Abstract
This study examined the presence of autistic traits in a sample of adult women diagnosed with different Eating Disorders (ED), and explored the concurrent role of autistic traits and sensory sensitivity in influencing both their eating disorder symptomatology and their autism-related eating behaviours. Seventy-five women with different ED (Anorexia Nervosa, Bulimia Nervosa, Binge-Eating Disorder, Other Specified Feeding or Eating Disorder) completed the Eating Attitude Test (EAT-26), the Autism Quotient (AQ), the Ritvo Autism Asperger Diagnostic Scale-Revised (RAADS-R), the Sensory Perception Quotient - Short Form 35 item (SPQ-SF35) and the Swedish Eating Assessment for Autism Spectrum Disorders (SWEAA). Twelve percent of participants scored above the cut-off on both the AQ and the RAADS-R, while 68% scored above the cut-off on the RAADS-R only. A mediation analysis revealed that the association between sensory sensitivity (SPQ-SFR35) and scores on both the EAT-26 and the SWEAA was significantly mediated by the presence of autistic traits (RAADS-R). These findings, first, confirm the presence of autistic traits in individuals with ED; second, they show that a lower sensory threshold (i.e., a higher sensory sensitivity) is associated with a higher presence of autistic traits which were, in turn, positively associated with dysfunctional eating behaviours typical of ED and ASD. This study ultimately highlights the importance of further research on autistic traits across all diagnostic categories of ED.
Keywords: Eating disorders, Autism-related eating behaviours, Autistic traits, Sensory sensitivity, SWEAA.
Subject terms: Neuroscience, Psychiatric disorders
Introduction
Over the last few decades, considerable interest has been directed to the study of the relationship between Autism Spectrum Disorders (ASD) and Eating Disorders (ED). ASD are defined as a group of neurodevelopmental conditions characterized by difficulties in social interaction and communication, restricted and repetitive patterns of behaviours and interests, and altered sensory sensitivity1. ED, on the other hand, are characterized by abnormal eating habits and attitudes towards food and body image, that significantly impact an individual’s physical and psychological well-being. The DSM-5-TR1 lists specific diagnostic categories such as Anorexia Nervosa (AN), Bulimia Nervosa (BN), Binge-Eating Disorder (BED), Avoidant/Restrictive Food Intake Disorder (ARFID), and provides a diagnostic category, the Other Specified Feeding or Eating Disorder (OSFED), that acknowledges individuals who are experiencing eating-related challenges, distress and/or impairment but don’t meet the full criteria for a specific diagnosis.
The relationship between Autism and Eating disorders: not only anorexia nervosa
The majority of the studies addressing the similarities between ASD and ED focused on AN, pointing out the presence of common psychopathological features such as: atypical social cognition, difficulties in processing emotions, weak central coherence (i.e., the difficulty in approaching daily life events and problems in a global perspective), cognitive rigidity2, impaired theory of mind (i.e. the ability to interpret other individuals’ mental states), altered set shifting (the ability to shift one’s own attention between different actions)3, difficulties in feeling and manifesting empathy4, high levels of alexithymia5,6, and social anxiety7,8.
Supporting these observations, the co-occurrence of AN and ASD within the same family has been reported3,9, and further research has emerged focusing specifically on the presence of autistic traits in individuals with AN. It is now recognized that autism features extend beyond those with a clinical diagnosis, existing on a continuum within the general population and manifesting at milder levels as sub-threshold autistic characteristics10–12. Screening measures indicate that patients with AN score similarly to individuals with ASD on the Autism Spectrum Quotient (AQ), a validated self-report measure of autistic traits13, thus demonstrating the presence of difficulties with social skills, communication and flexibility in AN14. However, despite these insights, only a limited number of studies have investigated the presence of autistic traits in ED other than AN15. A recent study found that patients with AN, BN, and BED showed subthreshold autistic traits more frequently than healthy control subjects, in a transdiagnostic fashion16. The authors highlighted that the presence of autistic traits might not be simply considered as an effect of severe malnutrition, since these traits were also observed among patients without a restrictive eating disorder. Consistently, it has also been proved that a considerable amount of remitted patients with AN continued to show higher autistic symptomatology in comparison with healthy controls17.
In consideration of the diagnostic transition theory of ED (i.e. the fluctuation over time of clinical presentation between ED diagnostic categories during the course of illness)18–20, it seems relevant to investigate the presence of autistic traits and autism-related features across the spectrum of ED regardless of patients’ categorial diagnosis. As a matter of fact, the presence of reduced cognitive flexibility has been pointed out both in patients diagnosed with AN and with BN21, and the presence of theory of mind difficulties has been observed in patients with BN and OSFED22. Additionally, in a study investigating autistic features in subjects with Orthorexia Nervosa (a proposed eating disorder characterized by an excessive preoccupation with eating healthy food), the participants showed a higher prevalence of autistic traits, particularly in the “inflexibility and adherence to routine” and “restricted interests and rumination” domains23. Finally, there is increasing research on the connection between autistic phenotypes and ARFID24,25, a condition impacting individuals with extreme and narrow food preferences, leading to a failure to meet nutritional needs through oral intake. Possible causes include a lack of interest in eating, alterations in sensory sensitivity, or food avoidance due to the fear of aversive consequences, such as gastrointestinal distress1.
From a psychopathological point of view, it has been hypothesized that rather than exerting a causative role, autistic traits may act as maintaining factors of pathological eating behaviours, through cognitive rigidity, anhedonia and social difficulties26. In fact, it appears that, once triggered, pathological eating behaviours tend to persist in a rigid fashion, suggesting that ASD-like neuropsychological traits may perpetuate the typical behaviours of ED4. Another possibility is that the challenges in interpersonal relationships intrinsic to ASD might lead the individual to social isolation, ultimately promoting the emergence and maintenance of behaviours and cognitive patterns associated with ED17.
