magstim Rapid Plus stimulator (3.5T) | Plymouth, MN, USA | https://www.magstim.com/row-en/product/rapid-family/ |
| MagVenture MagPro X100 stimulator | MagVenture, Farum, Denmark | https://magventure.com/products/magpro-x100/ |
Experimental model and study participant details
Thirteen observers (6 males, 7 females, including author HHL) participated in 4 experimental sessions, which is higher than the typical number of observers in previous TMS studies in visual perception.12,21,23,38,39,40,63 We conducted bootstrapping based on our pilot study (n=3) to calculate the required sample size. With 10,000 iterations, bootstrapping results indicated that we would need 10 observers to reach a 3-way interaction among adaptation, attention, and TMS (power=90%). All observers were naïve to the purpose of the experiment and provided informed consent before participating in the experiment. All observers were free from neurological disorders and had normal or corrected-to-normal vision. This study followed the protocol of the safety guidelines for TMS research and was approved by the University Committee on Activities Involving Human Subjects at New York University (study number: i14-00788_CR11).
Method details
Apparatus
The stimuli were presented on a gamma calibrated ViewPixx LCD monitor with 120 Hz refresh rate and 1920 × 1080 resolution. EyeLink 1000 (Eyelink SR) was used to monitor observers’ gaze (right eye) to make sure that observers were fixating at the fixation cross throughout the task and ensure that we were measuring a covert attentional effect. If observers moved their eyes (deviation > 1 dva) or blinked during the trial, the trial would stop and be repeated at the end of the block.
Stimuli
The stimuli were generated using MATLAB (MathWorks, Natick, MA) and the Psychophysics toolbox.93,94 The fixation cross consisted of two perpendicular lines (length=0.25 degree; width=0.06 degree) at the center of the screen. The Gabor patches (2 cpd) were presented on the left and right visual field, and the position was matched to the center of the reported phosphene by each observer [range: 4.24 – 14.34 dva away from the center]. The size of the Gabors were adjusted according to the cortical magnification factor96: M = M0(1+0.42E+0.000055E3)-1. The attentional cues consisted of four solid black dots (0.1 dva wide), which surround the two Gabors (1 dva from the Gabor’s edge, 2 above/below, 2 left/right).
Transcranial magnetic stimulation and phosphene mapping
The TMS pulses were given by a 70 mm figure-of-eight coil positioned at the occipital cortex with a Magstim Rapid Plus stimulator (3.5T) and triggered with MATLAB Arduino board. (Three observers received the pulses from an MCF-B70 coil with a MagVenture MagPro X100 stimulator instead). Stimulation intensity was the same throughout the experimental sessions for each observer and determined by the individual’s phosphene threshold (for 10 observers, 58%–65% of maximum Magstim stimulator output, mean = 61.3%, SD = 2.36%, and for the other 3 observers, 49%–58% of maximum MagVenture stimulator output, mean = 52%, SD = 5.2%, equipment was updated at NYU-TMS facility).
The phosphene mapping procedure was as the one used in previous studies.12,22,23,27,28,38,39,40 Observers were seated 57 cm from the monitor in a dark room and were instructed to fixate at a dark-blue fixation at the center of a black background. A train of seven TMS pulses at 30 Hz and 65% intensity of the maximum output was applied on the occipital area of the scalp. Observers were instructed to draw the outline of the perceived phosphene on the screen using the mouse and the coil location was recorded accordingly. The center of the phosphene drawing was used as the coordinates of the Gabor’s location in the psychophysics task, where one Gabor was presented in the phosphene region (i.e., the stimulated region), and the other was presented in the symmetric region in the other hemifield (Figure 2A). The phosphene threshold was determined by two pulses spaced 50 ms apart at the same coil location. The intensity of the TMS pulse was adjusted accordingly until observers reported seeing phosphenes 50% of the time. The same phosphene mapping procedure was administered at the beginning of each session. The observer’s head was calibrated to match Brainsight Neuronavigation software’s 3D head template, which ensured that the stimulation was given to the same location with the millimeter level of precision. The anatomical coordinate of the coil position was saved using Brainsight Neuronavigation software. The stimulating coordinates and the intensity used for each observer are reported in the supplemental information.
