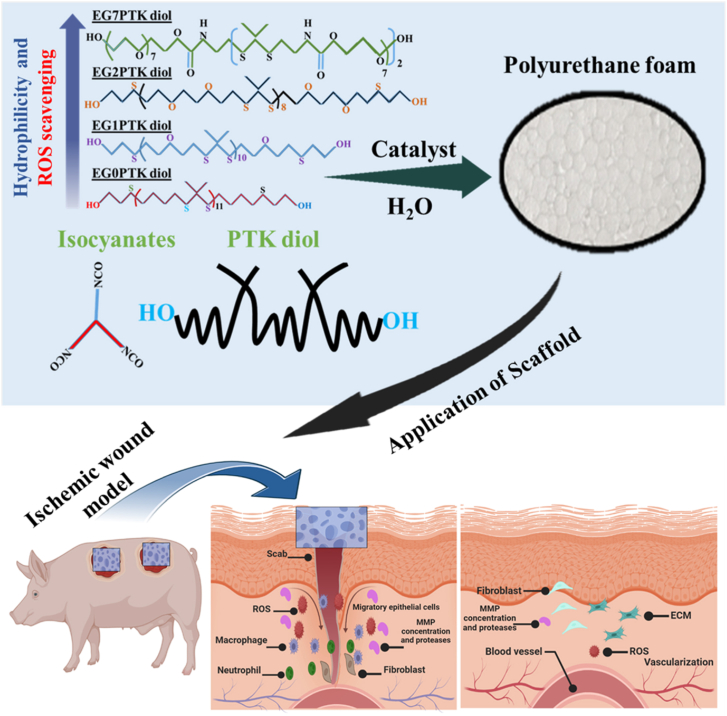
Porous, resorbable synthetic biomaterials such as polyesters provide a cost-effective framework for the healing of skin wounds. However, it must be optimized to reduce acidic breakdown and improve immunological and regenerative responses. Recently, Craig L. Duvall1 and his team published his work in Science Translational Medicine, which delineates the synthesis, characterization, and application of ethylene glycol (EG7) polythioketal urethane (PTK-UR). Here, they integrate seven ethylene glycol repeats unites in the PTK-UR polymer backbone to enhance its hydrophilicity and biological compatibility, facilitating optimal wound-healing processes in porcine skin models. These implants promote extracellular matrix (ECM) deposition, reepithelialization, and remodeling while effectively transitioning to a healing phenotype from a moderate inflammatory response.
Chronic wounds involve a complicated pathological process, which poses difficulties in their clinical treatment. Numerous external and internal factors can hinder the healing process, impede the transition between adjacent phases, and delay the healing process. In nonhealing wounds, polymorphic mononuclear leukocytes proliferate and produce reactive oxygen species (ROS), proteolytic enzymes, and inflammatory cytokines, all contributing to persistent and low-grade inflammation. Ischemia-reperfusion injury, which induces ROS production and tissue damage, frequently exacerbates non-healing lesions caused by vascular insufficiencies2. Oxidative stress, resulting from excess ROS, hampers essential wound healing processes, including angiogenesis, deposition of ECM, and the healing ability of keratinocytes and fibroblast cells3. Standard techniques for managing chronic wounds include tissue debridement, effective moisture control, and the application of contemporary wound dressings, with or without biologically active agents, to avoid infection. Dermal replacements offer a quick way to heal wounds while eliminating the difficulties connected with donor sites, which are frequent with typical skin grafts that require removing a thin layer of skin from another part of the body. Using these substitutes eliminates the need to harvest skin from the patient, which consequently prevents the patient from experiencing any additional discomfort or harm. In 2002, Integra Bilayer Wound Matrix was first approved for treating burn wounds; since then, its use has been expanded to a wide range of other types of wounds4. However, inadequate incorporation of integra BWM's has resulted in problems including wound reopening, silicone detachment, bleeding, tissue death, and bacterial infection in certain cases. Synthetic scaffolds, particularly polyurethane (PU) based foams like PE-UR NovoSorb, are more cost-effective and appealing. However, an autocatalytic, hydrolytic breakdown mechanism that works independently of cellular activity limits polyester (PE)-based materials. Applying the more durable PE formulations can help to reduce the biodegradation process of the material. However, this process increases the probability of the foreign body fibrous encapsulated response compared to non-degradable implants. Craig L. Duvall et al.1 developed a variety of PTK diols with different numbers of EG repeats among thioketal (TK) bonds in polymer backbones to maximize the potential of synthetic scaffolds by eliminating the drawbacks of PE-URs, and better understanding the structure–function correlations of PTK-URs as shown in the (Fig. 1). The objective was to modify the materials so that they could utilize ROS cellular-generated breakdown mechanism, as oxidative stress is a feature of chronic injuries with a persistent type 1 immune reaction. Various in vitro and in vivo tests are used to thoroughly investigate EG7's ROS reactivity. The results show that EG7's increased hydrophilicity significantly improves its reactivity towards ROS, resulting in fast degradation. This degradation is critical for lowering chronic inflammation and accelerating natural wound healing. Several PTK diols were created to investigate the effects of hydrophilicity on reactive oxygen species-mediated scaffold degradation, vascularization, immunological response, and tissue healing quality. It seems that the EG7 design, which was better hydrophilic than the PTK-UR foams that had been used in the past, was the most successful composition.
Figure 1.
Chemical structures showing controlled variation of the number (0–7) of ethylene glycol (EG) units in the polythioketal (PTK) diol backbone and their application of animal wound model.
