The rationale for this project arises from some considerations. Clinical trials often use surrogate endpoints (response rates) rather than direct measurement of benefit (survivals) as primary objective of the study. When both are considered, in clinical studies focusing on CAR T therapy in Hematology they are often estimated starting from infusion (analysis per protocol [PP]) and not from leukapheresis (analysis per intention-to-treat [ITT]) or in a modified ITT modality, i.e. counting only patients who achieved the infusion timepoint [1–4].
Studies on CAR T-cell therapy lend to possible biases when they do not consider patients who undergo leukapheresis but finally are not infused for a variety of competing risks (e.g., time of leukapheresis and enrollment, use of bridging therapy [BT], lymphodepleting regimens and, and in the near future, autologous versus allogeneic products, different standard of care clinical practice or patient populations). This makes difficult to perform a robust comparison of both the different available CAR T products and the drugs which share the same indication. Starting from these premises, real-world studies and national registries could be useful tools in determining a true PP versus ITT analysis as a mirror of the real benefit and feasibility of CAR T therapy if it is considered a therapeutic path, including competitive risks. As a consequence, to test our thesis, we conceived a project to provide a comprehensive overview of the benefits of CAR T-cell therapy in patients with relapsed/refractory (r/r) large B-cell lymphomas (LBCL) in the real-world setting recomputing results in both PP and ITT basis to show that both modalities bring useful information, and to propose a new CAR T analysis specific endpoint with a dedicated event-free survival (CAR T EFS) for assessment of the actual drug benefit and feasibility, also as a driver for future research.
To reach our aim, a systematic review was conducted for real-world studies on the effectiveness of anti-CD19 CAR T-cell products (axicabtagene ciloleucel and tisagenlecleucel) administered to r/r LBCL patients between 2017 (after first approval) and October 2022 (Supplementary Fig. S1). Researchers and cooperative groups worldwide (n = 10) provided original data and re-estimated endpoints in both PP and ITT modalities (taking into account also subjects who entering in the therapeutic journey with leukapheresis but finally did not undergo infusion) when lacking in the original research: overall and complete response rates (ORR, CRR), progression-free and overall survivals (PFS, OS) and duration of response (Table 1). We asked to estimate the new endpoint we defined, i.e. ITT CAR T EFS, starting from leukapheresis to patients’ exit from therapeutic pathway due to any cause, whatever happened first, also before infusion (date of decision to not infuse). Meta-analysis was performed on ORR and CRR separately in both ITT and PP populations with random-effect models.
Table 1.
Studies overview and pooled analysis.
| Study | Location | ITT Median follow up months (95%CI) | ITT Population N | Not infused patients % | PP population N | ORR_ITT (95%CI) | ORR_PP (95%CI) | CRR_ITT (95%CI) | CRR_PP (95%CI) | mPFS_ITT months (95%CI) | mPFS_PP months (95%CI) | mEFS_ITT months (95%CI) | mEFS_PP months (95%CI) | mOS_ITT months (95%CI) | mOS_PP months (95%CI) | mDoR months (95%CI) | BT % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bachy et al. [6] | EU | 13.1 (12.6–13.4) | 809 | 9.9 | 729 | 67% (0.63–0.7) | 74% (0.71–0.77) | 47% (0.43–0.5) | 52% (0.48–0.55) | NA | 5.7 (4.1–7.5) | NA | NA | 16.8 (13.3–21.6) | 19 (NA) | 11 (7.6–17.2) | 78 |