Psychophysics-TMS task
After the phosphene mapping and before performing the psychophysics-TMS task in each session, we assessed the semi-saturation point and the asymptote of the CRF (i.e., the c50 and dmax) by titrating the tilt angle and the contrast level for each observer (Figures 1A and 1B). They participated in thresholding tasks without adaptation and attention manipulation. We first conducted an adaptive staircase procedure to determine the tilt level (0.5°–6° relative to vertical) that corresponds to approximately 75% orientation discrimination accuracy when the Gabor patches were presented with 80% contrast using the Palamedes toolbox.95 Then, using the tilt level obtained from this tilt staircase task, we conducted a contrast staircase and varied the contrast of the Gabor from 5% to 30% to again achieved approximately 75% accuracy using the same toolbox to derive the semi-saturation point (c50; Figure 1A) of the CRF for each individual. The dmax contrast was fixed at 80% (Figure 1B) based on pilot data and previous studies with similar stimulus parameters.12,13,22,97 The orientations of the left and right Gabors were independent of each other. We tested observers’ performance at these two contrasts across attention, adaptation, and TMS conditions to infer contrast gain and response gain mechanisms.
For the psychophysics-TMS task, Figure 2B shows the timeline and Figure 2C shows the experimental procedure. The adaptation and non-adaptation sessions were administered on different days to ensure that the adaptation effect did not carry-over to other conditions. The order of the adaptation and non-adaptation sessions was counterbalanced between observers.
In the adaptation blocks, observers were adapted to two cortically-magnified 100%-contrast Gabor patches (2cpd) on a mid-gray background flickering for 60 seconds in a counter phase manner at 10 Hz at the beginning of each block, followed by 2 seconds of top-up before each trial started (Figure 2C). This top-up was applied to ensure that the adaptation continued throughout the block. In the non-adaptation blocks, the procedure was the same but without the flickering Gabors; instead, a mid-gray screen was presented for 20 seconds followed by 2 seconds of blank at the beginning of each trial. Observers were instructed to fixate at the center and pay attention to the flickering Gabors during the adaptation phase.
After 400 ms of inter-stimulus interval (ISI), a valid, neutral, or invalid peripheral cue (40 ms) presented around a Gabor (1 dva away from the Gabor edge), followed by a 60 ms ISI, then two Gabor patches presenting at the center of placeholders (i.e., the center of the phosphene outline) on the left and right visual fields for 100 ms. Observers’ task was a two-alternative forced-choice orientation discrimination task (either counterclockwise or clockwise relative to the vertical) of the Gabor patch being indicated by the response cue, via button press. Note that the pre-cue was uninformative; in a random half of the trials, the response cue indicated the same location as pre-cue (valid trials), while in the other half of the trials, the response cue indicated the other location (invalid trials).
During the target presentation, observers received two single pulses (separated by 50 ms, 20Hz) of TMS with the power at the sub-threshold level (Figure 2B). This inter-pulse interval we used was consistent with in previous studies (12,22,23,39,98,99,see28 for review). We presented the two stimuli in the TMS stimulated region and its symmetric location in the other hemifield; the stimulus was presented for each observer according to their phosphene location (Figure 2A), like in previous studies.12,22,23,39 In half of the trials, the response cue instructed observers to report the orientation of the stimulus at the stimulated region (contralateral to TMS; target-stimulated, which was equally likely to be a valid trial, invalid trial or neutral trial), and in the other half, the symmetric region (ipsilateral; distractor-stimulated, which was equally likely to be a valid trial, invalid trial or neutral trial). A feedback tone (400 Hz, 150 ms) was given after an incorrect response.
We used a lower intensity for the psychophysics-TMS task to ensure that no phosphenes were perceived during the main experimental task (conducted on mid-gray background). During the psychophysics-TMS task, if the stimulated region matched the response-cued region, it was a target-stimulated condition; otherwise, it was a distractor-stimulated condition (Figure 2C).
