Abstract
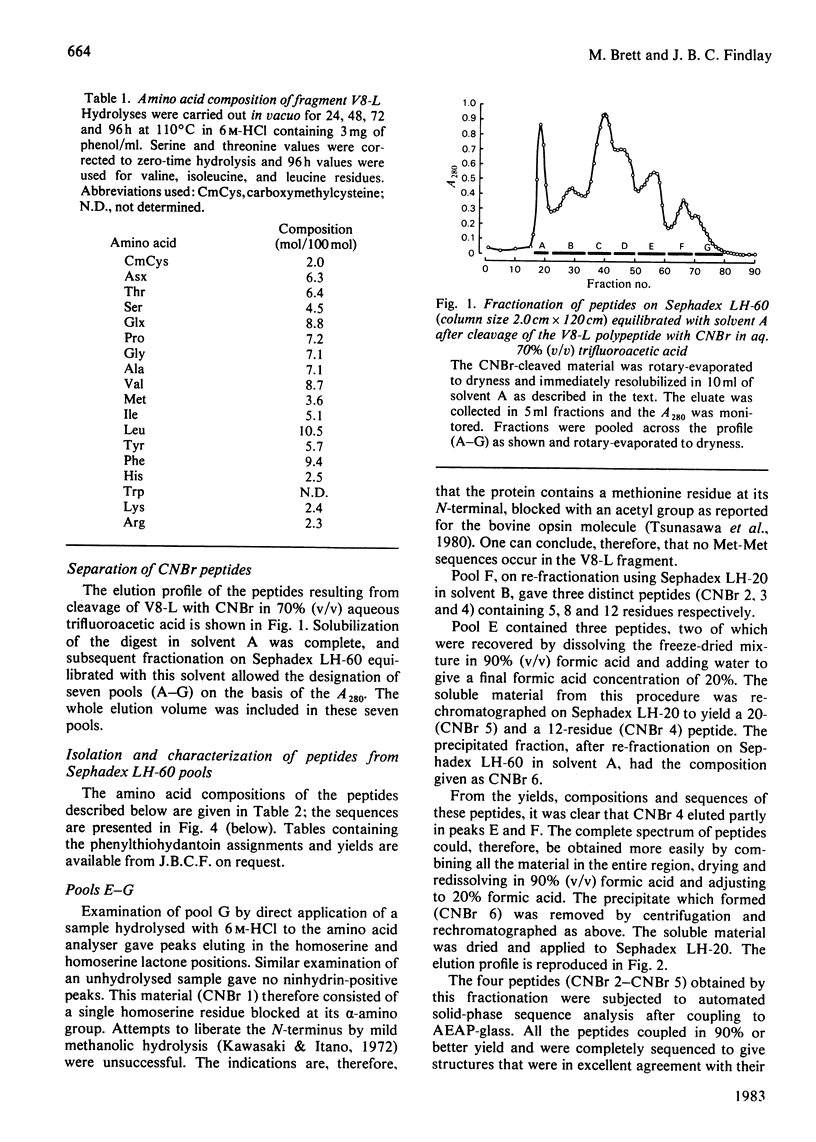
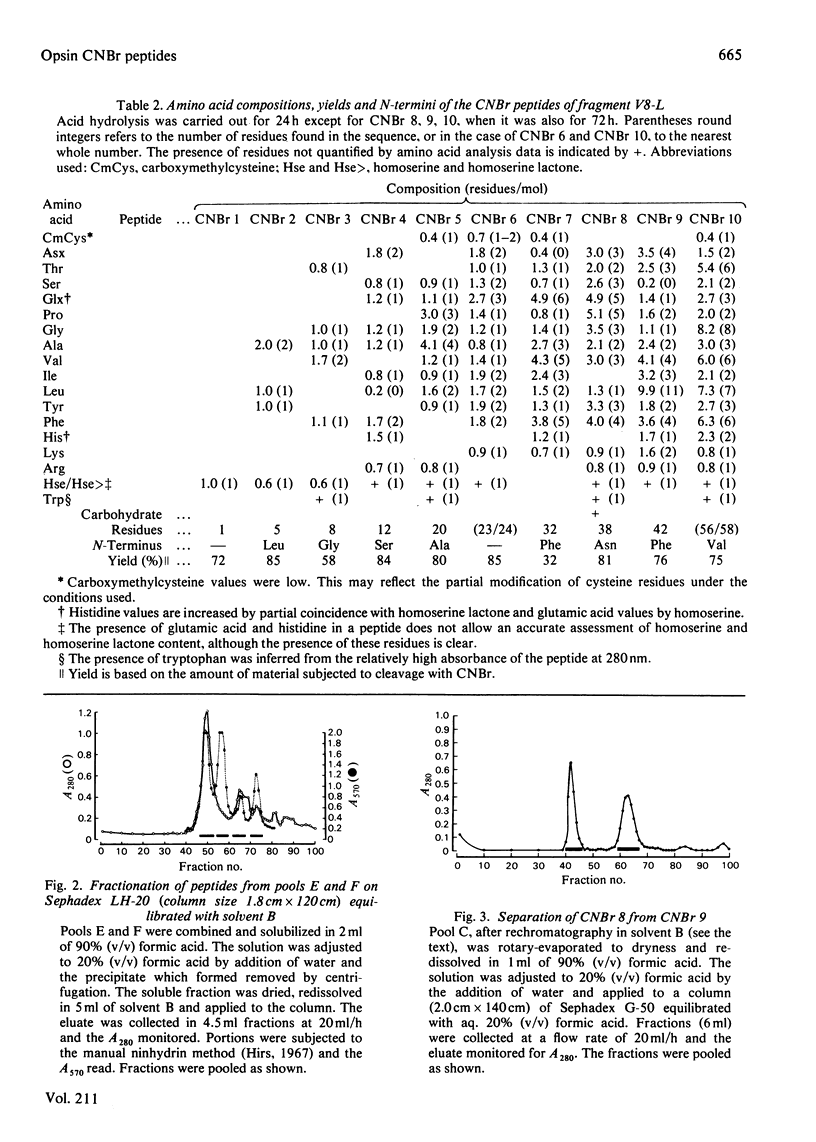
Ovine rhodopsin may be cleaved in situ by Staphylococcus aureus V8 proteinase into two membrane-bound fragments designated V8-L (27 000 mol.wt.) and V8-S (12 000 mol.wt.). After purification of the proteolysed complex by affinity chromatography in detergent using concanavalin A immobilized on Sepharose 4B, the two polypeptide fragments may be separated by gel-permeation chromatography on Sephadex LH-60. Digestion of the N-terminal-derived V8-L fragment with CNBr in 70% (v/v) trifluoroacetic acid resulted in a peptide mixture that could be fractionated by procedures involving gel-permeation chromatography in organic and aqueous solvents and the use of differential solubility. The complete or partial sequences of all ten peptides are reported.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhown A. S., Bennett J. C., Morgan P. H., Mole J. E. Use of fluorescamine as an effective blocking reagent to reduce the background in protein sequence analyses by the Beckman automated sequencer. Anal Biochem. 1981 Mar 15;112(1):158–162. doi: 10.1016/0003-2697(81)90274-8. [DOI] [PubMed] [Google Scholar]
- Bonting S. L., de Grip W. J., Rotmans J. P., Daemen F. J. Use of photoreceptor membrane suspensions for the study of rhodopsin and associated enzyme activities. Exp Eye Res. 1974 Jan;18(1):77–88. doi: 10.1016/0014-4835(74)90045-1. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Brett M., Findlay J. B. Investigation of the organization of rhodopsin in the sheep photoreceptor membrane by using cross-linking reagents. Biochem J. 1979 Jan 1;177(1):215–223. doi: 10.1042/bj1770215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay J. B., Brett M., Pappin D. J. Primary structure of C-terminal functional sites in ovine rhodopsin. Nature. 1981 Sep 24;293(5830):314–317. doi: 10.1038/293314a0. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Brew K. The complete amino-acid sequence of human -lactalbumin. Eur J Biochem. 1972 May;27(1):65–86. doi: 10.1111/j.1432-1033.1972.tb01812.x. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Moore A., Pappin D. J. Structural studies on the chromophore attachment site of rhodopsin following bleaching. FEBS Lett. 1982 Feb 8;138(1):67–70. doi: 10.1016/0014-5793(82)80396-7. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Papermaster D. S., Hargrave P. A. Rhodopsin carbohydrate. Structure of small oligosaccharides attached at two sites near the NH2 terminus. J Biol Chem. 1979 Sep 10;254(17):8201–8207. [PubMed] [Google Scholar]