Sensory sensitivity and autistic traits in ED
Sensory sensitivity alterations have been reported in both ASD1 and ED, although the literature on sensory sensitivity in the latter is less extensive.
Most studies on ASD have identified various changes in both overall sensory sensitivity and specific sensory domains, as well as a dysregulation in pain response27; notably, it has also been found that perceptual abnormalities predict the severity of autistic traits, such as difficulties in social interactions28. Some authors have even attempted to explain the pathophysiology of ASD by focusing on how individuals perceive sensations both from the external environment and within their own bodies (i.e., sensory perception and interoception)29.
Similarly, a general and domain-specific (gustative, kinetic/vestibular, somatosensory/tactile) sensory hyper-responsivity has been found to be positively correlated with ED severity, evaluated with the Eating Attitude Test-26 (EAT-26) and the Eating Disorder Inventory-2 (EDI-2), two validated screening questionnaires for ED27. Other studies have demonstrated that many of the identified sensory alterations tend to persist despite ED being treated, especially abnormalities in taste, smell, visual perception and discrimination, and somatosensory integration27,30,31. Additionally, it has been proposed that central symptoms of ED may reflect a deficit in multisensorial processing32, and that in patients with AN (and possibly in patients with other ED) sensory hypersensitivity may explain the altered body image perception that patients experience, ultimately representing a potential risk factor for the development of ED33.
Few studies directly investigated the potential relationship between autistic traits, sensory sensitivity and eating disorders phenomenology in patients with ED and individuals with ASD. Kinnaird and colleagues conducted a comparative analysis involving two distinct cohorts of individuals diagnosed with AN, distinguished by varying degrees of autistic traits (assessed via the AQ). They found that the cohort characterized by elevated autistic traits exhibited greater levels of hypersensitivity and a higher probability of experiencing bodily sensations as adverse, which in turn leads to avoiding those sensory experiences34. On the other hand, previous research by our group has reported the following findings. First, adults with ASD without intellectual disability, compared to neurotypical healthy controls, showed a higher prevalence of eating disturbances typical of the autistic spectrum, as evaluated by the Swedish Eating Assessment for Autism Spectrum Disorders (SWEAA)35, but also of other ED symptoms and concerns, as per EAT-2636. Second, a direct comparison between women with ASD without intellectual disability and patients with ED indicated that while the group with ASD did not reach the same level of ED symptomatology, both groups scored similarly on the SWEAA, suggesting shared alimentary difficulties characteristic of autism; notably, in both groups these scores were higher than those of neurotypical healthy controls37. Third, sensory sensitivity alterations in individuals with ASD were associated both with ED symptomatology, evaluated by the EAT-26, and with autistic-eating behaviours, as per SWEAA; in particular, hypersensitivity in the vision domain was linked to higher levels of both ED symptoms and autistic eating behaviours, while hyposensitivity in the taste domain was associated with higher levels of ED symptoms only38. Overall, our previous findings point towards an association between the sensory sensitivity threshold and dysfunctional eating behaviours.
In view of this background, the present study aimed to examine the presence of autistic traits in a sample of adult women diagnosed with ED, and the concurrent role of autistic traits and sensory sensitivity in both their eating disorder symptomatology and their autism-related eating behaviours.
Methods
Participants
Seventy-five consecutive women with different ED (AN, BN, BED, OSFED) were recruited at the tertiary-level outpatient clinic of ASST Santi Paolo e Carlo in Milan, Italy. The diagnosis of ED was made by a psychiatrist according to DSM-5-TR criteria1. The presence of any medical or psychiatric comorbidity was evaluated during the diagnostic process through a complete anamnestic interview (which includes questions on medical history). Exclusion criteria were: (i) age less than 18 years old; (ii) inability to understand the researcher’s instruction and/or diagnosed intellectual disabilities; (iii) presence of psychotic disorders; (iv) biological sex and self-declared gender different than female. The study was approved by the ethics committee of S. Paolo General Hospital and was performed in accordance with the Declaration of Helsinki. All participants signed an online written informed consent before completing the questionnaire and were free to withdraw from the study at any time without giving any further explanation.
Procedure
First, sociodemographic information was collected, including age, education level, employment status, and living environment. Second, participants completed the following validated self-report questionnaires:
The Autism Quotient (AQ), a 50-item questionnaire measuring the degree to which an adult without intellectual disabilities presents autistic traits; a Total Score of 32 or more is indicative of the presence of ASD traits13.
The Ritvo Autism Asperger Diagnostic Scale-Revised (RAADS-R), an 80-item instrument initially designed to assist clinicians diagnosing ASD in adults, and useful to identify autistic traits; a Total Score of 66 or more suggests that the participants should be further assessed for ASD39.
The Eating Attitude Test – 26 items (EAT-26), which measures symptoms and concerns specific to eating disorders; a participant with a Total Score of 20 or higher is deemed at risk of having an Eating Disorder and is consequently advised to undergo a specialized clinical evaluation. Three further subscales were calculated: (i) Dieting: evaluates the participant’s attention to calories ingested and burned doing physical exercise, their desire to be thin, and the sense of guilt after eating (e.g., “I feel extremely guilty after eating”); (ii) Bulimia: assess the presence of bulimic and binge-eating symptoms and concern about food (e.g., “I have gone on eating binges where I feel that I may not be able to stop”); (iii) Oral control: investigates the participants’ self-control over eating and the perceived pressure from others to gain weight (e.g. “I feel that other pressure me to eat”)40,41.
