Abstract
Reaction conditions are described that permit the enzyme-assisted semi-synthetic replacement of residue B30 of pig insulin (or of analogue) to proceed in very high yield in 2 h or less. Immobilized trypsin may be used as catalyst, and excess amino acid ester may be recycled after a simple extraction. Alanine-B30 may be replaced by a variety of nucleophiles, including threonine O-t-butyl ether t-butyl ester, in which case the yield of crude product is about 99%. De-protection of the B30 threonyl ester analogue of insulin thus formed then affords human insulin in an overall yield of about 92%, based on pig starting material. The product has full biological potency, as determined by depression of blood glucose concentration in rats, and showed the expected behaviour on radioimmunoassay.
Full text
PDF


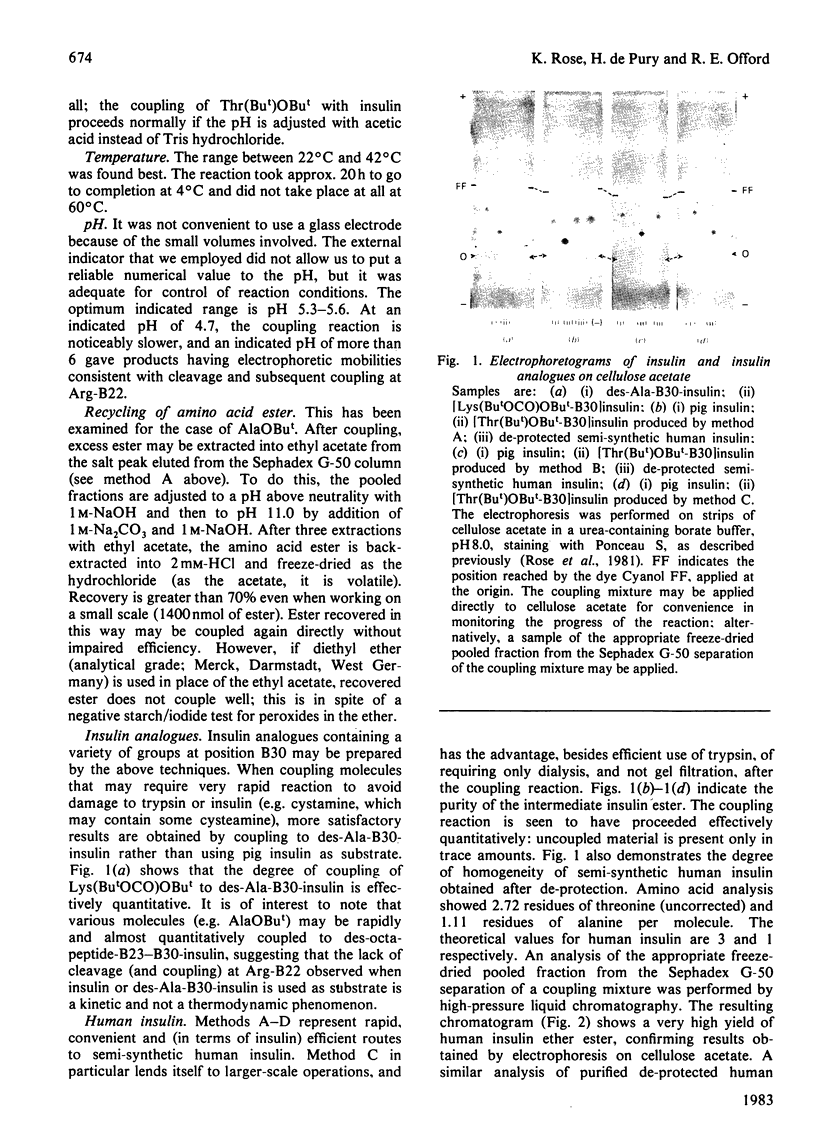
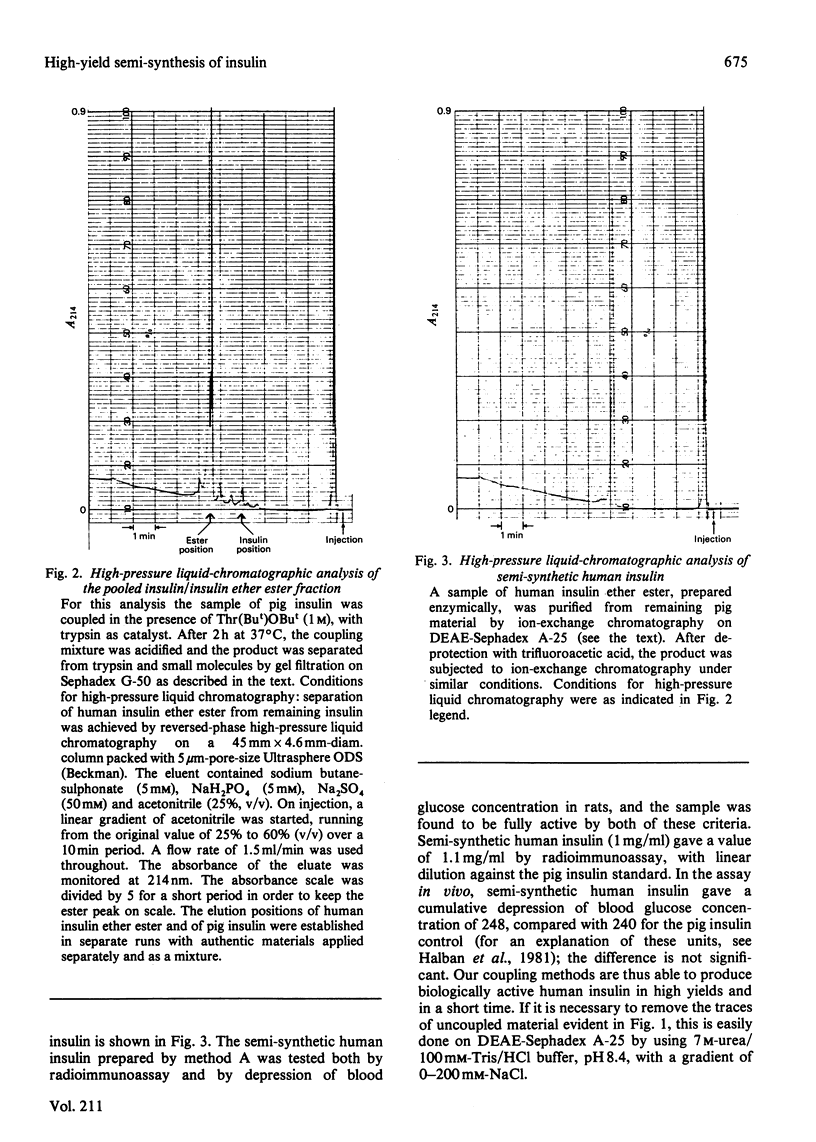

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Jonczyk A., Gattner H. G. Eine neue Semisynthese des Humaninsulins. Tryptisch-katalysierte Transpeptidierung von Schweineinsulin mit L-Threonin-tert-butylester. Hoppe Seylers Z Physiol Chem. 1981 Dec;362(12):1591–1598. [PubMed] [Google Scholar]
