Abstract
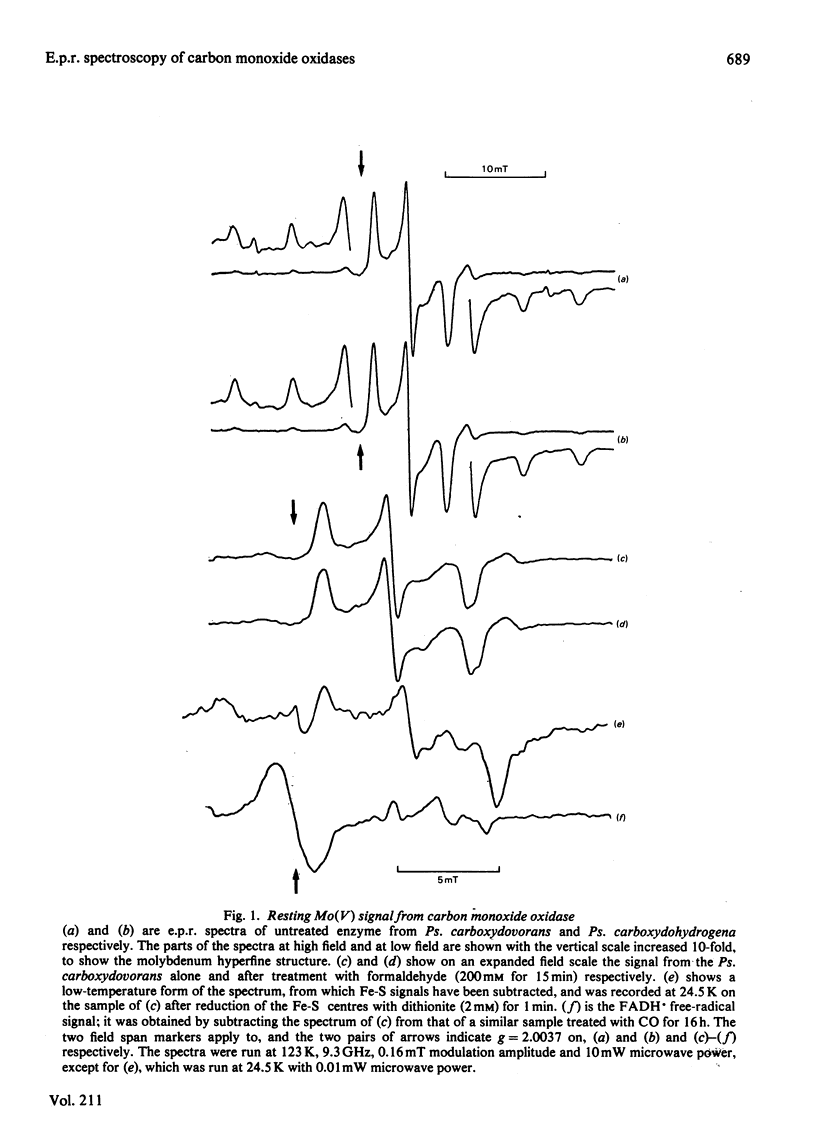
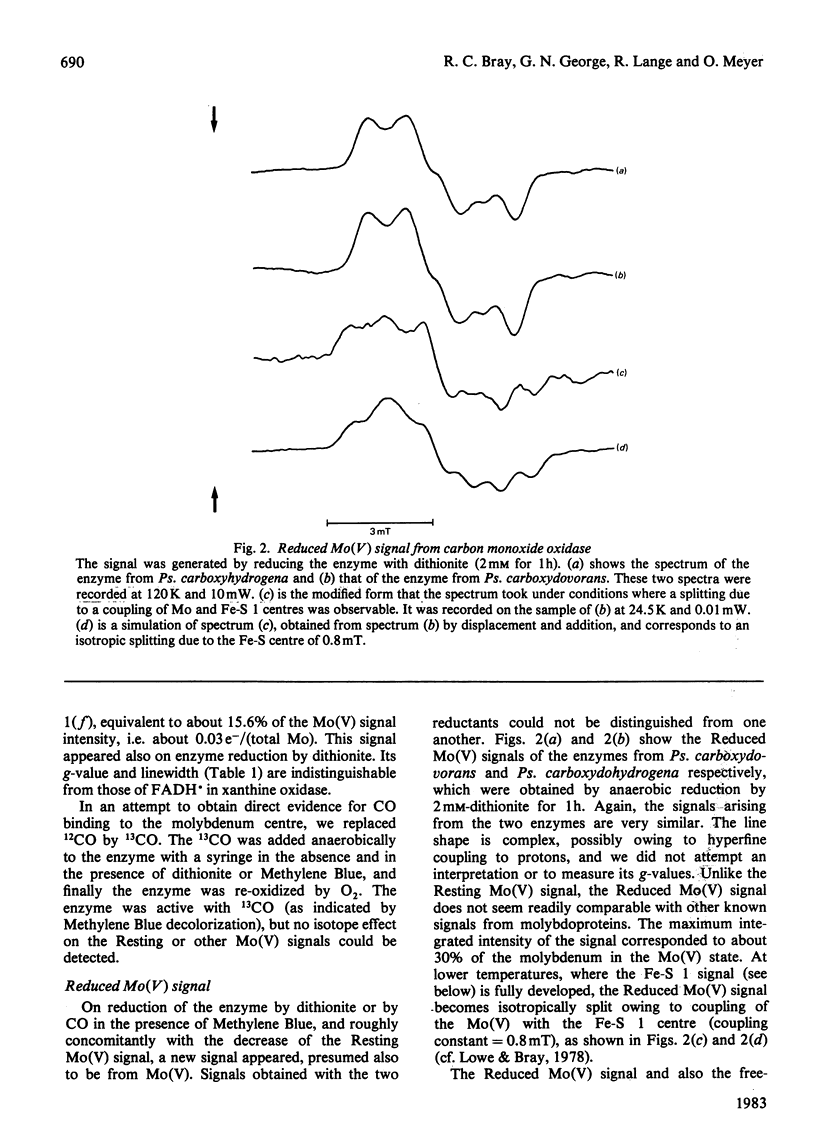
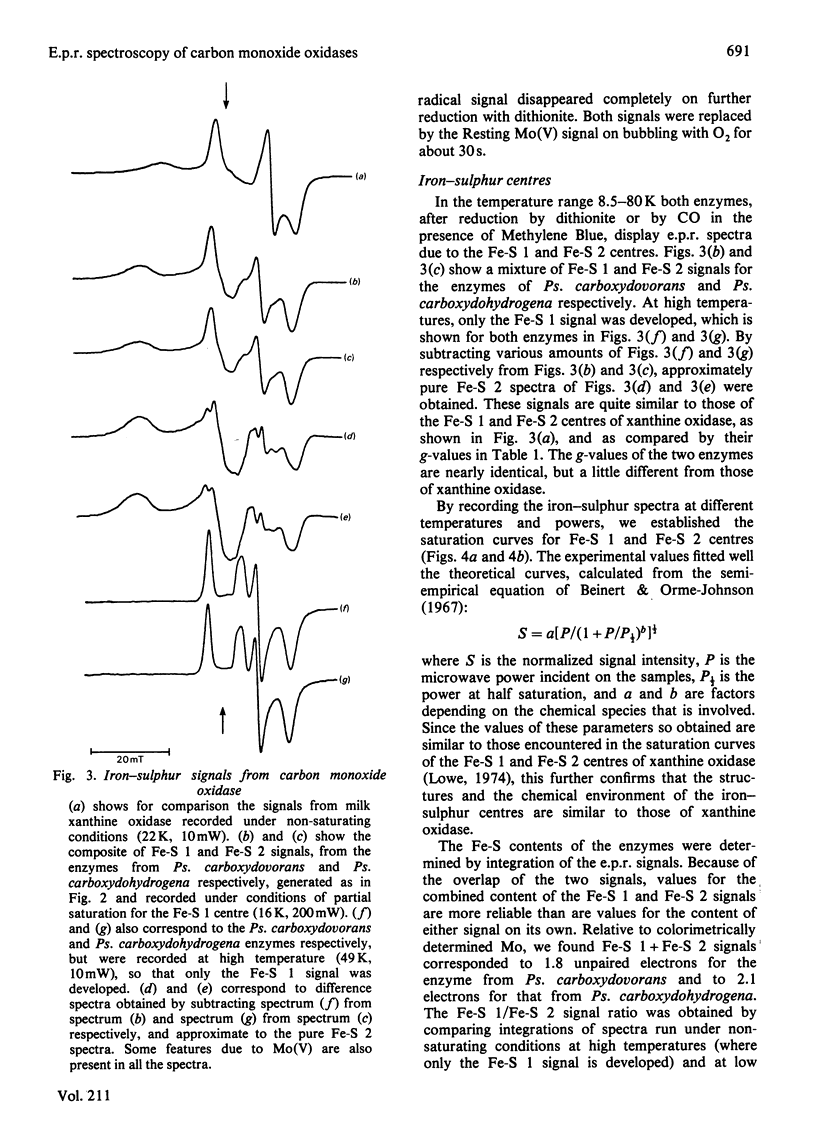
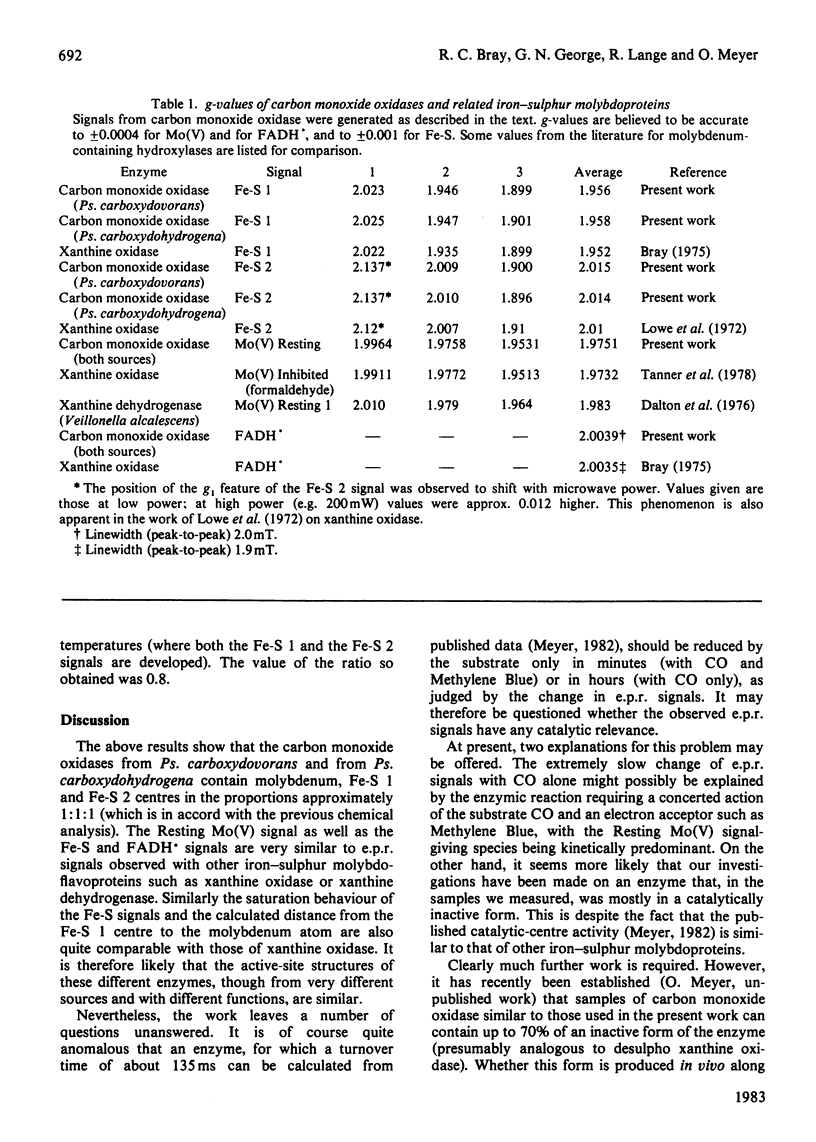
E.p.r. spectra were obtained at 8-120 K for carbon monoxide oxidases isolated from the carboxydotrophic bacteria Pseudomonas carboxydovorans and Pseudomonas carboxydohydrogena. Spectra from the two enzymes are extremely similar to one another. Under appropriate conditions each enzyme shows signals from Mo(V) atoms in two different chemical environments, as well as showing signals from two distinct iron-sulphur centres, presumed to be [2Fe-2S] clusters, and weak FADH X free-radical signals. Parameters of most of the signals were measured, and they show considerable similarities to those of the corresponding signals from xanthine oxidase and related enzymes. Though the signals from carbon monoxide oxidases appear and disappear under reducing and oxidizing conditions, we have so far failed to demonstrate the kinetic competence of any of them. It seems likely that this was due to the presence in the enzyme preparation examined of high amounts of desulpho carbon monoxide oxidase together with another non-functional form of the enzyme giving a stable 'Resting' Mo(V) e.p.r. signal.