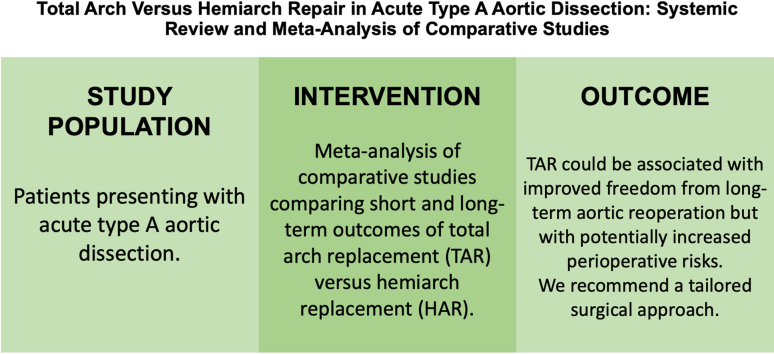
Abstract
Background
We aimed to compare the short- and long-term outcomes of total arch replacement (TAR) vs hemiarch replacement (HAR) in the management of acute type A aortic dissection.
Methods
We searched the literature for studies directly comparing TAR to HAR in acute type A aortic dissection. Hazard ratios (HRs) were extracted from digitized Kaplan-Meier curves.
Results
A total of 6526 patients were identified, of which 2060 (32%) had received a TAR. A total of 37% of patients were female, and the mean age (standard deviation) of the cohort was 59.8 ± 11.8 years. TAR patients had a higher prevalence of preoperative malperfusion (34% vs 26%). The TAR group had higher odds of 30-day mortality (4404 patients; odds ratio [OR] 1.79, 95% confidence interval [CI] 1.29-2.49), renal failure requiring dialysis (3475 patients; OR 1.34, 95% CI 1.02-1.76), and a trend toward higher rates of stroke (3292 patients; OR 1.49, 95% CI 0.93-2.39). No significant differences were observed in prevalence of permanent spinal cord injury, visceral ischemia, or reoperation for bleeding. The TAR group had a non–statistically significant increase in long-term mortality (4408 patients; HR 1.25, 95% CI 0.99-1.57), but showed a trend toward improved freedom from long-term aortic reoperation (1359 patients; HR 0.53; 95% CI 0.18-1.59). In a subgroup analysis, the hazard ratio of long-term mortality favoured TAR in only the subgroup of studies in which the difference in malperfusion was > 10% between groups.
Conclusions
TAR could be associated with improved freedom from long-term aortic reoperation but with potentially increased perioperative risks. We recommend a tailored surgical approach.
Graphical abstract
Résumé
Contexte
Nous souhaitions comparer les résultats à court et à long terme du remplacement total de l’arc aortique à ceux du remplacement de l’hémi-arc aortique dans la prise en charge de la dissection aortique aiguë de type A.
Méthodologie
Nous avons scruté la littérature à la recherche d’études comparant directement le remplacement total de l’arc aortique et le remplacement de l’hémi-arc aortique dans le traitement de la dissection aortique aiguë de type A. Des rapports de risques instantanés (RRI) ont été établis à partir de courbes de Kaplan-Meier numérisées.
Résultats
La recherche a permis de recenser 6526 patients, dont 2060 (32 %) avaient reçu un remplacement total de l’arc aortique. Au total, 37 % des patients étaient des femmes; l’âge moyen (écart-type) de la cohorte était 59,8 ± 11,8 ans. Les patients qui ont reçu un remplacement total de l’arc aortique affichaient une prévalence accrue de malperfusion préopératoire (34 % vs 26 %). Le groupe de remplacement total de l’arc de l’aorte présentait un risque accru de mortalité en 30 jours (4404 patients; rapport de cotes [RC] : 1,79; intervalle de confiance [IC] à 95 % : 1,29-2,49) et d’insuffisance rénale nécessitant une dialyse (3475 patients; RC : 1,34; IC à 95 % : 1,02-1,76) ainsi qu’une tendance à avoir un taux élevé d’AVC (3292 patients; RC : 1,49; IC à 95 % : 0,93-2,39). Aucune différence notable n’a été observée dans la prévalence des lésions médullaires permanentes, d’ischémie viscérale ou de réopération pour saignement. Le groupe de remplacement total de l’arc aortique affichait une hausse non statistiquement significative du taux de mortalité à long terme (4408 patients; RRI : 1,25; IC à 95 % : 0,99-1,57), mais avait tendance à subir moins de réopération aortique à long terme (1359 patients; RRI : 0,53; IC à 95 % : 0,18-1,59). Dans une analyse par sous-groupes, le rapport de risques instantanés de la mortalité à long terme favorisait le remplacement total de l’arc aortique seulement pour le sous-groupe des études dans lequel la différence de la malperfusion était supérieure à 10 % entre les groupes.
Conclusions
Le remplacement total de l’arc aortique pourrait être associé à une meilleure absence de réopération aortique à long terme, mais possiblement avec un risque péri-opératoire accru. Nous recommandons une approche chirurgicale adaptée.
Acute type A aortic dissection (ATAAD) remains a challenging condition associated with high rates of morbidity and mortality.1 Improved surgical techniques and perioperative care have led to a steady decline in mortality after emergent surgery for ATAAD. Nevertheless, postoperative risks remain high, and further improvement in the surgical management of this condition is urged.2
A particular element of debate in the management of ATAAD is the extent of surgery into the dissected aortic arch. Some surgeons advocate for a more conservative tear-oriented approach (most often involving hemiarch repair), to minimize perioperative mortality and morbidities; others propose a more aggressive total arch replacement (TAR), with liberal addition of elephant trunk techniques, to improve long-term prognosis by obliterating the false lumen and limiting late aneurysm formation at the distal aorta that may either require high-risk aortic reintervention or cause death by rupture.3, 4, 5, 6, 7, 8, 9
Previous meta-analyses attempted to investigate this question, but they either were designed to include a combination of comparative and single-cohort studies or covered heterogenous groups of patients.10, 11, 12, 13 In fact, some of the studies included in the previous meta-analyses had a comparison arm of hemiarch repair that comprised any type of proximal aortic repair (root, ascending, hemiarch, or combination of hemiarch and antegrade descending aorta stenting) and therefore were not necessarily limited to hemiarch repair.
The present systemic review and meta-analysis aims to formally assess the available literature and included studies with well defined comparison arms of total arch vs hemiarch repair.
Methods
Search strategy
We performed our literature search in MEDLINE, Embase, Web of Science, clinicaltrials.gov, and the Cochrane Library, using a combination of medical subject headings (MeSH) terms and keywords (Supplemental Table S1). To be included, studies had to cover adult patients with a history of ATAAD involving the aortic arch or extending into the descending thoracic aorta, and had to directly compare (i) extended aortic arch replacement using TAR, conventional elephant trunk technique, frozen elephant trunk technique, or TAR in combination with another simultaneous stent-deployment technique vs (ii) hemiarch aortic repair, including hemiarch repair alone or hemiarch in combination with another simultaneous stent-deployment technique.
Data extraction
Studies identified by our search strategy were processed through the Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Two authors reviewed studies at all levels of screening. Disagreements were resolved by consensus among all reviewers (F.H., R.A., J.A.). Data were extracted from the included studies using a spreadsheet designed specifically for this meta-analysis.
Study quality assessment
We used the Newcastle-Ottawa Scale for observational studies to assess the quality of the studies included in the systematic review and meta-analysis.14 The Newcastle-Ottawa Scale assesses the following 3 domains: (i) selection of the included studies; (ii) comparability of the study groups; and (iii) assessment and reporting of the outcomes (Supplemental Table S2).