| Bastos-Oreiro et al. [7] | EU | 11.0 (9–14) | 198 | 3.0 | 192 | 34% (0.27–0.41) | 35% (0.28–0.42) | 28% (0.22–0.35) | 29% (0.23–0.36) | 5.1 (3.5–6.7) | 5.6 (3.7–7.5) | 5.1 (3.53–6.67) | 3.3 (2.05–4.61) | 14.5 (NA) | 15 (NA) | 6.4 (0.7–22) | 82 |
| Ghafouri et al. [8]a | USA | 39.5 (36.6–49.9) | 62 | 14.5 | 53 | 68% (0.55–0.79) | 79% (0.66–0.89) | 55% (0.42–0.68) | 64% (0.50–0.77) | 5.57 (4–18.5) | 6.67 (3.6–22.7) | 5.23 (4–14.3) | 6.67 (3.6–22.7) | 11.1 (7.87–27.2) | 17.7 (7.9-NR) | 20.2 (10–31.9) | NA |
| Iacoboni et al. [9] | EU | 9.7 (0.1–24.5) | 91 | 17.6 | 75 | 49% (0.39–0.60) | 60% (0.48–0.71) | 26% (0.18–0.37) | 32% (0.22–0.44) | 4.6 (4.1–6.9) | 3 (2.6–4.7) | NA | 2.75 (1.2–4.3) | 11.1 (7.9-NR) | 10.7 (7.4–NR) | 8.9 (2.2-NR) | 87 |
| Kittai et al. [10]a | USA | 35.6 (29.9–41.7) | 215 | 10.2 | 193 | 68% (0.61–0.74) | 76% (0.69–0.82) | 42% (0.36–0.49) | 47% (0.40–0.54) | 5.43 (4.23–7.53) | 5.7 (3.57–10) | 4.97 (3.97–6.83) | 5.2 (3.3–9.2) | 19.7 (12.9–38.3) | 26.1 (15.5-NR) | 15.1 (11.8–19.8) | NA |
| Kwon et al. [11] | EU | 9.2 (8–11.5) | 307 | 15.0 | 261 | 48% (0.42–0.54) | 57% (0.50–0.62) | 32% (0.27–0.38) | 38% (0.32–0.44) | 4.8 (4.5–5.6) | 3.5 (3–6) | 4.8 (4.5–5.6) | 3.5 (3–6) | 11.7 (12.9–38.3) | 11.8 (1.3–31.4) | 14.1 (5.8-NR) | 80 |
| Mian et al. [12] | USA | 5.9 (4.5–10.8) | 38 | 28.9 | 27 | 58% (0.41–0.74) | 81% (0.62–0.94) | 32% (0.18–0.49) | 44% (0.25–0.65) | 5.59 (4.3-NR) | 9.07 (3.65-NR) | 5.59 (4.3-NR) | 9.07 (3.65-NR) | 10.9 (6.08-NR) | 13 (7.7-NR) | 7.69 (2.73-NR) | 50 |
| Nastoupil et al. [13] | USA | 13.8 (11.8–16.2) | 298 | 7.7 | 275 | 76% (0.70–0.80) | 82% (0.77–0.86) | 59% (0.53–0.65) | 64% (0.58–0.70) | 7.16 (5.65–12.4) | 8.31 (6.01–15.1) | NA | NA | NR | NR | NR (6.2 -NR) | 53 |
| Pinnix et al. [14] | USA | 11.1 (9.9–12.3) | 148 | 16.2 | 124 | 64% (0.56–0.72) | 77% (0.68–0.84) | 40% (0.32–0.48) | 48% (0.39–0.57) | 4.8 (3.7–6.0) | 6.2 (4.1–8.3) | NA | NA | 16.7 (7.1–26.2) | 21.9 (NA) | NA | 50 |
| Casadei et al. [15]a | EU | 16 (13.8–21.8) | 80 | 26.3 | 59 | 38% (0.27–0.49) | 51% (0.37–0.64) | 21% (0.13–0.32) | 29% (0.18–0.42) | 7.9 (6.23–14.9) | 5.6 (3.07–16.5) | 4.5 (3.77–7.47) | 5.6 (3.07–16.5) | 14.9 (13.4-NR) | 17.2 (12.6-NR) | 12.5 (5.1-NR) | 80 |
| Pooled analysisb | 29.7 (25.3–32.8) | 357 | 14.6 | 305 | 61% (0.56–0.66) | 71% (0.66–0.76) | 40% (0.35–0.45) | 47% (0.41–0.53) | 6.2 (4.9–7.9) | 5.67 (4.1–8.33) | 4.9 (4.1–6.2) | 5.2 (3.97–7.07) | 17.2 (13.4–26.6) | 24.1 (16.2–33.1) | 13.4 (10.9–18.9) |
Bold values: re-estimations by the principal investigator of the project (LA) or new information provided by authors after request. In the other cells, data already present in the original publication.
BT bridging therapy, CRR complete response rate, ITT intention-to-treat, mDoR median duration of response, mEFS median event-free survival, mPFS median progression-free survival, mOS median overall survival, NA Not available, NR Not reached, ORR overall response rate, PP per protocol.
aUpdated data.
bGhafouri et al. [8], Kittai et al. [10] and Casadei et al. [15].
For subgroup analyses with the help of metagregression, studies were categorized according to the CAR T product used, presence/absence of primary mediastinal B-cell lymphoma (PMBCL, 50%) patients and according to study location (5 in Europe versus 5 in USA). When Researchers shared the whole database, we performed also a pooled analysis, always preferable to meta-analysis because having individual patient data allows to create a homogeneous database and reduce bias. (ALMAIDEA2022CUP:J33C22001420001).