- Hargrave P. A. The amino-terminal tryptic peptide of bovine rhodopsin. A glycopeptide containing two sites of oligosaccharide attachment. Biochim Biophys Acta. 1977 May 27;492(1):83–94. doi: 10.1016/0005-2795(77)90216-1. [DOI] [PubMed] [Google Scholar]
- Horn M. J., Laursen R. A. Solid-phase edman degradation: attachment of carboxyl-terminal homoserine peptides to an insoluble resin. FEBS Lett. 1973 Nov 1;36(3):285–288. doi: 10.1016/0014-5793(73)80392-8. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki I., Itano H. A. Methanolysis of the pyrrolidone ring of amino-terminal pyroglutamic acid in model peptides. Anal Biochem. 1972 Aug;48(2):546–556. doi: 10.1016/0003-2697(72)90110-8. [DOI] [PubMed] [Google Scholar]
- Klip A., Darszon A., Montal M. Labelling of rhodopsin moieties confined to the membrane lipid bilayer. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1350–1358. doi: 10.1016/s0006-291x(76)80163-5. [DOI] [PubMed] [Google Scholar]
- Liang C. J., Yamashita K., Muellenberg C. G., Shichi H., Kobata A. Structure of the carbohydrate moieties of bovine rhodopsin. J Biol Chem. 1979 Jul 25;254(14):6414–6418. [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- Mullen E., Akhtar M. Topographic and active-site studies on bovine rhodopsin. FEBS Lett. 1981 Sep 28;132(2):261–264. doi: 10.1016/0014-5793(81)81174-x. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Stryer L. Letter to the editor: Light dissociates enzymatically-cleaved rhodopsin into two different fragments. J Mol Biol. 1975 Jul 5;95(3):477–481. doi: 10.1016/0022-2836(75)90204-1. [DOI] [PubMed] [Google Scholar]
- Saari J. C. The accessibility of bovine rhodopsin in photoreceptor membranes. J Cell Biol. 1974 Nov;63(2 Pt 1):480–491. doi: 10.1083/jcb.63.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale G. J., Towner P., Akhtar M. Functional rhodopsin complex consisting of three noncovalently linked fragments. Biochemistry. 1977 Dec 13;16(25):5641–5649. doi: 10.1021/bi00644a040. [DOI] [PubMed] [Google Scholar]