Data synthesis
The data were synthesized using Stata 17.0 (StataCorp, College Station, TX). All outcomes were calculated with extended arch as the intervention, and hemiarch as the reference. Dichotomous outcomes were presented as odds ratios (ORs) with 95% confidence intervals (CIs). Continuous outcomes were presented as mean differences with 95% CI. For long-term outcomes, hazard ratios (HRs) with 95% CIs were used. HRs either were collected from studies reporting it using Cox regression, or if not explicitly reported, the HRs were extracted from digitized Kaplan-Meier curves following the method by Tierney et al. and Parmar et al.15,16 For all outcomes, we used the random-effects model by DerSimonian-Laird and calculated 95% prediction intervals. Heterogeneity was assessed using Cochran’s Q and I2. Publication bias was evaluated using funnel plots and Egger’s test (Supplemental Figs. S1-S5). Subgroup analyses were performed to account for the difference in preoperative malperfusion between intervention arms in the individual studies and to compare extended arch replacement with descending aorta graft to hemiarch repair without descending aorta graft (Supplemental Figs. S6-S9). All statistical tests were 2-sided. P values < 0.05 were considered statistically significant, except P < 0.1 for heterogeneity analyses. This report was written in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Supplemental Table S3).17
Study selection
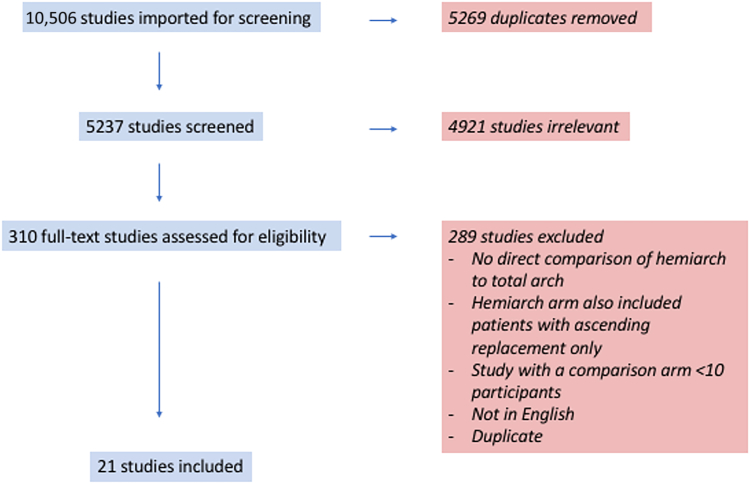
The literature search identified a total of 10,506 articles (Fig. 1). After exclusion of duplicates and title and/or abstract screening to remove irrelevant studies, 310 articles were deemed appropriate to undergo a full-text review. After the full-text review, 289 studies were excluded because they did not meet the inclusion criteria. Therefore, 22 observational studies (1 paper with 2 study arms) remained, which fulfilled the predetermined inclusion criteria, including a total of 6526 patients, of whom 2060 underwent TAR and 4466 underwent hemiarch replacement (HAR).4,9,18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 We included the following 2 study arms from the paper by Xue and colleagues: arm 1, presenting Island arch replacement vs HAR; and arm 2, presenting frozen elephant trunk vs HAR.34 The quality of the included studies was assessed using the Newcastle-Ottawa Scale (Supplemental Table S2).
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) diagram summarizing the screening process through the different phases of a systematic review.
Study characteristics
The 22 included studies were published between 2002 and 2020, describing patients treated for ATAAD. All studies were retrospective; one study was a report of an international registry,21 one was a study of a national registry,20 and all other studies were single-centre experiences. The total mean follow-up duration was 44 months. The mean age of the total cohort was 59.8 ± 11.8 years, and 37% of the patients were female. The total arch patients had a higher prevalence of preoperative malperfusion (34% vs 26%). Table 1, Table 2, Table 3 describe the study and baseline characteristics. We have noted that 4 studies excluded patients with arch tears,20,32,34 (and arm 136), and one study was comprised exclusively of patients with arch tears.33 The other studies included patients with or without arch tears, and in these studies, the decision to perform a TAR was based on the following: (i) purely the presence of tear in the arch, in 3 studies22,27,28; (ii) presence of tear in the arch or other indication for TAR, such as arch aneurysm, circumferential arch dissection, malperfusion, or connective-tissue disease, in 8 studies9,18,19,23,25,31,35 (and arm 234); and (iii) the discretion of the primary surgeon, in 5 studies.4,24,26,29,30
Table 1.
Preoperative patient characteristics in the individual studies
| Study (y) | Patients | Mean FU, mo | Group | Patients | Female | Age, y, mean ± SD | Hypertension | Diabetes | Pre-existing CAD | History of CVA | Chronic kidney disease | Previous cardiac surgery | Any connective-tissue disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Easo et al.20 (2012) | 658 | 1.0 | TA | 140 (21.3) | 54 | 58.3 ± 11.9 | NR | NR | NR | NR | NR | NR | NR |
| Fleischman et al.22 (2018) | 195 | 31.0 | TA | 39 (20) | 10 | 62 ± 13 | 32 (82.1) | 4 (10.3) | 1 (2.6) | NR | NR | 7 (17.9) | 0 |
| HA | 156 (80) | 46 | 61 ± 14 | 127 (81.4) | 21 (13.5) | 15 (9.6) | NR | NR | 21 (13.5) | 4 (2.6) | |||
| Hata et al.26 (2016) | 64 | 72.0 | TA | 12 (18.8) | 4 | 57.1 ± 12.1 | NR | NR | NR | NR | NR | NR | NR |