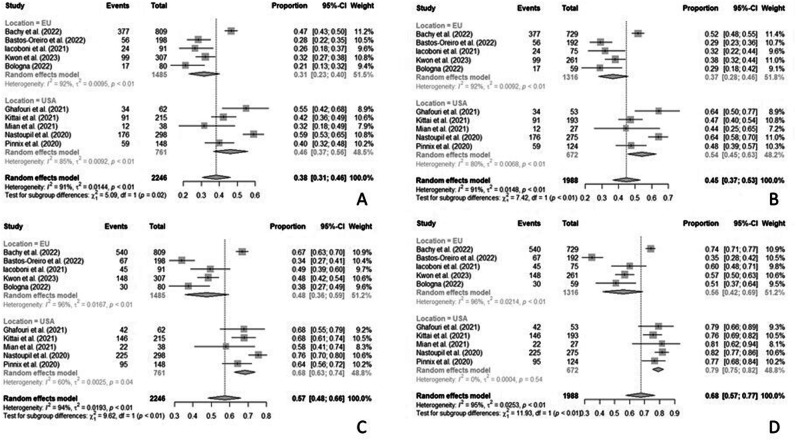
Our analysis indicates that rates of patients who did not achieve the infusion timepoint ranged from 3.0% to 28.9%. As a consequence, ORR and CR rates change whether they are considered on ITT (ORR ranged from 34 to 76% and CRR from 21 to 59%, respectively) or on PP basis (ORR ranged from 35 to 82% and CRR from 29 to 64%, respectively). The rate of patients who received BT ranged in Europe between 78.7% to 87.0%, whereas in the USA it ranged between 50.0% to 58.0%. The 50% of studies did not record the exit date for subjects who finally did not receive CAR T-cell, reflecting the inability to calculate the ITT EFS. Three studies reported a median PP PFS shorter than the ITT PFS, and in all studies but one median PP OS was higher than ITT OS. Median DoR ranges from 20.2 months to not reached (Table 1). Meta-analyses comprise 2 246 patients for the ITT population and 1 988 patients for the PP population, with a 10% of not infused patients on average. Metanalyses for ITT ORR and CRR resulted in 57% (95% CI 48–66) and 38% (95% CI 31–46), respectively. The PP ORR and CRR were 68% (95% CI 57–77) and 45% (95% CI 37–53), respectively. For both rates in both populations, heterogeneity was never below 90% (Supplementary Table S1). Subgroup analysis showed a significant difference for CRR and ORR in both PP and ITT populations, with better results for studies conducted in USA which also reached a slightly lower heterogeneity that drops to 0.0% in the PP population (Supplementary Table S1; Fig. 1).The presence of PMBCL patients in study cohort did not impact significantly on response rates estimated in both PP and ITT modalities (Supplementary Table S1).The type of CAR T product chosen differed significantly between groups for CRR and ORR in the PP population. Axicabtagene ciloleucel (3 studies) and axicabtagene ciloleucel or tisagenlecleucel (6 studies) groups showed higher response rates compared to tisagenlecleucel (1 study). However considering only axicabtagene ciloleucel studies showed a decrease of heterogeneity for CRR (Supplementary Fig. S2). Meta-regressions did not indicate a statistically significant influence of the different ratio of infused/not infused patients among studies, or of the different lengths of the median follow-up, this to the advantage of the robustness of the results. Of note, rate of patients who underwent BT had a significant influence on CRR of both PP and ITT populations (b = −0.635; P = 0.034 for ITT population and b = −0.850; P = 0.006 for PP population) and on ORR in PP population (b = −0.575; P = 0.024). The negative value of the coefficient b indicates that when the percentage of patients who underwent BT increase, the response rate decreases by about 60% (Supplementary Table S2 and Supplementary Fig. S3). At a median follow-up (ITT) of 29.7 months (95% CI 25.3–32.8), the pooled analysis led to an ITT ORR of 61% with a PP ORR of 71%. ITT CRR was 40% whereas the PP one resulted 47%, findings closer to those already published than those obtained with the meta-analysis. Median ITT OS resulted as 17.2 months while median PP OS was 24.1 months (Table 1). This rests on the fact that most patients who did not reach the infusion timepoint exited the CAR T pathway for death; moreover, infused subjects, included by definition in the PP analysis, may have taken advantage of further salvage treatments beyond CART. In contrast, both ITT and PP mPFS (6.2 versus 5.7 months) and mEFS (4.9 versus 5.2 months) were similar. PFS is slightly longer in the ITT population only as a consequence of the time elapsed between leukapheresis and infusion. This translates, de facto, into a non-significant difference between the two populations of patients, which points towards the reliability of PP PFS as a descriptor of CART effectiveness. On the other hand, an ITT mEFS lower than the PP mEFS, albeit without statistical significance, conveys the message that more dropout events have happened between leukapheresis and infusion. This means that more attention should be paid in terms of both patients’ selection and patients management in this crucial period of the CAR T pathway.