The Swedish Eating Assessment for Autism Spectrum Disorders (SWEAA), a 65-item scale assessing the presence of autism-related eating behaviours; participants are asked to reply to each item on a 5-point Likert Scale, ranging from 0 (Never) to 4 (Always). A Total Score and the following subscales were calculated: (i) Perception, assessing sensitivity to sensory input related to food, such as smell, taste, texture or sound (e.g. “I am oversensitive to certain flavours”; (ii) Motor Control, investigating issues in different aspects of movement that can influence eating behaviour, such as problems chewing or spilling, as well as table manners (e.g. “I spill while I eat”); (iii) Purchase of Food, assessing the grade of control over purchases, such as brands or type of groceries (e.g. “My food must be of a certain brand”); (iv) Eating Behaviour, assessing participants’ selectivity in eating, their limited repertoire and their difficulties in trying new foods (e.g. “I only eat a limited menu, maximum of 10 dishes”); (v) Mealtime Surrounding, investigating routines around mealtime such as where to eat and how cutlery is placed (e.g. “I find it difficult to change seats at the dining table); (vi) Social Situation at Mealtime, assessing difficulties in adapting their own behaviour to that of others or enjoying company during a meal (e.g. “I look down at my food most of the time during a meal”); (vii) Other Behaviour Associated with Disturbed Eating, evaluating the presence of other typical symptoms of an eating disorder (e.g. “I induce vomiting after meals”); (viii) Hunger/Satiety, assessing the ability to perceive when hungry or full (e.g. “I feel when I am hungry”) (ix) Simultaneous Capacity, evaluating the difficulties to do two things simultaneously during a meal (e.g. “I find it difficult to do two things simultaneously during a meal, like chewing and cutting the food); (x) Pica, investigating whether participants eat inedible things (e.g. “I eat things that others consider inedible, such as mortar or soil”), (xi) Autism Quotient, including items specifically selected by the SWEAA authors from the validated questionnaire Autism Quotient (AQ, averaging items 61–65, not included in the Total Score)35.
The Sensory Perception Quotient - Short Form 35 item (SPQ-SF35), investigating hyper- or hyposensitivity in the five modes of perception, able to discriminate between adults with ASD and neurotypical adults. A Total Score and five subscales (Vision, Taste, Smell, Touch, Hearing) were calculated, with higher scores meaning higher sensory threshold, thus suggesting a pattern of hyposensitivity in the sensory domain assessed (e.g. “I would notice if someone added 5 grains of salt to my cup of water”)42.
Statistical analysis
A priori power analysis conducted in G.Power 3.1 (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower) indicated that, with a power (1-β) = 0.80 and a significance threshold α = 0.05, to detect an effect size F = 0.2 (considered low according to Cohen’s criteria43), the total sample size should be N = 70. Hence, for the present study, a total of 75 participants was recruited.
Statistical analysis was conducted with the software Statistical Package for Social Science (SPSS), version 27 (https://www.ibm.com/products/spss-statistics) and in RStudio 2023.12 (https://posit.co/download/rstudio-desktop/); significance threshold was set at α ≤ 0.05, and all tests were 2-tailed. First, the normality distribution of the variables of interest (Total Scores of SPQ-SF35, EAT-26, SWEAA and RAADS-R) was tested using the Kolmogorov-Smirnov test. Consequently, descriptive statistics were calculated for the entire sample, including sociodemographic and psychometric variables; given the wide age range of our sample (from 18 to 57 years old)20, Pearson’s correlation analysis was conducted to ensure that age did not influence our variables of interest. Second, two mediation analysis were conducted with: SPQ-SF35 as predictor, RAADS-R as mediator, and EAT-26 and SWEAA as dependent variable, separately. Only the RAADS-R, and not the AQ, has been included in the mediation analysis because of its high specificity (100%) and sensitivity (97%)39, and because the AQ was originally validated in a sample mainly composed of male subjects44, whereas our sample is only composed by female individuals. Moreover, the choice of sensory sensitivity as predictor and autistic traits as mediator is rooted in the recent literature arguing that perceptual abnormalities predict the severity of autistic traits28,29.
Results
Sociodemographic and psychometric information
The mean age of subjects was 25.56 (SD = 9.58, min: 18, max: 57); age was not correlated with the Total Scores of the SPQ-SF35, EAT-26, SWEAA and RAADS-R (all p > 0.05); all participants were females. Further sociodemographic details are reported in Table 1.
Table 1.
Sociodemographic and clinical features.