| HA | 52 (81.3) | 25 | 66.3 ± 12.7 | NR | NR | NR | NR | NR | NR | NR | |||
| Keeling et al.18 (2017) | 342 | 1.0 | HA | 518 (78.7) | 194 | 59.1 ± 13.3 | NR | NR | NR | NR | NR | NR | NR |
| HA | 299 (87.4) | 85 | 55.4 ± 13.9 | 266 (89) | 31 (10.4) | 24 (8) | 20 (6.7) | 11 (3.7) | 11 (3.7) | NR | |||
| Kim et al.4 (2011) | 188 | 47.5 | TA | 44 (23.4) | 18 | 55 ± 12.1 | 24 (54.5) | 2 (4.5) | NR | 1 (2.3) | NR | NR | 1 (2.3) |
| HA | 144 (76.6) | 75 | 57.6 ± 11.5 | 92 (63.9) | 6 (4.2) | NR | 4 (2.8) | NR | NR | 7 (4.9) | |||
| Kimura et al.27 (2020) | 706 | 61.2 | TA | 135 (19.1) | 47 | 62 ± 5.8 | 101 (74.8) | 8 (5.9) | NR | NR | 3 (2.2) | NR | 15 (11.1) |
| HA | 571 (80.9) | 294 | 65.8 ± 4.9 | 407 (71.3) | 49 (8.6) | NR | NR | 14 (2.5) | NR | 2 (0.4) | |||
| Larsen et al.21 (2017) | 1241 | 60.0 | TA | 334 (26.9) | 81 | 59.1 ± 13.6 | 218 (65.3) | 18 (5.4) | 52 (15.6) | NR | 7 (2.1) | 33 (9.9) | 13 (3.9) |
| HA | 907 (73.1) | 323 | 60.8 ± 14.1 | 674 (74.3) | 79 (8.7) | 143 (15.8) | NR | 42 (4.6) | 93 (10.3) | 24 (2.6) | |||
| Lio et al.24 (2016) | 92 | 30.5 | TA | 33 (35.9) | 5 | 61 ± 12 | 30 (90.9) | 1 (3) | 0 | NR | 0 | 3 (9.1) | NR |
| HA | 59 (64.1) | 16 | 66 ± 10 | 51 (86.4) | 2 (3.4) | 2 (3.4) | NR | 1 (1.7) | 1 (1.7) | NR | |||
| Ohtsubo et al.25 (2002) | 47 | 42.0 | TA | 24 (51.1) | 11 | 68 ± 11.1 | NR | NR | NR | 3 (12.5) | NR | NR | 4 (16.7) |
| HA | 23 (48.9) | 16 | 69 ± 11.1 | NR | NR | NR | 2 (8.7) | NR | NR | 0 | |||
| Trivedi et al.19 (2016) | 259 | 60.0 | TA | 92 (35.5) | 34 | 58.9 ± 11.2 | 73 (79.3) | 6 (6.5) | 13 (14.1) | 13 (14.1) | 2 (2.2) | 9 (9.8) | NR |
| HA | 167 (64.5) | 74 | 63.3 ± 13.3 | 128 (76.6) | 20 (12) | 43 (25.7) | 10 (6) | 2 (1.2) | 18 (10.8) | NR | |||
| Yang et al.23 (2019) | 472 | 63.6 | TA | 150 (31.8) | 46 | 57 ± 5.2 | 107 (71.3) | 9 (6) | 15 (10) | 3 (2) | 26 (17.3) | 18 (12) | 7 (4.7) |
| HA | 322 (68.2) | 96 | 60.5 ± 5.8 | 230 (71.4) | 21 (6.5) | 71 (22) | 9 (2.8) | 40 (12.4) | 31 (9.6) | 19 (5.9) | |||
| Dai et al.36 (2015) | 93 | 64.0 | TA | 52 (55.9) | 23 | 49.8 ± 9.6 | 49 (94.2) | 1 (1.9) | NR | NR | 2 (3.8) | NR | NR |
| HA | 41 (44.1) | 16 | 49.1 ± 10.4 | 40 (97.6) | 1 (2.4) | NR | NR | 1 (2.4) | NR | NR | |||
| Driever et al.28 (2003) | 42 | 28.8 | TA | 30 (71.4) | 11 | 57.5 ± 11.1 | NR | NR | NR | NR | NR | NR | NR |
| HA | 12 (28.6) | 4 | 57.5 ± 11.1 | NR | NR | NR | NR | NR | NR | NR | |||
| Fichadiya et al.35 (2019) | 95 | 36.0 | TA | 28 (29.5) | 6 | 56 ± 14 | NR | NR | NR | NR | 8 (28.6) | NR | NR |
| HA | 67 (70.5) | 30 | 60 ± 16 | NR | NR | NR | NR | 5 (7.5) | NR | NR | |||
| Inoue et al.30 (2016) | 334 | 39.0 | TA | 161 (48.2) | 64 | 63 ± 12 | NR | NR | NR | NR | NR | NR | 19 (11.8) |
| HA | 173 (51.8) | 111 | 71 ± 12 | NR | NR | NR | NR | NR | NR | 6 (3.5) | |||
| Kim et al.31 (2018) | 94 | 50.0 | TA | 32 (34) | 16 | 53 ± 11 | NR | NR | NR | NR | NR | NR | NR |
| HA | 62 (66) | 30 | 61 ± 14 | NR | NR | NR | NR | NR | NR | NR | |||
| Omura et al.9 (2016) | 197 | 60.0 | TA | 88 (44.7) | 26 | 61 ± 13 | NR | NR | NR | NR | 3 (3.4) | NR | NR |
| HA | 109 (55.3) | 59 | 70 ± 11 | NR | NR | NR | NR | 3 (2.8) | NR | NR | |||
| Shi et al.32 (2014) | 155 | 46.3 | TA | 84 (54.2) | 27 | 53.9 ± 12.2 | 67 (79.8) | 19 (22.6) | 6 (7.1) | 2 (2.4) | 4 (4.8) | NR | 22 (26.2) |
| HA | 71 (45.8) | 18 | 55.9 ± 10.1 | 55 (77.5) | 12 (16.9) | 7 (9.9) | 2 (2.8) | 5 (7) | NR | 10 (14.1) | |||
| Shimamura et al.29 (2018) | 300 | 31.7 | TA | 29 (9.7) | 10 | 62.1 ± 13.9 | NR | NR | NR | 1 (3.4) | NR | NR | 1 (3.4) |
| HA | 271 (90.3) | 118 | 66.1 ± 13.5 | NR | NR | NR | 30 (11.1) | NR | NR | 4 (1.5) | |||
| Vallabhajosyula et al.33 (2016) | 61 | 51.9 | TA | 31 (50.8) | 11 | 59 ± 12 | 28 (90.3) | 2 (6.5) | 4 (12.9) | 5 (16.1) | 2 (6.5) | 7 (22.6) | NR |
| HA | 30 (49.2) | 10 | 58 ± 11 | 26 (86.7) | 2 (6.7) | 0 | 1 (3.3) | 3 (10) | 3 (10) | NR | |||
| Xue et al.,34(arm 1 (2020) | 260 | 45.6 | TA | 54 (20.8) | 4 | 56.5 ± 14.4 | 42 (77.8) | 1 (1.9) | 1 (1.9) | 4 (7.4) | 0 | NR | 2 (3.7) |
| HA | 206 (79.2) | 72 | 55.2 ± 14.4 | 132 (64.1) | 10 (4.9) | 3 (1.5) | 4 (1.9) | 3 (1.5) | NR | 6 (2.9) | |||
| Xue et al.34, arm 2 (2020) | 631 | 45.6 | TA | 425 (67.4) | 86 | 49.6 ± 11.8 | 284 (66.8) | 11 (2.6) | 10 (2.4) | 5 (1.2) | 9 (2.1) | NR | 15 (3.5) |
| HA | 206 (32.6) | 72 | 55.2 ± 14.4 | 132 (64.1) | 10 (4.9) | 3 (1.5) | 4 (1.9) | 3 (1.5) | NR | 6 (2.9) |
Values are n, or n (%), unless otherwise indicated.
FU, follow-up; CAD, coronary artery disease; CVA, cerebrovascular accident; HA, hemiarch; NR, not reported; SD, standard deviation; TA, total arch.
Table 2.