TMS over occipital cortex affects the contralateral hemifield.12 Thus, the distractor-stimulated condition can be considered as a control condition (similar to a no-TMS condition), and the target-stimulated condition was the one in which TMS should disrupt target processing. Importantly, in our experimental design, observers could not know whether they were experiencing a valid or invalid cue trial (as the cue was uninformative) and whether they were in a target-stimulated or distractor-stimulated trial until the response cue appeared. Thus, the current experimental design eliminated the need for a sham condition, which can produce a different somatosensory experience than the sham100,101 and can bring expectation and placebo effects (see102).
The whole experiment consisted of 4 sessions, and each session contained 10 blocks of 48 trials. Each observer completed 1920 trials in total, which included of 80 trials per condition (two different levels of contrast: c50 and dmax; three attentional conditions: valid, neutral, and invalid; two adaptation conditions: adaptation and non-adaptation; two stimulated conditions: target-stimulated and distractor-stimulated (Figure 2C).
Quantification and statistical analysis
Task performance indexed by d’ [z(hit rate) – z(false alarm rate)] across conditions. The correct discrimination of clockwise trials were considered as hits and incorrect discrimination of counter-clockwise trials were considered as false alarms.12,22,43,97,103,104
Repeated-measures ANOVA along with effect size (η2) were computed in R92 and used to assess statistical significance. Partial η2 was provided for all F tests, where η2=0.01 indicates small effect, η2=0.06 indicates a medium effect, and η2=0.14 indicates a large effect. Cohen’s d was computed for each post-hoc t-test, where d=0.2 indicates a small effect, d=0.5 indicates a medium effect, and d=0.8 indicates a large effect.105
Published: October 11, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111155.
Contributor Information
Hsing-Hao Lee, Email: hsinghaolee@nyu.edu.
Marisa Carrasco, Email: marisa.carrasco@nyu.edu.
Supplemental information
References
- 1.Lennie P. The cost of cortical computation. Curr. Biol. 2003;13:493–497. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 2.Carrasco M. Visual attention: The past 25 years. Vis. Res. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruckmaier M., Tachtsidis I., Phan P., Lavie N. Attention and capacity limits in perception: A cellular metabolism account. J. Neurosci. 2020;40:6801–6811. doi: 10.1523/JNEUROSCI.2368-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster M.A. Visual adaptation. Annu. Rev. Vis. Sci. 2015;1:547–567. doi: 10.1146/annurev-vision-082114-035509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dao D.Y., Lu Z.-L., Dosher B.A. Adaptation to sine-wave gratings selectively reduces the contrast gain of the adapted stimuli. J. Vis. 2006;6:6. doi: 10.1167/6.7.6. [DOI] [PubMed] [Google Scholar]
- 6.Carandini M., Ferster D. A tonic hyperpolarization underlying contrast adaptation in cat visual cortex. Science. 1997;276:949–952. doi: 10.1126/science.276.5314.949. [DOI] [PubMed] [Google Scholar]
- 7.Pestilli F., Ling S., Carrasco M. A population-coding model of attention’s influence on contrast response: Estimating neural effects from psychophysical data. Vis. Res. 2009;49:1144–1153. doi: 10.1016/j.visres.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Z.-L., Lesmes L.A., Dosher B.A. Spatial attention excludes external noise at the target location. J. Vis. 2002;2:4. doi: 10.1167/2.4.4. [DOI] [PubMed] [Google Scholar]