| Variable | Value | |
|---|---|---|
| Age, mean (SD) | 25.56 (9.58) | |
| Diagnosis, N (%) | Anorexia Nervosa | 37 (49.3) |
| Bulimia Nervosa | 16 (21.3) | |
| Binge-Eating Disorders | 10 (13.3) | |
| OSFED | 12 (16) | |
| BMI per diagnosis, mean (SD) | Anorexia Nervosa | 15.56 (3.64) |
| Bulimia Nervosa | 18.39 (6.05) | |
| Binge-Eating Disorders | 31.67 (10.59) | |
| OSFED | 18.09 (6.08) | |
| Education, N (%) | Middle School | 11 (14.67) |
| 3-year professional license | 4 (5.33) | |
| Diploma | 46 (61.33) | |
| Bachelor’s degree | 9 (12.00) | |
| Master’s degree | 5 (6.67) | |
| Employment, N (%) | Student | 46 (61.33) |
| Employed | 25 (33.33) | |
| Unemployed | 4 (5.33) | |
| Living condition, N (%) | Living Alone | 9 (12) |
| Living with parents | 51 (68) | |
| Living with partner | 10 (13) | |
| Living with flatmates | 5 (6) | |
| AQ Total Score, mean (SD) | 23.23 (6.74) | |
| AQ Total Score, N (%) | Below cut-off | 66 (88) |
| Above cut-off | 9 (12) | |
| AQ Social skills, mean (SD) | 4.4 (2.56) | |
| AQ Attention switching, mean (SD) | 6.6 (1.93) | |
| AQ Attention to detail, mean (SD) | 5.5 (2.24) | |
| AQ Comunication, mean (SD) | 3.3 (2.01) | |
| AQ Imagination, mean (SD) | 3.3 (1.85) | |
| RAADS-R Total Score, mean (SD) | 84.84 (41.96) | |
| RAADS-R Total Score, N (%) | Below cut-off | 24 (32) |
| Above cut-off | 51 (68) | |
| RAADS-R Social Relatedness, mean (SD) | 39.39 (21.02) | |
| RAADS-R Circumscribed Interests, mean (SD) | 18.18 (9.44) | |
| RAADS-R Language, mean (SD) | 6.6 (4.72) | |
| RAADS-R Sensory-motor, mean (SD) | 20.20 (12.82) | |
| EAT-26 Total Score, mean (SD) | 36.36 (19.00) | |
| EAT-26 Total Score, N (%) | Below cut-off | 20 (26.67) |
| Above cut-off | 55 (73.33) | |
| EAT-26 Dieting, mean (SD) | 20.20 (10.81) | |
| EAT-26 Bulimia and Food Preoccupation, mean (SD) | 7.7 (5.03) | |
| EAT-26 Oral Control, mean (SD) | 8.8 (5.81) | |
| SWEAA Total Score, mean (SD) | 1.52 (5.55) | |
| SWEAA Perception, mean (SD) | 1.72 (7.83) | |
| SWEAA Motor Control, mean (SD) | 0.96 (9.65) | |
| SWEAA Purchase of Food, mean (SD) | 2.34 (3.07) | |
| SWEAA Eating Behaviour, mean (SD) | 1.74 (7.82) | |
| SWEAA Mealtime Surrounding, mean (SD) | 1.91 (9.81) | |
| SWEAA Social Situation at Mealtime, mean (SD) | 1.46 (4.57) | |
| SWEAA Other Behaviour Associated with Disturbed Eating, mean (SD) | 1.07 (0.65) | |
| SWEAA Hunger/Satiety, mean (SD) | 1.54 (5.05) | |
| SWEAA Simultaneous Capacity, mean (SD) | 0.88 (8.21) | |
| SWEAA Pica, mean (SD) | 0.05 (0.28) | |
| SPQ-SF35 Total Score, mean (SD) | 52.52 (14.95) | |
| SPQ-SF35 Vision, mean (SD) | 10.10 (3.46) | |
| SPQ-SF35 Smell, mean (SD) | 15.15 (4.70) | |
| SPQ-SF35 Taste, mean (SD) | 4.4 (2.66) | |
| SPQ-SF35 Touch, mean (SD) | 13.57 (5.61) | |
| SPQ-SF35 Hearing, mean (SD) | 8.8 (2.82) | |
AQ = Autism Quotient; BMI = Body Mass Index; EAT-26 = Eating Attitude Test – 26 items; OSFED = Other Specified Feeding or Eating Disorder; N = numerosity; RAADS-R = Ritvo Autism Asperger Diagnostic Scale-Revised; SD = Standard deviation. SPQ-SF35 = Sensory Perception Quotient - Short Form 35 items; SWEAA = Swedish Eating Assessment for Autism spectrum disorders.
There was a higher prevalence of patients with AN, whereas autistic traits were slightly more frequent in patients with BN. In particular, our sample was composed of 37 (49.3%) women diagnosed with AN (average BMI: 15.56 ± 3.64), 16 (21.3%) with BN (average BMI: 18.39 ± 6.05), 10 (13.3%) with BED (average BMI: 31.67 ± 10.59), and 12 (16%) with OSFED (average BMI: 18.09 ± 6.08). Nine (12%) women scored above the cut-off at the AQ and 51 (68%) scored above the cut-off at the RAADS-R. All 9 participants who were positive at the AQ were positive also at the RAADS-R; 42 women were positive at the RAADS-R but not at the AQ, and 24 patients scored below the cut-off to both the AQ and the RAADS-R (Table 1). Regarding the specific ED diagnosis, 7 (18.9%) patients diagnosed with AN scored above the cut-off at AQ and 26 (70.3%) at RAADS; only 1 subject (6.3%) of the BN subgroup scored above the cut-off at AQ, whereas 12 subjects (75%) scored above the RAADS threshold; in the BED population no patient scored above the cut-off at the AQ, while 6 patients (60%) exceeded the threshold of RAADS; lastly, in the OSFED population, only 1 (8.3%) patient scored above the cut-off at AQ, whereas 7 (58.3%) did for RAADS (Table 2).
Table 2.