Intraoperative characteristics in the individual studies
| Study (y) | Group | Patients | Descending aortic repair | Aortic root replacement | CABG | CPB time, min, mean ± SD | Circulatory arrest duration, min, mean ± SD | Antegrade cerebral perfusion | Retrograde cerebral perfusion |
|---|---|---|---|---|---|---|---|---|---|
| Easo et al.20 (2012) | TA | 140 (21.3) | 48 (34.3) | 41 (29.3) | 15 (10.7) | 44.8 ± 29.7 | NR | 118 (84.3) | NR |
| HA | 518 (78.7) | 0 | 113 (21.8) | 49 (9.5) | 24.3 ± 14.4 | NR | 347 (67) | NR | |
| Fleischman et al.22 (2018) | TA | 39 (20) | 7 (17.9) | 5 (12.8) | 3 (7.7) | 214 ± 64.4 | NR | 30 (76.9) | NR |
| HA | 156 (80) | 4 (2.6) | 15 (9.6) | 21 (13.5) | 167.5 ± 57.0 | NR | 124 (79.5) | NR | |
| Hata et al.26 (2016) | TA | 12 (18.8) | NR | 3 (25) | 2 (16.7) | 206 ± 46 | NR | 12 (100) | 0 |
| HA | 52 (81.3) | NR | 4 (7.7) | 9 (17.3) | 121.5 ± 41.7 | NR | 0 | 0 | |
| Keeling et al.18 (2017) | TA | 43 (12.6) | 10 (23.3) | 4 (9.3) | 5 (11.6) | 257.9 ± 82.7 | 65.1 ± 30.1 | 43 (100) | 0 |
| HA | 299 (87.4) | 5 (1.7) | 38 (12.7) | 40 (13.4) | 195 ± 69.1 | 35 ± 19.2 | 299 (100) | 0 | |
| Kim et al.4 (2011) | TA | 44 (23.4) | 5 (11.4) | 0 | 3 (6.8) | NR | NR | 27 (61.4) | 17 (38.6) |
| HA | 144 (76.6) | 0 | 8 (5.6) | 14 (9.7) | NR | NR | 42 (29.2) | 99 (68.8) | |
| Kimura et al.27 (2020) | TA | 135 (19.1) | NR | 6 (4.4) | NR | 241 ± 103 | NR | 135 (100) | 0 |
| HA | 571 (80.9) | NR | 28 (4.9) | NR | 141 ± 49 | NR | 35 (6.1) | 0 | |
| Larsen et al.21 (2017) | TA | 334 (26.9) | 29 (8.7) | 249 (74.6) | 19 (5.7) | 218.5 ± 65.9 | NR | 232 (69.5) | 53 (15.9) |
| HA | 907 (73.1) | 3 (0.3) | 595 (65.6) | 86 (9.5) | 188 ± 64.4 | NR | 376 (41.5) | 231 (25.5) | |
| Lio et al.24 (2016) | TA | 33 (35.9) | 4 (12.1) | 2 (6.1) | 0 | 249 ± 87 | NR | NR | NR |
| HA | 59 (64.1) | 0 | 5 (8.5) | 5 (8.5) | 175 ± 63 | NR | NR | NR | |
| Ohtsubo et al.25 (2002) | TA | 24 (51.1) | NR | 4 (16.7) | NR | 292 ± 20 | 48 ± 4.2 | 24 (100) | 0 |
| HA | 23 (48.9) | NR | 0 | NR | 190 ± 9.7 | 32 ± 2.6 | 5 (21.7) | 0 | |
| Trivedi et al.19 (2016) | TA | 92 (35.5) | 28 (30.4) | 23 (25) | 12 (13) | 269.6 ± 87 | NR | 86 (93.5) | 29 (31.5) |
| HA | 167 (64.5) | 3 (1.8) | 53 (31.7) | 35 (21) | 205.5 ± 58.9 | NR | 13 (7.8) | 154 (92.2) | |
| Yang et al.23 (2019) | TA | 150 (31.8) | 18 (12) | 98 (65.3) | 6 (4) | 227 ± 62.2 | 43.5 ± 15.6 | 54 (36) | 5 (3.3) |
| HA | 322 (68.2) | 11 (3.4) | 178 (55.3) | 20 (6.2) | 217.5 ± 54.1 | 32 ± 9.6 | 91 (28.3) | 194 (60.2) | |
| Dai et al.36 (2015) | TA | 52 (55.9) | NR | 1 (1.9) | 6 (11.5) | 153 ± 23.1 | NR | NR | NR |
| HA | 41 (44.1) | NR | 0 | 6 (14.6) | 150 ± 19.4 | NR | NR | NR | |
| Driever et al.28 (2003) | TA | 30 (71.4) | 0 | NR | NR | NR | 36 ± 14 | NR | NR |
| HA | 12 (28.6) | NR | NR | NR | NR | NR | NR | NR | |
| Fichadiya et al.35 (2019) | TA | 28 (29.5) | 28 (100) | 9 (32.1) | 4 (14.3) | 192 ± 51 | NR | NR | NR |
| HA | 67 (70.5) | 0 | 21 (31.3) | 8 (11.9) | 178 ± 57 | NR | NR | NR | |
| Inoue et al.30 (2016) | TA | 161 (48.2) | NR | 16 (9.9) | 10 (6.2) | 294 ± 136 | NR | 161 (100) | 0 |
| HA | 173 (51.8) | NR | 23 (13.3) | 12 (6.9) | 210 ± 90 | NR | 173 (100) | 0 | |
| Kim et al.31 (2018) | TA | 32 (34) | NR | NR | NR | NR | 92 ± 41 | NR | NR |
| HA | 62 (66) | NR | NR | NR | NR | 43 ± 15 | NR | NR | |
| Omura et al.9 (2016) | TA | 88 (44.7) | NR | NR | 4 (4.5) | 244 ± 88 | NR | 86 (97.7) | NR |
| HA | 109 (55.3) | NR | NR | 3 (2.8) | 187 ± 71 | NR | 61 (56) | NR | |
| Shi et al.32 (2014) | TA | 84 (54.2) | 84 (100) | 24 (28.6) | 6 (7.1) | 164.7 ± 19.6 | 29.3 ± 4.3 | 84 (100) | 0 |
| HA | 71 (45.8) | 71 (100) | 24 (33.8) | 7 (9.9) | 103.6 ± 20.9 | 30.6 ± 4.9 | 71 (100) | 0 | |
| Shimamura et al.29 (2018) | TA | 29 (9.7) | 0 | NR | NR | 338 ± 123 | 96.3 ± 19.6 | 0 | 29 (100) |
| HA | 271 (90.3) | 0 | NR | NR | 260 ± 85 | 58.5 ± 15 | 0 | 271 (100) | |
| Vallabhajosyula et al.33 (2016) | TA | 31 (50.8) | 0 | 5 (16.1) | NR | 313 ± 80 | 78 ± 45 | 10 (32.3) | 0 |
| HA | 30 (49.2) | 30 (100) | 1 (3.3) | NR | 239 ± 34 | 60 ± 15 | 0 | 24 (80) | |
| Xue et al.,34arm 1 (2020) | TA | 54 (20.8) | 54 (100) | 52 (96.3) | 6 (11.1) | 243 ± 72.1 | 31.7 ± 8.6 | 46 (85.2) | 2 (3.7) |
| HA | 206 (79.2) | 0 | 202 (98.1) | 16 (7.8) | 234 ± 70 | 27 ± 11.3 | 126 (61.2) | 4 (1.9) | |
| Xue et al.,34arm 2 (2020) | TA | 425 (67.4) | 425 (100) | 416 (97.9) | 32 (7.5) | 260.8 ± 77.3 | 35.4 ± 10 | 364 (85.6) | 10 (2.4) |
| HA | 206 (32.6) | 0 | 202 (98.1) | 16 (7.8) | 234 ± 70 | 27 ± 11.3 | 126 (61.2) | 4 (1.9) |
Values are n (%), unless otherwise indicated.
CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; HA, hemiarch; NR, not reported; SD, standard deviation; TA, total arch.
Table 3.