- Schechter I., Burstein Y., Zemell R., Ziv E., Kantor F., Papermaster D. S. Messenger RNA of opsin from bovine retina: isolation and partial sequence of the in vitro translation product. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2654–2658. doi: 10.1073/pnas.76.6.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. B., Shelton J. R. An examination of conditions for the cleavage of polypeptide chains with cyanogen bromide: application to catalase. Arch Biochem Biophys. 1969 Mar;130(1):551–556. doi: 10.1016/0003-9861(69)90069-1. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner P., Sale G. J., Akhtar M. Identification of the active site polypeptide in labeled photoreceptor membranes digested with papain. FEBS Lett. 1977 Apr 1;76(1):51–55. doi: 10.1016/0014-5793(77)80118-x. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Mandel P., Virmaux N. Composition of the rhodopsin-core obtained by proteolysis of retinal rod outer segments with papain, and its regenerability after photobleaching. Exp Eye Res. 1974 Sep;19(3):259–265. doi: 10.1016/0014-4835(74)90144-4. [DOI] [PubMed] [Google Scholar]
- Tsunasawa S., Narita K., Shichi H. The N-terminal residue of bovine rhodopsin is acetylmethionine. Biochim Biophys Acta. 1980 Jul 24;624(1):218–225. doi: 10.1016/0005-2795(80)90241-x. [DOI] [PubMed] [Google Scholar]
- Wachter E., Machleidt W., Hofner H., Otto J. Aminopropyl glass and its p-phenylene diisothiocyanate derivative, a new support in solid-phase Edman degradation of peptides and proteins. FEBS Lett. 1973 Sep 1;35(1):97–102. doi: 10.1016/0014-5793(73)80585-x. [DOI] [PubMed] [Google Scholar]
- Williams D. G., Jenkins R. E., Tanner M. J. Structure of the anion-transport protein of the human erythrocyte membrane. Further studies on the fragments produced by proteolytic digestion. Biochem J. 1979 Aug 1;181(2):477–493. doi: 10.1042/bj1810477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]
- van Breugel P. J., Daemen F. J., Bonting S. L. Biochemical aspects of the visual process, XXXIII. A convenient purification procedure of rhodopsin by means of affinity chromatography. Exp Eye Res. 1977 Jun;24(6):581–585. doi: 10.1016/0014-4835(77)90115-4. [DOI] [PubMed] [Google Scholar]