- 9.Pestilli F., Viera G., Carrasco M. How do attention and adaptation affect contrast sensitivity? J. Vis. 2007;7:9. doi: 10.1167/7.7.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perini F., Cattaneo L., Carrasco M., Schwarzbach J.V. Occipital transcranial magnetic stimulation has an activity-dependent suppressive effect. J. Neurosci. 2012;32:12361–12365. doi: 10.1523/JNEUROSCI.5864-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner J.L., Sun P., Waggoner R.A., Ueno K., Tanaka K., Cheng K. Contrast adaptation and representation in human early visual cortex. Neuron. 2005;47:607–620. doi: 10.1016/j.neuron.2005.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández A., Carrasco M. Extinguishing exogenous attention via transcranial magnetic stimulation. Curr. Biol. 2020;30:4078–4084. doi: 10.1016/j.cub.2020.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling S., Carrasco M. Sustained and transient covert attention enhance the signal via different contrast response functions. Vis. Res. 2006;46:1210–1220. doi: 10.1016/j.visres.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastner S., De Weerd P., Ungerleider L.G. Texture segregation in the human visual cortex: A functional MRI study. J. Neurophysiol. 2000;83:2453–2457. doi: 10.1152/jn.2000.83.4.2453. [DOI] [PubMed] [Google Scholar]
- 15.Walsh V., Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nat. Rev. Neurosci. 2000;1:73–80. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- 16.Vernet M., Thut G. Electroencephalography during transcranial magnetic stimulation: current modus operandi. Transcranial Magn. Stimul. 2014;89:197–232. [Google Scholar]
- 17.Valero-Cabré A., Amengual J.L., Stengel C., Pascual-Leone A., Coubard O.A. Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci. Biobehav. Rev. 2017;83:381–404. doi: 10.1016/j.neubiorev.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqi S.H., Kording K.P., Parvizi J., Fox M.D. Causal mapping of human brain function. Nat. Rev. Neurosci. 2022;23:361–375. doi: 10.1038/s41583-022-00583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silvanto J., Cattaneo Z. Common framework for “virtual lesion” and state-dependent TMS: the facilitatory/suppressive range model of online TMS effects on behavior. Brain Cognit. 2017;119:32–38. doi: 10.1016/j.bandc.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley C., Nydam A.S., Dux P.E., Mattingley J.B. State-dependent effects of neural stimulation on brain function and cognition. Nat. Rev. Neurosci. 2022;23:459–475. doi: 10.1038/s41583-022-00598-1. [DOI] [PubMed] [Google Scholar]
- 21.Silvanto J., Muggleton N.G., Cowey A., Walsh V. Neural adaptation reveals state-dependent effects of transcranial magnetic stimulation. Eur. J. Neurosci. 2007;25:1874–1881. doi: 10.1111/j.1460-9568.2007.05440.x. [DOI] [PubMed] [Google Scholar]
- 22.Fernández A., Hanning N.M., Carrasco M. Transcranial magnetic stimulation to frontal but not occipital cortex disrupts endogenous attention. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2219635120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanning N.M., Fernández A., Carrasco M. Dissociable roles of human frontal eye fields and early visual cortex in presaccadic attention. Nat. Commun. 2023;14:5381. doi: 10.1038/s41467-023-40678-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sack A.T., Paneva J., Küthe T., Dijkstra E., Zwienenberg L., Arns M., Schuhmann T. Target engagement and brain state dependence of transcranial magnetic stimulation: Implications for clinical practice. Biol. Psychiatr. 2023;95:536–544. doi: 10.1016/j.biopsych.2023.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Ahrens M.-M., Veniero D., Freund I.M., Harvey M., Thut G. Both dorsal and ventral attention network nodes are implicated in exogenously driven visuospatial anticipation. Cortex. 2019;117:168–181. doi: 10.1016/j.cortex.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Amemiya T., Beck B., Walsh V., Gomi H., Haggard P. Visual area V5/hMT+ contributes to perception of tactile motion direction: A TMS study. Sci. Rep. 2017;7 doi: 10.1038/srep40937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dugué L., Marque P., VanRullen R. Transcranial magnetic stimulation reveals attentional feedback to area V1 during serial visual search. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dugué L., Beck A.-A., Marque P., VanRullen R. Contribution of FEF to attentional periodicity during visual search: A TMS study. eNeuro. 2019;6 doi: 10.1523/ENEURO.0357-18.2019. ENEURO.0357-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vetter P., Grosbras M.-H., Muckli L. TMS over V5 disrupts motion prediction. Cerebr. Cortex. 2015;25:1052–1059. doi: 10.1093/cercor/bht297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cirillo J., Byblow W.D. Threshold tracking primary motor cortex inhibition: The influence of current direction. Eur. J. Neurosci. 2016;44:2614–2621. doi: 10.1111/ejn.13369. [DOI] [PubMed] [Google Scholar]