Preoperative malperfusion and presence of arch tear
| Study (y) | Group | Patients | Any malperfusion | Visceral malperfusion | Neurologic malperfusion | Peripheral malperfusion | Coronary malperfusion | Tamponade | Arch tear (non-resectable by hemiarch) |
|---|---|---|---|---|---|---|---|---|---|
| Easo et al.20 (2012) | TA | 140 (21.3) | NR | NR | NR | NR | NR | NR | 0 |
| HA | 518 (78.7) | NR | NR | NR | NR | NR | NR | 0 | |
| Fleischman et al.22 (2018) | TA | 39 (20) | 10 (25.6) | 4 (10.3) | NR | 6 (15.4) | NR | 7 (17.9) | NR |
| HA | 156 (80) | 23 (14.7) | 11 (7.1) | NR | 12 (7.7) | NR | 20 (12.8) | NR | |
| Hata et al.26 (2016) | TA | 12 (18.8) | 3 (25) | NR | NR | NR | NR | 5 (41.7) | NR |
| HA | 52 (81.3) | 8 (15.4) | NR | NR | NR | NR | 28 (53.8) | NR | |
| Keeling et al.18 (2017) | TA | 43 (12.6) | 6 (14) | 6 (14) | NR | NR | NR | NR | NR |
| HA | 299 (87.4) | 55 (18.4) | 55 (18.4) | NR | NR | NR | NR | NR | |
| Kim et al.4 (2011) | TA | 44 (23.4) | 11 (25) | 2 (4.5) | 3 (6.8) | 6 (13.6) | 1 (2.3) | NR | 33 (75) |
| HA | 144 (76.6) | 11 (7.6) | 1 (0.7) | 4 (2.8) | NR | 1 (0.7) | NR | 35 (24.3) | |
| Kimura et al.27 (2020) | TA | 135 (19.1) | 54 (40) | 4 (3) | 27 (20) | NR | NR | NR | 135 (100) |
| HA | 571 (80.9) | 195 (34.2) | 7 (22) | 67 (12) | NR | NR | NR | 0 | |
| Larsen et al.21 (2017) | TA | 334 (26.9) | NR | NR | NR | NR | NR | NR | 47 (14.1) |
| HA | 907 (73.1) | NR | NR | NR | NR | NR | NR | 147 (16.2) | |
| Lio et al.24 (2016) | TA | 33 (35.9) | NR | NR | NR | NR | NR | NR | NR |
| HA | 59 (64.1) | NR | NR | NR | NR | NR | NR | NR | |
| Ohtsubo et al.25 (2002) | TA | 24 (51.1) | NR | NR | NR | NR | NR | 10 (41.7) | NR |
| HA | 23 (48.9) | NR | NR+ | NR | NR | NR | 12 (52.2) | NR | |
| Trivedi et al.19 (2016) | TA | 92 (35.5) | 76 (82.6) | 21 (22.8) | 31 (33.7) | 19 (20.7) | 5 (0) | NR | 45 (48.9) |
| HA | 167 (64.5) | 68 (40.7) | 9 (5.4) | 21 (12.6) | 23 (13.8) | 15 (0) | NR | 31 (18.6) | |
| Yang et al.23 (2019) | TA | 150 (31.8) | 30 (20) | 17 (11.3) | 11 (7.3) | NR | 2 (1.3) | 6 (4) | NR |
| HA | 322 (68.2) | 62 (19.3) | 34 (10.6) | 17 (5.3) | NR | 11 (3.4) | 36 (11.2) | NR | |
| Dai et al.36 (2015) | TA | 52 (55.9) | 2 (3.8) | 1 (1.9) | 1 (1.9) | NR | NR | 1 (1.9) | 0 |
| HA | 41 (44.1) | 1 (2.4) | 1 (2.4) | 0 | NR | NR | 2 (4.9) | 0 | |
| Driever et al.28 (2003) | TA | 30 (71.4) | NR | NR | NR | NR | NR | NR | NR |
| HA | 12 (28.6) | NR | NR | NR | NR | NR | NR | NR | |
| Fichadiya et al.35 (2019) | TA | 28 (29.5) | 12 (42.9) | NR | 7 (25) | 4 (14.3) | NR | 3 (10.7) | NR |
| HA | 67 (70.5) | 18 (26.9) | NR | 3 (4) | 3 (4.5) | NR | 25 (37.3) | NR | |
| Inoue et al.30 (2016) | TA | 161 (48.2) | NR | NR | NR | NR | NR | NR | 73 (45.3) |
| HA | 173 (51.8) | NR | NR | NR | NR | NR | NR | 10 (5.8) | |
| Kim et al.31 (2018) | TA | 32 (34) | NR | NR | NR | NR | NR | NR | NR |
| HA | 62 (66) | NR | NR | NR | NR | NR | NR | NR | |
| Omura et al.9 (2016) | TA | 88 (44.7) | 35 (39.8) | 4 (4.5) | 14 (15.9) | 19 (21.6) | 5 (5.7) | NR | 53 (60.2) |
| HA | 109 (55.3) | 46 (42.2) | 7 (6.4) | 25 (22.9) | 14 (12.8) | 7 (6.4) | NR | NR | |
| Shi et al.32 (2014) | TA | 84 (54.2) | NR | NR | NR | NR | NR | 12 (14.3) | 0 |
| HA | 71 (45.8) | NR | NR | NR | NR | NR | 13 (18.3) | 0 | |
| Shimamura et al.29 (2018) | TA | 29 (9.7) | 8 (27.6) | NR | NR | NR | NR | 5 (17.2) | NR |
| HA | 271 (90.3) | 67 (24.7) | NR | NR | NR | NR | 60 (22.1) | NR | |
| Vallabhajosyula et al.33 (2016) | TA | 31 (50.8) | 7 (22.6) | NR | NR | NR | NR | 3 (9.7) | 31 (100) |
| HA | 30 (49.2) | 10 (33.3) | NR | NR | NR | NR | 7 (23.3) | 30 (100) | |
| Xue et al.,34 arm 1 (2020) | TA | 54 (20.8) | 17 (31.5) | 5 (9.3) | 4 (7.4) | 6 (11.1) | 2 (3.7) | 2 (3.7) | 0 |
| HA | 206 (79.2) | 52 (25.2) | 5 (2.4) | 19 (9.2) | 15 (7.3) | 13 (6.3) | 36 (17.5) | 0 | |
| Xue et al.,34 arm 2 (2020) | TA | 425 (67.4) | 135 (31.8) | 17 (4) | 41 (9.6) | 58 (13.6) | 19 (4.5) | 23 (5.4) | 161 (37.9) |
| HA | 206 (32.6) | 52 (25.2) | 5 (2.4) | 19 (9.2) | 15 (7.3) | 13 (6.3) | 36 (17.5) | 0 |
Values are n (%).
HA, hemiarch; NR, not reported; TA, total arch.
Results
Short-term outcomes
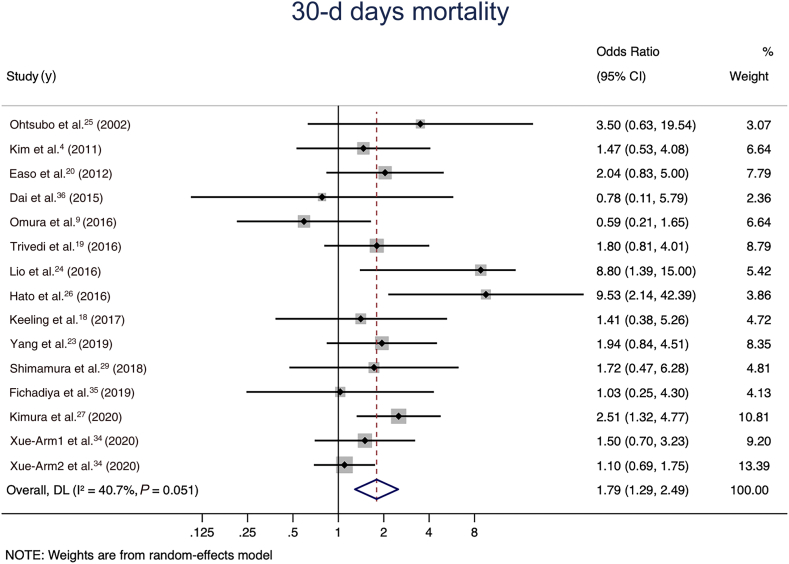
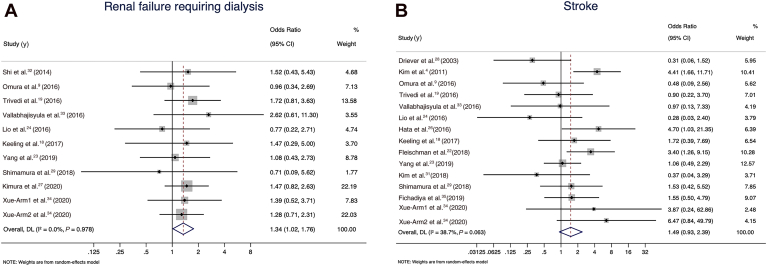
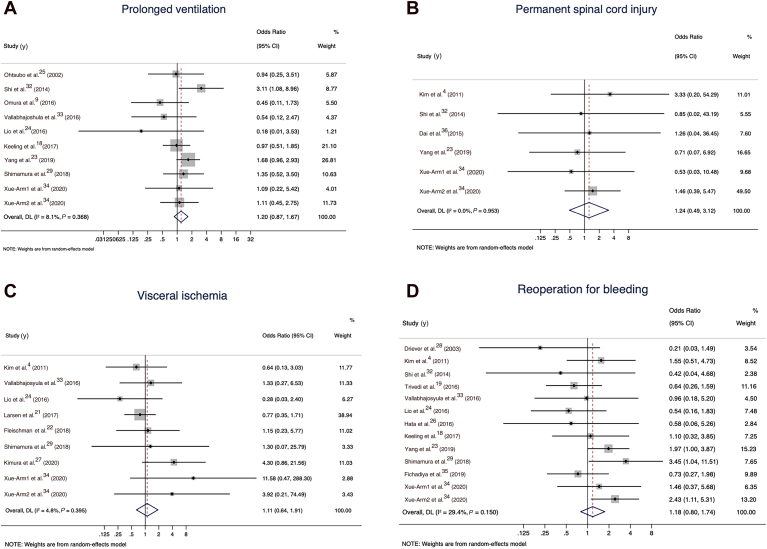