- Morihara K., Oka T., Tsuzuki H. Semi-synthesis of human insulin by trypsin-catalysed replacement of Ala-B30 by Thr in porcine insulin. Nature. 1979 Aug 2;280(5721):412–413. doi: 10.1038/280412a0. [DOI] [PubMed] [Google Scholar]
- Morihara K., Oka T., Tsuzuki H., Tochino Y., Kanaya T. Achromobacter protease I-catalyzed conversion of porcine insulin into human insulin. Biochem Biophys Res Commun. 1980 Jan 29;92(2):396–402. doi: 10.1016/0006-291x(80)90346-0. [DOI] [PubMed] [Google Scholar]
- Obermeier R., Geiger R. A new semisynthesis of human insulin. Hoppe Seylers Z Physiol Chem. 1976 Jun;357(6):759–767. doi: 10.1515/bchm2.1976.357.1.759. [DOI] [PubMed] [Google Scholar]
- Rose K., Rees A. R., Drake C. S., Offord R. E. The role of the arginine-B22 residue in insulin action. Biochem J. 1981 Jun 1;195(3):765–768. doi: 10.1042/bj1950765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E. W., Gattner H. G. Verbesserte Darstellung von Des-alanylB30-insulin. Hoppe Seylers Z Physiol Chem. 1978 Jul;359(7):799–802. [PubMed] [Google Scholar]