Prevalence of autistic traits across ED diagnosis.
| Diagnosis | Autistic traits | AQ | RAADS-R | ||
|---|---|---|---|---|---|
| Prevalence | % | Prevalence | % | ||
| AN | No autistic traits | 30 | 81.1 | 11 | 29.7 |
| Autistic traits | 7 | 18.9 | 26 | 70.3 | |
| Total | 37 | 100 | 37 | 100 | |
| BN | No autistic traits | 15 | 93.8 | 4 | 25 |
| Autistic traits | 1 | 6.3 | 12 | 75 | |
| Total | 16 | 100 | 16 | 100 | |
| BED | No autistic traits | 10 | 100 | 4 | 40 |
| Autistic traits | 0 | 0 | 6 | 60 | |
| Total | 10 | 10 | 10 | 100 | |
| OSFED | No autistic traits | 11 | 91.7 | 5 | 41.7 |
| Autistic traits | 1 | 8.3 | 7 | 58.3 | |
| Total | 12 | 100 | 12 | 100 | |
AQ = Autism Quotient; RAADS-R = Ritvo Autism Asperger Diagnostic Scale-Revised; AN = Anorexia Nervosa; BN = Bulimia Nervosa; BED = Binge Eating Disorder; OSFED = Other Specified Feeding or Eating Disorder.
Mediation analysis
The Total Scores of the SPQ-SF35, EAT-26, SWEAA and RAADS-R resulted normally distributed according to the Kolmogorov-Smirnov test (all p = 0.200).
EAT-26
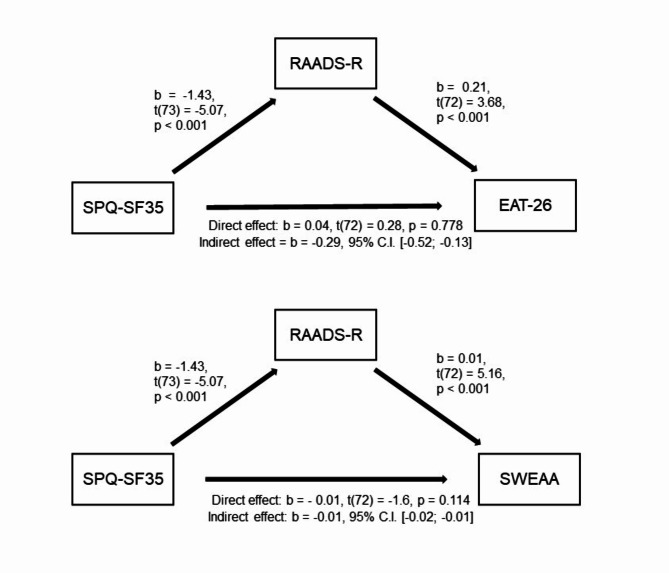
The mediation analysis showed: (i) significant associations between the SPQ-SF35 (predictor) and the RAADS-R (mediator) (b = -1.43, t(73) = -5.07, p < 0.001), and between the RAADS-R (mediator) and EAT-26 (dependent variable) (b = -0.25, t(72) = 3.68, p < 0.001); (ii) a non-significant Total Effect (i.e., not controlling for RAADS-R) of SPQ-SF35 on EAT-26 (b = − 0.25, t(73) = -1.71, p = 0.091); (iii) a non-significant Direct Effect (i.e. controlling for RAADS-R) of SPQ-SF35 on EAT-26, (b = − 0.04, t(72) = 0.28, p = 0.778); (iv) a significant Indirect Effect (i.e., mediated by the RAADS-R) of SPQ-SF35 on EAT-26 (b = -0.29, 95% C.I. [-0.52; -0.13]). The model was overall significant (R2 = 0.19, F(72) = 8.49, p < 0.001). Therefore, the relationship between the sensory sensitivity as per SPQ-SF35 and the eating disturbances as per EAT-26 was totally mediated by the presence of autistic traits, as per RAADS-R.
SWEAA
The mediation analysis showed: (i) significant associations between the SPQ-SF35 (predictor) and the RAADS-R (mediator) (b = -1.43, t(73) = -5.07, p < 0.001), and between the RAADS-R (mediator) and SWEAA (dependent variable) (b = 0.01, t(72) = 5.16, p < 0.001); (ii) a significant Total Effect (i.e., not controlling for RAADS-R) of SPQ-SF35 on SWEAA (b = − 0.02, t(73) = -4.23, p < 0.001); (iii) a non-significant Direct Effect (i.e., controlling for the RAADS-R) of SPQ-SF35 on SWEAA (b = − 0.01, t(72) = -1.6, p = 0.114); (iv) a significant Indirect Effect (i.e., mediated by the RAADS-R) of SPQ-SF35 on SWEAA (b = -0.01, 95% C.I. [-0.02; -0.01]). The model was overall significant (R2 = 0.41, F(72) = 25.42, p < 0.001). Therefore, the relationship between the sensory sensitivity as per SPQ-SF35 and the autism-related eating behaviour as per SWEAA was totally mediated by the presence of autistic traits, as per RAADS-R (Fig. 1).
Figure 1.
Mediation analysis. Autistic traits, as per RAADS-R, mediate the association between sensory sensitivity as per SPQ-SF35 and both eating disorder symptomatology (as per EAT-26, above) and autism-related eating behaviour (as per SWEAA, below). Abbreviations: EAT-26 = Eating Attitude Test – 26 items; RAADS-R = Ritvo Autism Asperger Diagnostic Scale-Revised; SPQ-SF35 = Sensory Perception Quotient - Short Form 35 items; SWEAA = Swedish Eating Assessment for Autism spectrum disorders.