A pooled analysis of all 15 comparative studies (N pooled = 4404 patients) reporting early mortality demonstrated a statistically significant increase in the odds of 30-day mortality in the total arch group in the random-effects model (OR 1.79, 95% CI 1.29 to 2.49; I2 value of 40.7%; Fig. 2). The total arch group also had higher rates of renal failure requiring dialysis (11 studies with N pooled = 3475; OR 1.34, 95% CI 1.02 to 1.76; I2 value of 0; Fig. 3A), and a trend toward higher rates of stroke (15 studies with N pooled = 3292; OR 1.49, 95% CI 0.93 to 2.39; I2 value of 38.7%; Fig. 3B). No significant differences were observed between the groups in terms of prolonged ventilation (10 studies with N pooled = 2557; OR 1.20, 95% CI 0.87 to 1.67; I2 value of 8.1; Fig. 4A), permanent spinal cord injury (6 studies with N pooled = 1799; OR 1.24, 95% CI 0.49 to 3.12; I2 value of 0; Fig. 4B), visceral ischemia (9 studies with N pooled = 3674; OR 1.11, 95% CI 0.64 to 1.91; I2 value of 4.8%; Fig. 4C), or reoperation for bleeding (13 studies with N pooled = 2961; OR 1.18, 95% CI 0.80 to 1.74; I2 value of 29.4%; Fig. 4D).
Figure 2.
30-day mortality for the total arch vs hemiarch groups in the random-effects model. CI, confidence interval; DL, DerSimonian and Laird estimation of summary effect.
Figure 3.
(A) Renal failure requiring dialysis for the total arch group vs hemiarch groups in the random-effects model. (B) Stroke for the total arch vs hemiarch groups in the random-effects model. CI, confidence interval.
Figure 4.
(A) Prolonged ventilation for the total arch group vs hemiarch groups in the random-effects model. (B) Permanent spinal cord injury for the total arch group vs hemiarch groups in the random-effects model. (C) Visceral ischemia for the total arch group vs hemiarch groups in the random-effects model. (D) Reoperation for bleeding for the total arch vs hemiarch groups in the random-effects model. CI, confidence interval.
Figure 5.
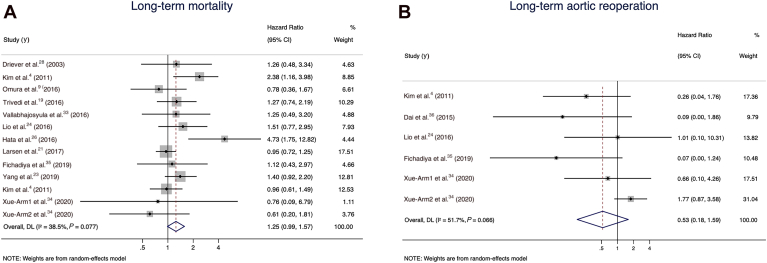
(A) Long-term mortality for the total arch group vs hemiarch groups in the random-effects model. (B) Long-term unplanned aortic reoperation for the total arch vs hemiarch groups in the random-effects model. CI, confidence interval.
Long-term outcomes
A pooled analysis of 13 comparative studies (N pooled = 4408 patients) reporting long-term mortality demonstrated a non–statistically significant increase in the hazards of long-term mortality in the total arch group in the random-effects model (HR 1.25, 95% CI 0.99 to 1.57; I2 value of 38.5%; Fig. 5A). Long-term unplanned aortic reoperation was reported in 6 studies (N pooled = 1359 patients) and showed a trend in favour of the total arch group (HR 0.53, 95% CI 0.18 to 1.59; I2 value of 51.7%; Fig. 5B). The rate of late aortic events was reported in 2 studies (N pooled = 903 patients) and was lower in the total arch group (HR 0.53, 95% CI 0.33 to 0.84; I2 value of 0).
Subgroup and sensitivity analysis
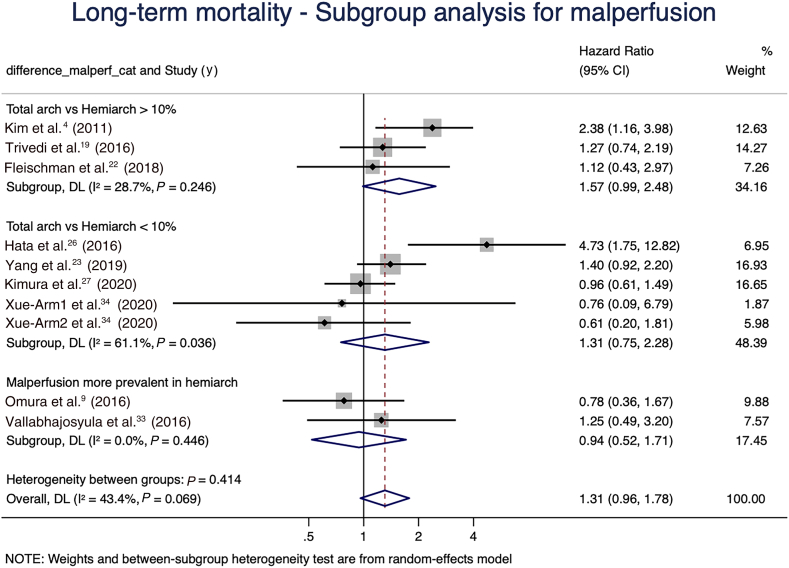
To account for confounders in the meta-analysis of long-term mortality, a subgroup analysis was performed based on the difference in preoperative malperfusion between intervention arms in the individual studies. Studies were subgrouped based on the percent difference in preoperative malperfusion between the intervention arms (proportion of malperfusion in total arch minus proportion of malperfusion in hemiarch), as follows: 4 studies had a difference > 10%; 7 had a difference < 10%; and 3 had a higher prevalence of malperfusion in the hemiarch group. The rest of the studies did not report data on malperfusion. The increased hazard of long-term mortality was observed mainly in the subgroup of studies in which the difference in malperfusion prevalence between the treatment arms was > 10% (Fig. 6).
Figure 6.
Subgroup analysis for long-term mortality, stratifying studies based on percent difference in preoperative malperfusion in the total arch vs hemiarch groups. CI, confidence interval; DL, DerSimonian and Laird estimation of summary effect.
We also performed a subgroup analysis of the studies that compared TAR with concomitant descending aorta graft vs HAR without descending aorta graft. No difference was present in short-term mortality, but results for long-term mortality (HR 0.72, 95% CI 0.39 to 1.32; I2 value of 0; Supplemental File S1) and long-term aortic reoperation (HR, 0.47; 95% CI, 0.10 to 2.27; I2 value of 63%; Supplemental File S1) showed a trend favouring patients with TAR with descending aorta graft. We also present the results for short-term mortality, long-term mortality, and long-term aortic reoperation based on the surgical approach intent for TAR (total arch only if tear in the arch; total arch if tear in the arch or other indication for TAR; total arch at the discretion of the surgeon; absence of arch tear in all patients; presence of arch tear in all patients) in Supplemental File S1.