- 31.Cirillo J., Semmler J.G., Mooney R.A., Byblow W.D. Conventional or threshold-hunting TMS? A tale of two SICIs. Brain Stimul. 2018;11:1296–1305. doi: 10.1016/j.brs.2018.07.047. [DOI] [PubMed] [Google Scholar]
- 32.Macdonald H.J., Coxon J.P., Stinear C.M., Byblow W.D. The fall and rise of corticomotor excitability with cancellation and reinitiation of prepared action. J. Neurophysiol. 2014;112:2707–2717. doi: 10.1152/jn.00366.2014. [DOI] [PubMed] [Google Scholar]
- 33.Opie G.M., Ridding M.C., Semmler J.G. Task-related changes in intracortical inhibition assessed with paired-and triple-pulse transcranial magnetic stimulation. J. Neurophysiol. 2015;113:1470–1479. doi: 10.1152/jn.00651.2014. [DOI] [PubMed] [Google Scholar]
- 34.Derosiere G., Vassiliadis P., Duque J. Advanced TMS approaches to probe corticospinal excitability during action preparation. Neuroimage. 2020;213 doi: 10.1016/j.neuroimage.2020.116746. [DOI] [PubMed] [Google Scholar]
- 35.Lefaucheur J.-P. Transcranial magnetic stimulation. Handb. Clin. Neurol. 2019;160:559–580. doi: 10.1016/B978-0-444-64032-1.00037-0. [DOI] [PubMed] [Google Scholar]
- 36.Sanger T.D., Garg R.R., Chen R. Interactions between two different inhibitory systems in the human motor cortex. J. Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silvanto J., Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dugué L., Marque P., VanRullen R. Theta oscillations modulate attentional search performance periodically. J. Cognit. Neurosci. 2015;27:945–958. doi: 10.1162/jocn_a_00755. [DOI] [PubMed] [Google Scholar]
- 39.Dugué L., Roberts M., Carrasco M. Attention reorients periodically. Curr. Biol. 2016;26:1595–1601. doi: 10.1016/j.cub.2016.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Y.-J., Shukla L., Dugué L., Valero-Cabré A., Carrasco M. Transcranial magnetic stimulation entrains alpha oscillatory activity in occipital cortex. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-96849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Georgeson M., Lerner P., Kingdom F. Binocular properties of contrast adaptation in human vision. Vis. Res. 2023;209 doi: 10.1016/j.visres.2023.108261. [DOI] [PubMed] [Google Scholar]
- 42.Vinke L.N., Bloem I.M., Ling S. Saturating nonlinearities of contrast response in human visual cortex. J. Neurosci. 2022;42:1292–1302. doi: 10.1523/JNEUROSCI.0106-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrmann K., Montaser-Kouhsari L., Carrasco M., Heeger D.J. When size matters: Attention affects performance by contrast or response gain. Nat. Neurosci. 2010;13:1554–1559. doi: 10.1038/nn.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron E., Tai J.C., Carrasco M. Covert attention affects the psychometric function of contrast sensitivity. Vis. Res. 2002;42:949–967. doi: 10.1016/s0042-6989(02)00039-1. [DOI] [PubMed] [Google Scholar]
- 45.Fernández A., Okun S., Carrasco M. Differential effects of endogenous and exogenous attention on sensory tuning. J. Neurosci. 2022;42:1316–1327. doi: 10.1523/JNEUROSCI.0892-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anton-Erxleben K., Herrmann K., Carrasco M. Independent effects of adaptation and attention on perceived speed. Psychol. Sci. 2013;24:150–159. doi: 10.1177/0956797612449178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon S.G., Kohn A. Moving sensory adaptation beyond suppressive effects in single neurons. Curr. Biol. 2014;24:R1012–R1022. doi: 10.1016/j.cub.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hallett M. Transcranial magnetic stimulation: A primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 49.Farzan F., Vernet M., Shafi M.M.D., Rotenberg A., Daskalakis Z.J., Pascual-Leone A. Characterizing and modulating brain circuitry through transcranial magnetic stimulation combined with electroencephalography. Front. Neural Circ. 2016;10:73. doi: 10.3389/fncir.2016.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergmann T.O., Hartwigsen G. Inferring causality from noninvasive brain stimulation in cognitive neuroscience. J. Cognit. Neurosci. 2021;33:195–225. doi: 10.1162/jocn_a_01591. [DOI] [PubMed] [Google Scholar]