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray R. C., Barber M. J., Lowe D. J. Electron-paramagnetic-resonance spectroscopy of complexes of xanthine oxidase with xanthine and uric acid. Biochem J. 1978 Jun 1;171(3):653–658. doi: 10.1042/bj1710653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Gutteridge S. Numbers and exchangeability with water of oxygen-17 atoms coupled to molybdenum (V) in different reduced forms of xanthine oxidase. Biochemistry. 1982 Nov 9;21(23):5992–5999. doi: 10.1021/bi00266a041. [DOI] [PubMed] [Google Scholar]
- Bray R. C. The reactions and the structures of molybdenum centers in enzymes. Adv Enzymol Relat Areas Mol Biol. 1980;51:107–165. doi: 10.1002/9780470122969.ch3. [DOI] [PubMed] [Google Scholar]
- Dalton H., Lowe D. J., Pawlik T., Bray R. C. Studies by electron-paramagnetic-resonance spectroscopy on the mechanism of action of xanthine dehydrogenase from Veillonella alcalescens. Biochem J. 1976 Feb 1;153(2):287–295. doi: 10.1042/bj1530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem J. 1970 Mar;116(5):851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. M., Hegeman G. D. Purification and some properties of carbon monoxide dehydrogenase from Pseudomonas carboxydohydrogena. J Bacteriol. 