Discussion
In this meta-analysis, we compared TAR vs HAR in the management of ATAAD. In the main analysis, we found that TAR could be associated with improved freedom from long-term aortic reoperation, but with potentially increased perioperative risks and a trend toward a lower late survival rate. However, when taking into account the within-study difference in preoperative malperfusion, we noticed that the difference in late survival rate was observed mostly in studies in which the proportion of malperfusion in the total arch group was substantially greater than that in the hemiarch group. To our knowledge, this meta-analysis is the first that has explored the effect of preoperative malperfusion on mortality following total arch vs HAR. Additionally, as opposed to previous meta-analyses that included a heterogenous group of patients in their conservative aortic replacement arm (patients receiving aortic root, ascending aorta, or HAR), our group with proximal arch replacement was limited to patients who underwent HAR.
Although limited by the small sample size, a subgroup analysis of the studies that compared TAR with concomitant descending aorta graft vs HAR without descending aorta graft showed no difference in the short-term mortality rate, but did show a trend toward improved freedom from long-term mortality and long-term aortic reoperation in the TAR with descending aorta graft group. Possibly, the deployment of descending aorta graft, most commonly in the form of frozen elephant trunk, was useful in mitigating the consequence of preoperative malperfusion, which is present more commonly in patients with TAR, as a byproduct of selection bias.
In their paper, Heuts and colleagues showed that TAR was associated with a higher risk of early mortality and postoperative renal failure, but the risk of stroke did not differ between the groups.12 We have found similar results for the early outcomes between the intervention arms. For their long-term outcomes, Heuts and colleagues did not find a statistically significant difference in survival after 1 and 5 years, but they noted a survival benefit for patients who underwent TAR after 10 years of follow-up. The authors did not demonstrate improved freedom from reoperation at long-term follow-up with the total arch approach. Similarly, Sá and colleagues observed that the aggressive approach with TAR seemed to confer a better survival rate over time, compared with the rate with the conservative approach, but it did not seem to be associated with a lower risk of reintervention.13 However, in their landmark analysis for reoperation, the authors revealed a possible benefit of the aggressive approach beyond 7 years after the initial procedure. In our study, we observed a trend toward improved freedom from long-term aortic reoperation in the group with TAR, which could stem from the higher proportion of patients receiving elephant trunks in the group with TAR.
We have noted that the indication for TAR varied widely among the included studies. In the studies in which TAR was performed in patients with arch tear or other indication for arch replacement, such as arch aneurysm, circumferential arch dissection, malperfusion, or connective-tissue disease, the patients had comparable early and long-term mortality to those with HAR, suggesting that patient selection is paramount in minimizing postoperative mortality in the acute and high-risk setting of an ATAAD, in which surviving the perioperative phase is the first-order priority.
The findings of this meta-analysis are in keeping with those of previous single-centre studies, showing that TAR, as compared to HAR, is associated with an increased initial short-term hazard (30-day mortality and in-hospital complications) but that TAR results in improved long-term outcomes, in both survival likelihood and freedom from reoperations. These results, however, should not be generalized blindly, and the decision to perform a TAR vs a HAR should be carefully tailored to both each individual patient and the resources available. The decision to perform a TAR should be based on the following: the presence of a clear surgical indication (tear in the arch, arch aneurysm, circumferential arch dissection, malperfusion, or connective-tissue disease); patients’ comorbidities and likelihood of living long enough to see a long-term benefit; the surgeon’s experience level; and the resources available to perform a TAR safely. Several of these considerations have been investigated and confirmed already (such as the relationship between a centre’s volume and surgical outcomes), but many remain to be studied.
Limitations
Our analysis has some limitations. All of the studies included in our analysis were retrospective, and they therefore were limited by the intrinsic biases they may carry, notably the selection bias (confounding by indication) created by patients having to meet certain criteria to undergo TAR. As in any meta-analysis that pools results from different studies, differences in the characteristics of the individual studies included in the analysis are expected. To account for the between-studies variabilities, we performed our meta-analysis using random-effects (rather than fixed effects). Some of the outcomes have residual heterogeneity. We have attempted to understand the source of this heterogeneity, and we have performed subgroup analyses taking into account malperfusion as a possible confounder and a surrogate for the severity of clinical status. However, accounting for all patients’ preoperative characteristics and surgeons’ expertise that could create residual confounding was not possible. Also, the subgroup analysis combined all types of malperfusion and did not stratify by type of malperfusion, owing to lack of statistical power. As for the surgeon experience and/or centre volume, the data from this metanalysis do not allow us to make any conclusion on this matter; however, several studies have shown improved outcomes in high-volumes centres.37,38
Conclusion
This meta-analysis has demonstrated that TAR is associated with a trend toward improved freedom from long-term aortic reoperation but also with potentially increased perioperative risks. Early and late postoperative mortality appeared to be confounded by preoperative malperfusion, which was more prevalent in the patients with TAR.
Acknowledgments
Ethics Statement
This meta-analysis was conducted on studies that either explicitly stated having or were understood to have complied with all appropriate ethical standards. Our analysis was rigorously conducted based on the available data.
Patient Consent
The authors confirm that patient consent is not applicable to this article. This is a meta-analysis of published comparative deidentified studies.
Funding Sources
The authors would like to acknowledge funding from the Heart and Stroke Foundation Grant in Aid supporting the TITAN:HEADSTART randomized clinical trial.
Disclosures
M.W.A.C. reports receiving support as the Ray and Margaret Elliott Chair in Surgical Innovation; and speaker honoraria from Medtronic, Edwards Lifesciences, Terumo Aortic, and Artivion. M.O. is partially supported by the Munk Chair in Advanced Therapeutics and the Antonio & Helga De Gasperis Chair in Clinical Trials and Outcomes Research. All the other authors have no competing interests to disclose.
Footnotes
See page 1085 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2024.04.012.