- 51.Taylor P.C., Nobre A.C., Rushworth M.F. FEF TMS affects visual cortical activity. Cerebr. Cortex. 2006;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- 52.Bolognini N., Ro T. Transcranial magnetic stimulation: disrupting neural activity to alter and assess brain function. J. Neurosci. 2010;30:9647–9650. doi: 10.1523/JNEUROSCI.1990-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gamboa Arana O.L., Palmer H., Dannhauer M., Hile C., Liu S., Hamdan R., Brito A., Cabeza R., Davis S.W., Peterchev A.V., et al. Intensity-and timing-dependent modulation of motion perception with transcranial magnetic stimulation of visual cortex. Neuropsychologia. 2020;147 doi: 10.1016/j.neuropsychologia.2020.107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pitcher D., Walsh V., Yovel G., Duchaine B. TMS evidence for the involvement of the right occipital face area in early face processing. Curr. Biol. 2007;17:1568–1573. doi: 10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 55.Pitcher D., Goldhaber T., Duchaine B., Walsh V., Kanwisher N. Two critical and functionally distinct stages of face and body perception. J. Neurosci. 2012;32:15877–15885. doi: 10.1523/JNEUROSCI.2624-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao A., Nobre A.C., Alexander I., Cowey A. Auditory evoked visual awareness following sudden ocular blindness: an EEG and TMS investigation. Exp. Brain Res. 2007;176:288–298. doi: 10.1007/s00221-006-0616-2. [DOI] [PubMed] [Google Scholar]
- 57.Center E.G., Knight R., Fabiani M., Gratton G., Beck D.M. Examining the role of feedback in TMS-induced visual suppression: A cautionary tale. Conscious. Cognit. 2019;75 doi: 10.1016/j.concog.2019.102805. [DOI] [PubMed] [Google Scholar]
- 58.Romei V., Murray M.M., Merabet L.B., Thut G. Occipital transcranial magnetic stimulation has opposing effects on visual and auditory stimulus detection: Implications for multisensory interactions. J. Neurosci. 2007;27:11465–11472. doi: 10.1523/JNEUROSCI.2827-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beauchamp M.S., Nath A.R., Pasalar S. fMRI-Guided transcranial magnetic stimulation reveals that the superior temporal sulcus is a cortical locus of the McGurk effect. J. Neurosci. 2010;30:2414–2417. doi: 10.1523/JNEUROSCI.4865-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackey W.E., Curtis C.E. Distinct contributions by frontal and parietal cortices support working memory. Sci. Rep. 2017;7:6188. doi: 10.1038/s41598-017-06293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor P.C.J., Nobre A.C., Rushworth M.F.S. Subsecond changes in top–down control exerted by human medial frontal cortex during conflict and action selection: A combined transcranial magnetic stimulation–electroencephalography study. J. Neurosci. 2007;27:11343–11353. doi: 10.1523/JNEUROSCI.2877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olk B., Peschke C., Hilgetag C.C. Attention and control of manual responses in cognitive conflict: Findings from TMS perturbation studies. Neuropsychologia. 2015;74:7–20. doi: 10.1016/j.neuropsychologia.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Silvanto J., Cattaneo Z., Battelli L., Pascual-Leone A. Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. J. Neurophysiol. 2008;99:2725–2730. doi: 10.1152/jn.01392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pitcher D., Parkin B., Walsh V. Transcranial magnetic stimulation and the understanding of behavior. Annu. Rev. Psychol. 2021;72:97–121. doi: 10.1146/annurev-psych-081120-013144. [DOI] [PubMed] [Google Scholar]