Discussion
The first aim of this study was to assess the prevalence of autistic traits in a sample of adult women diagnosed with ED. In our sample, we found that 9 (12%) women scored above the cut-off at both the AQ and the RAADS-R, while 51 (68%) scored above the cut-off at the RAADS-R. To the best of our knowledge, only three studies have previously used the RAADS-R to screen autistic traits in eating disorder populations, mostly considering restrictive ED45–47, whereas the AQ has been more widely used48–54. Our results are in line with the different psychometric properties of the two questionnaires: the AQ has lower sensitivity compared to the RAADS-R, which is both highly specific (100%) and sensitive (97%)39. Moreover, as previously stated, the AQ was originally validated in a sample prevalently composed of male subjects44, whereas our sample is female-only. Of note, similar results have been obtained in other studies with female-only or female-prevalent samples55,56.
Second, we investigated the association between autistic traits as per RAADS-R, sensory sensitivity as per SPQ-SF35, eating disorder symptomatology as per EAT-26, and autism-related eating behaviours as per SWEAA. We found that the association between sensory sensitivity and both the EAT-26 and the SWEAA was significantly mediated by the presence of autistic traits. In particular, a lower sensory threshold (i.e., a higher sensory sensitivity) was associated with a higher presence of autistic traits, which were, in turn, positively associated with the presence of eating anomalies. On the one hand, this is not surprising, considering that the SWEAA specifically evaluates the eating habits of ASD patients; on the other hand, our results are in line with previous literature on the subject and further supports the association between sensory sensitivity, autistic traits and ED. For example, this could imply the possibility that having a heightened sensitivity may lead to a different perception and enjoyment of food, which in turn can constitute a risk factor for the development of ED. Previous studies showed that vision has a role in food intake and satiety: eating while blindfolded can reduce the quantity of ingested food by 22% 57, and the size of the first bite is influenced by the identification of food properties through vision and smell58; moreover, hypersensitivity in the vision domain might influence body image perception and thus could contribute to the development and maintenance of ED. Hypersensitivity in the other sensory domains can be understood in light of autism-related features such as insistence on sameness, attention to details, and the sensory abnormalities commonly associated with ASD; in terms of eating habits, it might lead to selective eating behaviours, such as increased sensitivity to food texture and mixed flavours59.
Autistic traits can influence how individuals experience eating disorders, affecting their treatment needs. For example, patients with autistic traits often face difficulties in expressing their needs and communicating effectively during treatment60, and evidence suggests that they might benefit more from individual sessions rather than group sessions61. Although there is limited information about adaptations to existing eating disorder treatments for those with comorbid ASD, recognizing these traits allows for a more individualized approach, thus potentially improving engagement of patients and overall treatment effectiveness61.
Strengths and limits
We acknowledge the limitations of our study. First, all data were collected using self-report questionnaires, whereas for a formal diagnosis of ASD through a standardized clinician-administered interview (e.g., the Autism Diagnostic Observation Scale) is usually required; it is worth mentioning, however, that the research on subthreshold autistic traits is still ongoing, and, to the best of our knowledge, no clinician-administer standardized instrument with the aim of investigating these features has been validated yet. Second, the limited sample size prevented comparisons between subgroups defined by specific ED diagnosis and a detailed investigation of the relationship between SPQ-SF35, EAT-26 and SWEAA subscales. Moreover, male subjects and patients with ARFID could not be included due to the underrepresentation of such categories among ED; future studies should consider a larger and more balanced sample of participants. Third, other psychiatric conditions that may mediate or explain the presence of autistic traits in the study sample have not been considered. Finally, the sample used in the study consisted solely of female patients, which may affect the results due to the lack of diversity; however, our findings could be of interest for future research, considering the growing body of literature on the topic of gender-specific presentations in ASD, and notably about AN being considered a potential female-specific manifestation of ASD15.
The strength of this study lies in the comprehensive exploration of autistic traits in ED patients across various diagnoses (AN, BN, BED, OSFED). Although there was a higher representation of patients with AN compared to other ED categories in the sample (Table 2), our findings underscore the necessity for systematic investigations spanning all ED diagnostic categories. It is becoming progressively evident that identifying and measuring the levels of autistic traits in patients with ED is crucial not only for a better understanding of the pathophysiology of these disorders, but also to customize therapeutic interventions, addressing the cognitive flexibility issues and socio-emotional challenges they encounter. Additionally, the study establishes a foundation for examining the interplay between sensory sensitivity and dysfunctional eating behaviours in both ED and ASD. Subsequent research in this realm has the potential to improve the prognosis and outcomes for affected patients.
Acknowledgements
VN was partially supported by the “Borsa di Studio Rotary Dott. Gabriele Corbelli”- Milan Rotary Clubs.
Author contributions
All authors contributed to the study conception and design. Material preparation and data analysis were performed by GI, VN, FL, BM.Data collection was mainly performed by ACC, MO, AM, CAR, MT, LR, SA, SB. The first draft of the manuscript was written by GI, VN, FL, BM. RF, SA, SB, OG, BD administered the project and revised the manuscript for intellectual content. All authors agreed on the final version of the manuscript.
Funding
“Aldo Ravelli” Research Center for Neurotechnology and Experimental Brain Therapeutics partially supported the study.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gianmarco Ingrosso and Veronica Nisticò share first authorship
Contributor Information
Veronica Nisticò, Email: veronica.nistico@unimi.it.
Benedetta Demartini, Email: benedetta.demartini@asst-santipaolocarlo.it.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5-TR (American Psychiatric Association Publishing, 2022).