1981 Dec;148(3):904–911. doi: 10.1128/jb.148.3.904-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Bray R. C. Magnetic coupling of the molybdenum and iron-sulphur centres in xanthine oxidase and xanthine dehydrogenases. Biochem J. 1978 Mar 1;169(3):471–479. doi: 10.1042/bj1690471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Lynden-Bell R. M., Bray R. C. Spin-spin interaction between molybdenum and one of the iron-sulphur systems of xanthine oxidase and its relevance to the enzymic mechanism. Biochem J. 1972 Nov;130(1):239–249. doi: 10.1042/bj1300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O. Chemical and spectral properties of carbon monoxide: methylene blue oxidoreductase. The molybdenum-containing iron-sulfur flavoprotein from Pseudomonas carboxydovorans. J Biol Chem. 1982 Feb 10;257(3):1333–1341. [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Carbon monoxide:methylene blue oxidoreductase from Pseudomonas carboxydovorans. J Bacteriol. 1980 Jan;141(1):74–80. doi: 10.1128/jb.141.1.74-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Oxidation of carbon monoxide in cell extracts of Pseudomonas carboxydovorans. J Bacteriol. 1979 Feb;137(2):811–817. doi: 10.1128/jb.137.2.811-817.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Reisolation of the carbon monoxide utilizing hydrogen bacterium Pseudomonas carboxydovorans (Kistner) comb. nov. Arch Microbiol. 1978 Jul;118(1):35–43. doi: 10.1007/BF00406071. [DOI] [PubMed] [Google Scholar]
- Pick F. M., McGartoll M. A., Bray R. C. Reaction of formaldehyde and of methanol with xanthine oxidase. Eur J Biochem. 1971 Jan 1;18(1):65–72. doi: 10.1111/j.1432-1033.1971.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Swann J. C., Bray R. C. Multiple phases in the reduction of xanthine oxidase by substrates. Eur J Biochem. 1972 Apr 11;26(3):407–415. doi: 10.1111/j.1432-1033.1972.tb01781.x. [DOI] [PubMed] [Google Scholar]
- Tanner S. J., Bray R. C., Bergmann F. 13C hyperfine splitting of some molybdenum electron-paramagnetic-resonance signals from xanthine oxidase [proceedings]. Biochem Soc Trans. 1978;6(6):1328–1330. doi: 10.1042/bst0061328. [DOI] [PubMed] [Google Scholar]
- Wahl R. C., Warner C. K., Finnerty V., Rajagopalan K. V. Drosophila melanogaster ma-l mutants are defective in the sulfuration of desulfo Mo hydroxylases. J Biol Chem. 1982 Apr 10;257(7):3958–3962. [PubMed] [Google Scholar]