- 65.Cattaneo Z., Silvanto J. Time course of the state-dependent effect of transcranial magnetic stimulation in the TMS-adaptation paradigm. Neurosci. Lett. 2008;443:82–85. doi: 10.1016/j.neulet.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 66.Harris J.A., Clifford C.W.G., Miniussi C. The functional effect of transcranial magnetic stimulation: Signal suppression or neural noise generation? J. Cognit. Neurosci. 2008;20:734–740. doi: 10.1162/jocn.2008.20048. [DOI] [PubMed] [Google Scholar]
- 67.Siebner H.R., Hartwigsen G., Kassuba T., Rothwell J.C. How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex. 2009;45:1035–1042. doi: 10.1016/j.cortex.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruzzoli M., Marzi C.A., Miniussi C. The neural mechanisms of the effects of transcranial magnetic stimulation on perception. J. Neurophysiol. 2010;103:2982–2989. doi: 10.1152/jn.01096.2009. [DOI] [PubMed] [Google Scholar]
- 69.Stocker A.A., Simoncelli E. Sensory adaptation within a Bayesian framework for perception. Adv. Neural Inf. Process. Syst. 2005;18 [Google Scholar]
- 70.Pozzorini C., Naud R., Mensi S., Gerstner W. Temporal whitening by power-law adaptation in neocortical neurons. Nat. Neurosci. 2013;16:942–948. doi: 10.1038/nn.3431. [DOI] [PubMed] [Google Scholar]
- 71.Pinchuk-Yacobi N., Sagi D. Orientation-selective adaptation improves perceptual grouping. J. Vis. 2019;19:6. doi: 10.1167/19.9.6. [DOI] [PubMed] [Google Scholar]
- 72.Barlow H.B., Foldiak P. The Computing Neuron. Addison Wesley; 1989. Adaptation and decorrelation in the cortex; pp. 54–72. [Google Scholar]
- 73.Gutnisky D.A., Dragoi V. Adaptive coding of visual information in neural populations. Nature. 2008;452:220–224. doi: 10.1038/nature06563. [DOI] [PubMed] [Google Scholar]
- 74.Benucci A., Saleem A.B., Carandini M. Adaptation maintains population homeostasis in primary visual cortex. Nat. Neurosci. 2013;16:724–729. doi: 10.1038/nn.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carandini M., Heeger D.J. Normalization as a canonical neural computation. Nat. Rev. Neurosci. 2012;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reynolds J.H., Heeger D.J. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herrmann K., Heeger D.J., Carrasco M. Feature-based attention enhances performance by increasing response gain. Vis. Res. 2012;74:10–20. doi: 10.1016/j.visres.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jigo M., Heeger D.J., Carrasco M. An image-computable model of how endogenous and exogenous attention differentially alter visual perception. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2106436118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chica A.B., Valero-Cabré A., Paz-Alonso P.M., Bartolomeo P. Causal contributions of the left frontal eye field to conscious perception. Cerebr. Cortex. 2014;24:745–753. doi: 10.1093/cercor/bhs357. [DOI] [PubMed] [Google Scholar]
- 80.Valero-Cabré A., Pascual-Leone A., Rushmore R.J. Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMS-mediated behavioural disruptions. Eur. J. Neurosci. 2008;27:765–774. doi: 10.1111/j.1460-9568.2008.06045.x. [DOI] [PubMed] [Google Scholar]
- 81.Aschner A., Solomon S.G., Landy M.S., Heeger D.J., Kohn A. Temporal contingencies determine whether adaptation strengthens or weakens normalization. J. Neurosci. 2018;38:10129–10142. doi: 10.1523/JNEUROSCI.1131-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stocks N.G. Suprathreshold stochastic resonance in multilevel threshold systems. Phys. Rev. Lett. 2000;84:2310–2313. doi: 10.1103/PhysRevLett.84.2310. [DOI] [PubMed] [Google Scholar]
- 83.Schwarzkopf D.S., Silvanto J., Rees G. Stochastic resonance effects reveal the neural mechanisms of transcranial magnetic stimulation. J. Neurosci. 2011;31:3143–3147. doi: 10.1523/JNEUROSCI.4863-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Movshon J.A., Lennie P. Pattern-selective adaptation in visual cortical neurones. Nature. 1979;278:850–852. doi: 10.1038/278850a0. [DOI] [PubMed] [Google Scholar]