- 2.Brede, J. et al. For me, the Anorexia is just a Symptom, and the cause is the Autism’: Investigating restrictive eating disorders in Autistic Women. J. Autism Dev. Disord. 50, 4280–4296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huke, V., Turk, J., Saeidi, S., Kent, A. & Morgan, J. F. Autism spectrum disorders in eating disorder populations: A systematic review. Eur. Eat. Disord Rev. J. Eat. Disord Assoc.21, 345–351 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Kerr-Gaffney, J., Harrison, A. & Tchanturia, K. Cognitive and affective Empathy in Eating disorders: A systematic review and Meta-analysis. Front. Psychiatry. 10, 102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinnaird, E., Stewart, C. & Tchanturia, K. Investigating alexithymia in autism: A systematic review and meta-analysis. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 55, 80–89 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westwood, H., Kerr-Gaffney, J., Stahl, D. & Tchanturia, K. Alexithymia in eating disorders: systematic review and meta-analyses of studies using the Toronto Alexithymia Scale. J. Psychosom. Res.99, 66–81 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerr-Gaffney, J., Harrison, A. & Tchanturia, K. Social anxiety in the eating disorders: A systematic review and meta-analysis. Psychol. Med.48, 2477–2491 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Spain, D., Sin, J., Linder, K. B., McMahon, J. & Happé, F. Social anxiety in autism spectrum disorder: A systematic review. Res. Autism Spectr. Disord. 52, 51–68 (2018). [Google Scholar]
- 9.Gillberg, C. Are autism and anorexia nervosa related? Br. J. Psychiatry. 142, 428–428 (1983). [DOI] [PubMed] [Google Scholar]
- 10.Dell’Osso, L. et al. Defining the optimal threshold scores for adult Autism Subthreshold Spectrum (AdAS Spectrum) in Clinical and General Population. Clin. Pract. Epidemiol. Ment Health CP EMH. 16, 204–211 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dell’Osso, L. et al. The adult Autism Subthreshold Spectrum (AdAS) model: A neurodevelopmental approach to mental disorders. Off J. Ital. Soc. Psychopathol.24, 118–124 (2018). [Google Scholar]
- 12.Sulla, F. et al. The moderator effect of Subthreshold autistic traits on the relationship between quality of life and internet addiction. Healthcare. 11, 186 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J. & Clubley, E. The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 31, 5–17 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Westwood, H. & Tchanturia, K. Autism spectrum disorder in Anorexia Nervosa: an updated literature review. Curr. Psychiatry Rep.19, 41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpita, B., Muti, D., Cremone, I. M., Fagiolini, A. & Dell’Osso, L. Eating disorders and autism spectrum: Links and risks. CNS Spectr.27, 272–280 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Dell’Osso, L. et al. Subthreshold autism spectrum disorder in patients with eating disorders. Compr. Psychiatry. 81, 66–72 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Kerr-Gaffney, J., Halls, D., Harrison, A. & Tchanturia, K. Exploring relationships between Autism Spectrum disorder symptoms and eating disorder symptoms in adults with Anorexia Nervosa: A Network Approach. Front. Psychiatry. 11, 401 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellini, G. et al. Diagnostic crossover and outcome predictors in eating disorders according to DSM-IV and DSM-V proposed criteria: a 6-year follow-up study. Psychosom. Med.73, 270–279 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Milos, G., Spindler, A., Schnyder, U. & Fairburn, C. G. Instability of eating disorder diagnoses: Prospective study. Br. J. Psychiatry J. Ment Sci.187, 573–578 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaumberg, K. et al. Patterns of diagnostic transition in eating disorders: a longitudinal population study in Sweden. Psychol. Med.49, 819–827 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchanturia, K. et al. Cognitive flexibility in anorexia nervosa and bulimia nervosa. J. Int. Neuropsychol. Soc. JINS. 10, 513–520 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Medina-Pradas, C., Navarro, J. B., Alvarez-Moya, E. M., Grau, A. & Obiols, J. E. Emotional theory of mind in eating disorders. Int. J. Clin. Health Psychol.12, 189–202 (2012). [Google Scholar]
- 23.Carpita, B. et al. Investigating the relationship between orthorexia nervosa and autistic traits in a university population. CNS Spectr.27, 613–620 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Yule, S. et al. Nutritional Deficiency Disease secondary to ARFID symptoms Associated with Autism and the broad autism phenotype: A qualitative systematic review of Case reports and Case Series. J. Acad. Nutr. Diet.121, 467–492 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Thomas, J. J. et al. Avoidant/Restrictive food intake disorder: A three-Dimensional Model of Neurobiology with implications for etiology and treatment. Curr. Psychiatry Rep.19, 54 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tchanturia, K. et al. Exploring autistic traits in anorexia: a clinical study. Mol. Autism. 4, 44 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brand-Gothelf, A. et al. Sensory modulation disorder symptoms in anorexia nervosa and bulimia nervosa: A pilot study. Int. J. Eat. Disord. 49, 59–68 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Mayer, J. L. The relationship between autistic traits and atypical sensory functioning in Neurotypical and ASD adults: A Spectrum Approach. J. Autism Dev. Disord. 