Fig. 1. Forest plot of the subgroup analysis according to study location was conducted.
A CRR ITT; B CRR PP; C ORR ITT; D ORR PP. CRR complete response rate, ITT intention-to-treat, ORR overall response rate, PP per protocol.
The present research showed that limiting the analysis on the CAR T infused population alone could lead to a wrong choice of treatment as the response rates of the ITT population is also influenced by the percentage of people who undergo BT (with a proportion that do not achieve the infusion timepoint). The finding that the rate of patients who underwent BT negatively affects the response rate could be explained by a worse condition of patients needing BT to reach CAR T infusion; this effect on the response rate is present in both populations (ITT and PP). Another interesting result was that USA cohorts have a better response than those from Europe, and that the USA studies have a much lower heterogeneity compared to European ones confirming literature [5]. This could mean that higher heterogeneity in studies results could be better explained by substantial differences that may occur (e.g., timing of referral or leukapheresis, if and which BT is used, different lymphodepleting regimens or cellular product) in everyday clinical practice worldwide, also precluding a robust comparison among the different available CAR T products and the therapies which share the same indication.
This is the first study that analyzed and compared the benefit of CAR T-cell in real-world in both ITT and PP modalities on the same datasets, considering also patients who started CAR T pathway without reaching infusion timepoint. The importance of considering this new treatment option as a pathway should translate into implementation of CAR T patient registries that include the new endpoint we advocate for, i.e. ITT EFS, to establish population-level effectiveness of these products. In addition, there is also a need to improve the consistency of data collection requirements between countries (around 50% of the analyzed studies did not record the exit date due to any cause for subjects who finally did not receive CAR T-cell) and – ideally – to collect data at a cross-country level. Such collaboration increases the efficiency of data collection, quality of the evidence that is generated and a better management of the therapeutic pathway.
Supplementary information
Acknowledgements
Funding was provided by Alma Mater Studiorum - University of Bologna (ID grant: ALMA IDEA 2022 CUP:J33C22001420001). We thank Massimo Agostini for data entry and AIL Bologna OdV (prot 2CSAIL21 Argnani).
Author contributions
LA conceived and designed the study, wrote the paper and performed the analyses as principal biostatistician, collected the data, provided study materials, interpreted final results and approved the final manuscript. RM designed the study, edited the paper draft interpreted final results and approved the final manuscript. DG designed the study and edited the paper draft. RDS performed the analysis, wrote the paper, interpreted final results and approved the final manuscript. BC collected the data, provided study materials, interpreted final results and approved the final manuscript. FLL collected the data, provided study materials, interpreted final results and approved the final manuscript. MJ collected the data, provided study materials, interpreted final results and approved the final manuscript. TJV collected the data, provided study materials, interpreted final results and approved the final manuscript. ASK collected the data, provided study materials, interpreted final results and approved the final manuscript. MB collected the data, provided study materials, interpreted final results and approved the final manuscript. AG collected the data, provided study materials, interpreted final results and approved the final manuscript. AMG collected the data, provided study materials, interpreted final results and approved the final manuscript. MJT collected the data, provided study materials, interpreted final results and approved the final manuscript. MM collected the data, provided study materials, interpreted final results and approved the final manuscript. MJM collected the data, provided study materials, interpreted final results and approved the final manuscript. GI collected the data, provided study materials, interpreted final results and approved the final manuscript. PB collected the data, provided study materials, interpreted final results and approved the final manuscript. MK collected the data, provided study materials, interpreted final results and approved the final manuscript. RB collected the data, provided study materials, interpreted final results and approved the final manuscript. JLR collected the data, provided study materials, collected the data, provided study materials, interpreted final results and approved the final manuscript. AM collected the data, provided study materials, interpreted final results and approved the final manuscript. BH collected the data, provided study