- 85.Dhruv N.T., Tailby C., Sokol S.H., Lennie P. Multiple adaptable mechanisms early in the primate visual pathway. J. Neurosci. 2011;31:15016–15025. doi: 10.1523/JNEUROSCI.0890-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwon M., Legge G.E., Fang F., Cheong A.M.Y., He S. Adaptive changes in visual cortex following prolonged contrast reduction. J. Vis. 2009;9:20. doi: 10.1167/9.2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carrasco M., Loula F., Ho Y.-X. How attention enhances spatial resolution: Evidence from selective adaptation to spatial frequency. Percept. Psychophys. 2006;68:1004–1012. doi: 10.3758/bf03193361. [DOI] [PubMed] [Google Scholar]
- 88.Lamme V.A.F., Van Dijk B.W., Spekreijse H. Contour from motion processing occurs in primary visual cortex. Nature. 1993;363:541–543. doi: 10.1038/363541a0. [DOI] [PubMed] [Google Scholar]
- 89.Purpura K.P., Victor J.D., Katz E. Striate cortex extracts higher-order spatial correlations from visual textures. Proc. Natl. Acad. Sci. USA. 1994;91:8482–8486. doi: 10.1073/pnas.91.18.8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lamme V. The neurophysiology of figure-ground segregation in primary visual cortex. J. Neurosci. 1995;15:1605–1615. doi: 10.1523/JNEUROSCI.15-02-01605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poort J., Self M.W., Van Vugt B., Malkki H., Roelfsema P.R. Texture segregation causes early figure enhancement and later ground suppression in areas V1 and V4 of visual cortex. Cerebr. Cortex. 2016;26:3964–3976. doi: 10.1093/cercor/bhw235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.R Core Team . 2010. R: A language and environment for statistical computing. [Google Scholar]
- 93.Brainard D.H. The psychophysics toolbox. Spatial Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 94.Pelli D.G. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- 95.Prins N., Kingdom F.A.A. Applying the model-comparison approach to test specific research hypotheses in psychophysical research using the Palamedes toolbox. Front. Psychol. 2018;9:1250. doi: 10.3389/fpsyg.2018.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rovamo J., Virsu V. An estimation and application of the human cortical magnification factor. Exp. Brain Res. 1979;37:495–510. doi: 10.1007/BF00236819. [DOI] [PubMed] [Google Scholar]
- 97.Jigo M., Carrasco M. Differential impact of exogenous and endogenous attention on the contrast sensitivity function across eccentricity. J. Vis. 2020;20:11. doi: 10.1167/jov.20.6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chica A.B., Bartolomeo P., Valero-Cabré A. Dorsal and ventral parietal contributions to spatial orienting in the human brain. J. Neurosci. 2011;31:8143–8149. doi: 10.1523/JNEUROSCI.5463-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bolognini N., Convento S., Fusaro M., Vallar G. The sound-induced phosphene illusion. Exp. Brain Res. 2013;231:469–478. doi: 10.1007/s00221-013-3711-1. [DOI] [PubMed] [Google Scholar]
- 100.Mennemeier M.S., Triggs W.J., Chelette K.C., Woods A., Kimbrell T.A., Dornhoffer J.L. Sham transcranial magnetic stimulation using electrical stimulation of the scalp. Brain Stimul. 2009;2:168–173. doi: 10.1016/j.brs.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rossi S., Ferro M., Cincotta M., Ulivelli M., Bartalini S., Miniussi C., Giovannelli F., Passero S. A real electro-magnetic placebo (REMP) device for sham transcranial magnetic stimulation (TMS) Clin. Neurophysiol. 2007;118:709–716. doi: 10.1016/j.clinph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 102.Duecker F., Sack A.T. Rethinking the role of sham TMS. Front. Psychol. 2015;6:210. doi: 10.3389/fpsyg.2015.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang H., Morrone M.C., Alais D. Behavioural oscillations in visual orientation discrimination reveal distinct modulation rates for both sensitivity and response bias. Sci. Rep. 2019;9:1115. doi: 10.1038/s41598-018-37918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Locke S.M., Gaffin-Cahn E., Hosseinizaveh N., Mamassian P., Landy M.S. Priors and payoffs in confidence judgments. Atten. Percept. Psychophys. 2020;82:3158–3175. doi: 10.3758/s13414-020-02018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen J. Academic press; 2013. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The behavioral data have been uploaded the OSF database and are publicly available.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.