47, 316–327 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Quattrocki, E. & Friston, K. Autism, oxytocin and interoception. Neurosci. Biobehav Rev.47, 410–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunwald, M. et al. Haptic perception in anorexia nervosa before and after weight gain. J. Clin. Exp. Neuropsychol.23, 520–529 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Wagner, A. et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacol. Off Publ Am. Coll. Neuropsychopharmacol.33, 513–523 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Riva, G. & Dakanalis, A. Altered Processing and Integration of multisensory bodily representations and signals in eating disorders: A possible path toward the understanding of their underlying causes. Front. Hum. Neurosci.12, 49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calvo Sagardoy, R. et al. Heightened sensitivity to somatosensory stimuli in anorexia nervosa: An exploratory study with the SASTCA scale. Nutr. Hosp.31, 1413–1422 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Kinnaird, E. et al. Pragmatic sensory screening in Anorexia Nervosa and associations with autistic traits. J. Clin. Med.9, 1182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlsson, L., Råstam, M. & Wentz, E. The SWedish Eating Assessment for Autism spectrum disorders (SWEAA)-Validation of a self-report questionnaire targeting eating disturbances within the autism spectrum. Res. Dev. Disabil.34, 2224–2233 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Demartini, B. et al. Eating disturbances in adults with autism spectrum disorder without intellectual disabilities. Autism Res. Off J. Int. Soc. Autism Res.14, 1434–1443 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Nisticò, V. et al. Eating disturbances in eating disorders and in high-functioning autism spectrum disorders: A preliminary study. Eat. Weight Disord EWD. 27, 1555–1561 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Nisticò, V. et al. Brief report: sensory sensitivity is Associated with disturbed eating in adults with Autism Spectrum disorders without Intellectual Disabilities. J. Autism Dev. Disord. 10.1007/s10803-022-05439-9 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Ritvo, R. A. et al. The Ritvo Autism Asperger Diagnostic Scale-revised (RAADS-R): a scale to assist the diagnosis of Autism Spectrum Disorder in adults: An international validation study. J. Autism Dev. Disord. 41, 1076–1089 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dotti, A. & Lazzari, R. Validation and reliability of the Italian EAT-26. Eat. Weight Disord EWD. 3, 188–194 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Garner, D. M., Olmsted, M. P., Bohr, Y. & Garfinkel, P. E. The eating attitudes test: Psychometric features and clinical correlates. Psychol. Med.12, 871–878 (1982). [DOI] [PubMed] [Google Scholar]
- 42.Tavassoli, T., Hoekstra, R. A. & Baron-Cohen, S. The sensory perception quotient (SPQ): Development and validation of a new sensory questionnaire for adults with and without autism. Mol. Autism. 5, 29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Routledge, 1988). 10.4324/9780203771587
- 44.Allison, C., Auyeung, B. & Baron-Cohen, S. Toward brief red flags for Autism Screening: The short Autism Spectrum Quotient and the short quantitative checklist in 1,000 cases and 3,000 controls. J. Am. Acad. Child. Adolesc. Psychiatry. 51, 202–212e7 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Adamson, J. et al. Towards identifying a method of screening for autism amongst women with restrictive eating disorders. Eur. Eat. Disord Rev.30, 592–603 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babb, C. et al. A comparison of the eating disorder service experiences of autistic and non-autistic women in the UK. Eur. Eat. Disord Rev.30, 616–627 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vagni, D., Moscone, D., Travaglione, S. & Cotugno, A. Using the Ritvo Autism Asperger Diagnostic Scale-revised (RAADS-R) disentangle the heterogeneity of autistic traits in an Italian eating disorder population. Res. Autism Spectr. Disord. 32, 143–155 (2016). [Google Scholar]
- 48.Baron-Cohen, S. et al. Do girls with anorexia nervosa have elevated autistic traits? Mol. Autism. 4, 24 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Björnsdotter, M. et al. Grey matter correlates of autistic traits in women with anorexia nervosa. J. Psychiatry Neurosci.43, 79–86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Courty, A. et al. Levels of autistic traits in anorexia nervosa: A comparative psychometric study. BMC Psychiatry. 13, 222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinnaird, E., Stewart, C. & Tchanturia, K. The relationship of autistic traits to taste and olfactory processing in anorexia nervosa. Mol. Autism. 11, 25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Numata, N. et al. Associations between autism spectrum disorder and eating disorders with and without self-induced vomiting: an empirical study. J. Eat. Disord. 9, 5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Susanin, A., Cooper, M., Makara, A., Kuschner, E. S. & Timko, C. A. Autistic characteristics in youth with anorexia nervosa before and after treatment. Eur. Eat. Disord Rev. J. Eat. Disord Assoc.30, 664–670 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tchanturia, K., Adamson, J., Leppanen, J. & Westwood, H. Characteristics of autism spectrum disorder in anorexia nervosa: a naturalistic study in an inpatient treatment programme. Autism Int. J. Res. Pract.23, 123–130 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Karjalainen, L., Råstam, M., Paulson-Karlsson, G. & Wentz, E. Do autism spectrum disorder and anorexia nervosa have some eating disturbances in common? Eur. Child. Adolesc. Psychiatry. 28, 69–78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westwood, H. et al. Using the autism-spectrum quotient to measure autistic traits in Anorexia Nervosa: A systematic review and Meta-analysis. J. Autism Dev. Disord. 46, 964–977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linné, Y., Barkeling, B., Rössner, S. & Rooth, P. Vision and eating behavior. Obes. Res.10, 92–95 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Pereira, L. J. & van der Bilt, A. The influence of oral processing, food perception and social aspects on food consumption: A review. J. Oral Rehabil. 43, 630–648 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Chen, N., Watanabe, K., Kobayakawa, T. & Wada, M. Relationships between autistic traits, taste preference, taste perception, and eating behaviour. Eur. Eat. Disord Rev. J. Eat. Disord Assoc.30, 628–640 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kinnaird, E., Norton, C. & Tchanturia, K. Clinicians’ views on working with anorexia nervosa and autism spectrum disorder comorbidity: A qualitative study. BMC Psychiatry. 17, 292 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li, Z., Halls, D., Byford, S. & Tchanturia, K. Autistic characteristics in eating disorders: Treatment adaptations and impact on clinical outcomes. Eur. Eat. Disord Rev.30, 671–690 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.