Supplementary Material
References
- 1.Hagan P.G., Nienaber C.A., Isselbacher E.M., et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 2.Pape L.A., Awais M., Woznicki E.M., et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66:350–358. doi: 10.1016/j.jacc.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Halstead J.C., Meier M., Etz C., et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2007;133:127–135. doi: 10.1016/j.jtcvs.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 4.Kim J.B., Chung C.H., Moon D.H., et al. Total arch repair versus hemiarch repair in the management of acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg. 2011;40:881–887. doi: 10.1016/j.ejcts.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Li B., Ma W.G., Liu Y.M., Sun L.Z. Is extended arch replacement justified for acute type A aortic dissection? Interact Cardiovasc Thorac Surg. 2015;20:120–126. doi: 10.1093/icvts/ivu323. [DOI] [PubMed] [Google Scholar]
- 6.Mody P.S., Wang Y., Geirsson A., et al. Trends in aortic dissection hospitalizations, interventions, and outcomes among Medicare beneficiaries in the United States, 2000–2011. Circ Cardiovasc Qual Outcomes. 2014;7:920–928. doi: 10.1161/CIRCOUTCOMES.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanuki H., Ogino H., Minatoya K., et al. Is emergency total arch replacement with a modified elephant trunk technique justified for acute type A aortic dissection? Ann Thorac Surg. 2007;84:1585–1591. doi: 10.1016/j.athoracsur.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 8.Fattouch K., Sampognaro R., Navarra E., et al. Long-term results after repair of type A acute aortic dissection according to false lumen patency. Ann Thorac Surg. 2009;88:1244–1250. doi: 10.1016/j.athoracsur.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 9.Omura A., Miyahara S., Yamanaka K., et al. Early and late outcomes of repaired acute DeBakey type I aortic dissection after graft replacement. J Thorac Cardiovasc Surg. 2016;151:341–348. doi: 10.1016/j.jtcvs.2015.03.068. [DOI] [PubMed] [Google Scholar]
- 10.Smith H.N., Boodhwani M., Ouzounian M., et al. Classification and outcomes of extended arch repair for acute type A aortic dissection: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2017;24:450–459. doi: 10.1093/icvts/ivw355. [DOI] [PubMed] [Google Scholar]
- 11.Poon S.S., Theologou T., Harrington D., et al. Hemiarch versus total aortic arch replacement in acute type A dissection: a systematic review and meta-analysis. Ann Cardiothorac Surg. 2016;5:156–173. doi: 10.21037/acs.2016.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heuts S., Adriaans B.P., Kawczynski M.J., et al. Editor’s choice—extending aortic replacement beyond the proximal arch in acute type A aortic dissection: a meta-analysis of short term outcomes and long term actuarial survival. Eur J Vasc Endovasc Surg. 2022;63:674–687. doi: 10.1016/j.ejvs.2021.12.045. [DOI] [PubMed] [Google Scholar]
- 13.Sá M.P., Jacquemyn X., Tasoudis P.T., et al. Long-term outcomes of total arch replacement versus proximal aortic replacement in acute type A aortic dissection: meta-analysis of Kaplan-Meier-derived individual patient data. J Card Surg. 2022;37:4256–4266. doi: 10.1111/jocs.16852. [DOI] [PubMed] [Google Scholar]
- 14.Wells G., Shea B., O’Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta- analysis. http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf Available at:
- 15.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmar M.K., Torri V., Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keeling W.B., Leshnower B.G., Hunting J.C., Binongo J., Chen E.P. Hypothermia and selective antegrade cerebral perfusion is safe for arch repair in type A dissection. Ann Thorac Surg. 2017;104:767–772. doi: 10.1016/j.athoracsur.2017.02.066. [DOI] [PubMed] [Google Scholar]
- 19.Trivedi D., Navid F., Balzer J.R., et al. Aggressive aortic arch and carotid replacement strategy for type A aortic dissection improves neurologic outcomes. Ann Thorac Surg. 2016;101:896–903. doi: 10.1016/j.athoracsur.2015.08.073. discussion 903-5. [DOI] [PubMed] [Google Scholar]
- 20.Easo J., Weigang E., Hölzl P.P.F., et al. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection: analysis of the German Registry for Acute Aortic Dissection Type A. J Thorac Cardiovasc Surg. 2012;144:617–623. doi: 10.1016/j.jtcvs.2011.07.066. [DOI] [PubMed] [Google Scholar]
- 21.Larsen M., Trimarchi S., Patel H.J., et al. Extended versus limited arch replacement in acute type A aortic dissection. Eur J Cardiothorac Surg. 2017;52:1104–1110. doi: 10.1093/ejcts/ezx214. [DOI] [PubMed] [Google Scholar]
- 22.Fleischman F., Elsayed R.S., Cohen R.G., et al. Selective aortic arch and root replacement in repair of acute type A aortic dissection. Ann Thorac Surg. 2018;105:505–512. doi: 10.1016/j.athoracsur.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Yang B., Norton E.L., Shih T., et al. Late outcomes of strategic arch resection in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2019;157:1313–1321.e2. doi: 10.1016/j.jtcvs.2018.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lio A., Nicolò F., Bovio E., et al. Total arch versus hemiarch replacement for type A acute aortic dissection: a single-center experience. Tex Heart Inst J. 2016;43:488–495. doi: 10.14503/THIJ-15-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohtsubo S., Itoh T., Takarabe K., et al. Surgical results of hemiarch replacement for acute type A dissection. Ann Thorac Surg. 2002;74:S1853–S1856. doi: 10.1016/s0003-4975(02)04133-4. discussion S1857-63. [DOI] [PubMed] [Google Scholar]
- 26.Hata M., Orime Y., Wakui S., et al. Efficacy of limited proximal arch replacement for type A acute aortic dissection with critical complications. Gen Thorac Cardiovasc Surg. 2016;64:651–656. doi: 10.1007/s11748-016-0688-2. [DOI] [PubMed] [Google Scholar]
- 27.Kimura N., Momose N., Kusadokoro S., et al. Minimized perfusion circuit for acute type A aortic dissection surgery. Artif Organs. 2020;44:E470–E481. doi: 10.1111/aor.13724. [DOI] [PubMed] [Google Scholar]
- 28.Driever R., Botsios S., Schmitz E., Donovan J., Vetter H.O. Long-term effectiveness of operative procedures for Stanford type A aortic dissections. Cardiovasc Surg. 2003;11:265–272. doi: 10.1016/S0967-2109(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 29.Shimamura J., Yamamoto S., Oshima S., et al. Surgical outcomes of aortic repair via transapical cannulation and the adventitial inversion technique for acute type A aortic dissection. Eur J Cardiothorac Surg. 2018;54:369–374. doi: 10.1093/ejcts/ezy014. [DOI] [PubMed] [Google Scholar]
- 30.Inoue Y., Minatoya K., Oda T., et al. Surgical outcomes for acute type A aortic dissection with aggressive primary entry resection. Eur J Cardiothorac Surg. 2016;50:567–573. doi: 10.1093/ejcts/ezw111. [DOI] [PubMed] [Google Scholar]
- 31.Kim J.H., Choi J.B., Kim T.Y., Kim K.H., Kuh J.H. Simplified surgical approach to improve surgical outcomes in the center with a small volume of acute type A aortic dissection surgery. Technol Health Care. 2018;26:675–685. doi: 10.3233/THC-171169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi E., Gu T., Yu Y., et al. Early and midterm outcomes of hemiarch replacement combined with stented elephant trunk in the management of acute DeBakey type I aortic dissection: comparison with total arch replacement. J Thorac Cardiovasc Surg. 2014;148:2125–2131. doi: 10.1016/j.jtcvs.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 33.Vallabhajosyula P., Gottret J.P., Robb J.D., et al. Hemiarch replacement with concomitant antegrade stent grafting of the descending thoracic aorta versus total arch replacement for treatment of acute DeBakey I aortic dissection with arch tear. Eur J Cardiothorac Surg. 2016;49:1256–1261. doi: 10.1093/ejcts/ezv374. discussion 1261. [DOI] [PubMed] [Google Scholar]
- 34.Xue Y., Pan J., Cao H., et al. Different aortic arch surgery methods for type A aortic dissection: clinical outcomes and follow-up results. Interact Cardiovasc Thorac Surg. 2020;31:254–262. doi: 10.1093/icvts/ivaa095. [DOI] [PubMed] [Google Scholar]
- 35.Fichadiya A., Gregory A.J., Kotha V.K., et al. Extended-arch repair for acute type-A aortic dissection: perioperative and mid-term results. Eur J Cardiothorac Surg. 2019;56:714–721. doi: 10.1093/ejcts/ezz071. [DOI] [PubMed] [Google Scholar]
- 36.Dai X.F., Chen L.W., Wu X.J., Dong Y., Wang Q.M. Total aortic arch reconstruction with triple-branched stent graft or hemiarch replacement for acute Debakey type I aortic dissection: five-years experience with 93 patients. J Card Surg. 2015;30:749–755. doi: 10.1111/jocs.12608. [DOI] [PubMed] [Google Scholar]
- 37.Brescia A.A., Patel H.J., Likosky D.S., et al. Volume-outcome relationships in surgical and endovascular repair of aortic dissection. Ann Thorac Surg. 2019;108:1299–1306. doi: 10.1016/j.athoracsur.2019.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobaria V., Kwon O.J., Hadaya J., et al. Impact of center volume on outcomes of surgical repair for type A acute aortic dissections. Surgery. 2020;168:185–192. doi: 10.1016/j.surg.2020.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.