materials, interpreted final results and approved the final manuscript. EB collected the data, provided study materials, interpreted final results and approved the final manuscript. FM collected the data, provided study materials, interpreted final results and approved the final manuscript. RH collected the data, provided study materials, interpreted final results and approved the final manuscript. CT collected the data, provided study materials, interpreted final results and approved the final manuscript. SLG collected the data, provided study materials, interpreted final results and approved the final manuscript. AB collected the data, provided study materials, interpreted final results and approved the final manuscript. PLZ collected the data, provided study materials, interpreted final results and approved the final manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
GI served as consultant and received honoraria from Novartis, Roche, Kite/Gilead, Bristol-Myers Squibb, Abbvie, Janssen, Sandoz, Miltenyi, AstraZeneca. PB as consultant and received honoraria from Allogene, Amgen, Autolus, BMS/Celgene, Kite/Gilead, Incyte, Miltenyi Biomedicine, Novartis, Nektar, Pfizer, Pierre Fabre. JLR served as consultant and received honoraria from Johnson&Johnson, Kite/Gilead, Novartis, BMS and Sanofi. RH served as consultant and received honoraria from Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, Roche, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi. AMG served as consultant and received honoraria from Roche, BMS, Takeda, Janssen, Kyowa Kirin, Gilead/Kite, Incyte, Lilly, Miltenyi, Ideogen, Genmab, AbbVie, Sobi, AstraZeneca, GSK. MBO served as consultant and received honoraria from Roche, BMS, Novartis, Janssen, Kyowa Kirin, Gilead/Kite, Incyte, Lilly, Genmab, AbbVie, Sobi, AstraZeneca. The remaining authors declare no competing interests.
Ethics approval and consent to participate
Formal ethical approval was not needed as this is a secondary research and patient have already signed written informed consent for each study analyzed in the present manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-024-01183-8.
References
- 1.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 3.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N Engl J Med. 2022;386:629–39. [DOI] [PubMed] [Google Scholar]
- 5.Canales Albendea MÁ, Canonico PL, Cartron G, Deiters B, Jommi C, Marks R, et al. Comparative analysis of CAR T-cell therapy access for DLBCL patients: associated challenges and solutions in the four largest EU countries. Front Med. 2023;10:1128295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28:2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastos-Oreiro M, Gutierrez A, Reguera JL, Iacoboni G, López-Corral L, Terol MJ, et al. Best Treatment Option for Patients With Refractory Aggressive B-Cell Lymphoma in the CAR-T Cell Era: Real-World Evidence From GELTAMO/GETH Spanish Groups. Front Immunol. 2022;13:855730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghafouri S, Fenerty K, Schiller G, de Vos S, Eradat H, Timmerman J, et al. Real-World Experience of Axicabtagene Ciloleucel and Tisagenlecleucel for Relapsed or Refractory Aggressive B-cell Lymphomas: A Single-Institution Experience. Clin Lymphoma Myeloma Leuk. 2021;21:861–72. [DOI] [PubMed] [Google Scholar]
- 9.Iacoboni G, Villacampa G, Martinez-Cibrian N, Bailén R, Lopez Corral L, Sanchez JM, et al. Real-world evidence of tisagenlecleucel for the treatment of relapsed or refractory large B-cell lymphoma. Cancer Med. 2021;10:3214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kittai AS, Huang Y, Gordon M, Denlinger N, Mian A, Fitzgerald L, et al. Comorbidities Predict Inferior Survival in Patients Receiving Chimeric Antigen Receptor T Cell Therapy for Diffuse Large B Cell Lymphoma: A Multicenter Analysis. Transpl Cell Ther. 2021;27:46–52. [DOI] [PubMed] [Google Scholar]
- 11.Kwon M, Iacoboni G, Reguera JL, Corral LL, Morales RH, Ortiz-Maldonado V, et al. Axicabtagene ciloleucel compared to tisagenlecleucel for the treatment of aggressive B-cell lymphoma. Haematologica. 2023;108:110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mian A, Wei W, Winter AM, Khouri J, Jagadeesh D, Anwer F, et al. Outcomes and factors impacting use of axicabtagene ciloleucel in patients with relapsed or refractory large B-cell lymphoma: results from an intention-to-treat analysis. Leuk Lymphoma. 2021;62:1344–52. [DOI] [PubMed] [Google Scholar]
- 13.Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38:3119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinnix CC, Gunther JR, Dabaja BS, Strati P, Fang P, Hawkins MC, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020;4:2871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casadei B, Argnani L, Guadagnuolo S, Pellegrini C, Stefoni V, Broccoli A, et al. Real World Evidence of CAR T-Cell Therapies for the Treatment of Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma: A Monocentric Experience. Cancers. 2021;13:4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.