The presence of EG7 in the TK content led to a more rapid breakdown and decrease in mass compared to the other EG series, emphasizing the significance of the hydrophilicity in the process of (oxidative) degradation. TK bonds are more susceptible to breakage in a wet environment by highly polar oxidative radicals. It is essential to mention that although EG2 and EG7 PTK scaffolds have a similar amount of EG content overall, there were substantial variances (P < 0.001) in swelling ratio 619.72% for EG7 and 208.72% for EG2. This phenomenon can be attributed to the intrinsic hydrophobic nature of TK bonds and demonstrates the necessity for spacer groups throughout the backbone of polymers to be highly hydrophilic to permit TK bond accessibility and an improved ability to scavenge reactive oxygen in aqueous environments.
The diol chemistry also affects the function of the scaffold both in-vitro and in-vivo. In the EG7 PTK diol chemistry, a negative logP value was observed, indicating a strong affinity for water over octanol. In contrast, the logP value in the other EG series is greater than one. The in vivo investigation of EG7 PTK-UR and 900t PE-UR demonstrates a significant decrease in ROS concentration in mice treated with TK scaffolds. Moreover, the immunohistochemistry (IHC) analysis revealed a reduction in 8-OhdG staining in wound samples obtained from the pig's model. The PTK foam efficiently reduced levels of ROS and cellular damage, signifying the antioxidant potential of the foam fabricated with the PTK scaffold. The overall healing effects of EG7 foams maybe due to decreasing oxidative stress, which can reduce the expression of inflammatory genes and cytokines regulated by nuclear factor-κB, which inhibit the healing process. The histological findings show that EG7 PTK-URs, EG0/EG1/EG2, and PTK-UR are well integrated into tissue after their application on pig excisional wounds. The EG7 PTK-UR scaffolds showed a uniform distribution over the whole depth of the wound, measuring 3.1 ± 0.4 mm. In contrast, the other scaffolds, which were highly hydrophobic, exhibited lower levels of tissue incorporation (<2 mm). The enriched vascularization of EG7 PTK-UR scaffolds could be attributed to foam swelling/degradation, lower levels of myeloperoxidase, and antiangiogenic enzymes generally found in chronic wounds. The EG7 PTK-URs scaffold also improved the expression level of growth factors, including PDGF and TGFB3, which increase endothelial cell proliferation, migration, and maturation. It's concluded that The EG7 PTK-UR scaffolds increase wound healing by possessing hydrophilic properties that facilitate favorable biological interactions, good ROS scavenging, and fast clearance of breakdown products.
The study additionally discovered that the EG7 PTK-UR scaffold activated a more favorable immunological response from the host than the hydrophobic PTK-URs. The implantation of biomaterials and Tissue damage is closely associated with the initial movement of circulating monocytes, neutrophils, and macrophages to the area of injury. The initial inflammation responses produce ROS, such as hydroxyl radical (·OH), hypochlorite ion (ClO−), hydrogen peroxide (H2O2), and superoxide ion (O2−), as part of the innate immune responder. The EG7 formulations exhibited a significant decrease the density of macrophages, myofibroblasts, and T cells, which can be attributed to the non-degradable fibrous encapsulation of the implant. The active migration of macrophages, monocytes, neutrophils, and dendritic cells (DCs) to the place of biological material implantation might exacerbate inflammation and foreign body reactions, causing tissue and implant structural damage. DCs play a crucial role in connecting innate and adaptive immunity through pathogen recognition receptor-mediated interactions with biomaterials, which result in antigen presentation and T-cell activation. Additionally, hydrophilic surfaces can prevent the attachment and development of DCs, which in turn drops the proinflammatory response of T-cells5. Craig L. Duvall applied dermal implants in animals, discovering that EG7 PTK scaffolds had less invading DCs than the more hydrophobic group EG2 PTK-UR materials. The results displayed that hydrophobic scaffolds produce a greater persistent inflammatory response than the EG7 PTK-UR foam. There are key implications for the chronic wound-healing abilities of synthetic materials that relate to both the preliminary recruitment of immune cells and the duration of the immune response. The study further revealed that hydrophobic scaffolds cause a stronger and more long-lasting inflammatory response than EG7 PTK-UR foams. Moreover, it is important to note that both the initial recruitment of immune cell response and the gradual progress of the immune reaction have significant effects on the capability of synthetic biomaterials to heal wounds.
Reepithelialization is essential to wound healing and a main outcome in clinical trials. It decreases the threat of infection and patient morbidity by restoring the skin's epidermal barrier. The EG7 PTK-UR's have the capability to keep the wound wet and have much higher epithelial resurfacing than wounds treated with highly hydrophobic scaffolds. EG7 PTK-UR foams exhibited a considerably higher (2.7-fold) recruitment of T-cells compared to EG2 PTK scaffolds when implanted in the mouse dermal region. In the wounds treated with EG7 PTKUR scaffold, the expression of certain growth factors, such as PDGF (3-fold), IGF1 (2-fold), and TGFB3 (2-fold), was increased. Craig L. Duvall and his colleague investigated the EG7 PTK-UR scaffolds against the commercially available NovoSorb BTM and Integra BWM in pig wounds model to determine their therapeutic potential in wound healing. The Integra and EG7 PTK-UR wound healing rates were almost similar, but the wounds treated with EG7 PTK-UR foam exhibited significantly increased blood perfusion compared to Integra BWM. These findings show that EG7 PTK-UR foam has the benefit of promoting neovascularization. The EG7 PTK-UR foam has the advantage of more frequent and superior treatment outcomes for ischemic wounds compared to Integra. After 31 days, a considerable amount of NovoSorb was in the wound, suggesting the delayed biodegradation profile of the NovoSorb. This might trigger an extended host foreign body reaction. The high stability of the NovoSorb within the body may contribute to the high level of pro-inflammatory cytokine genes, including CSF2 (5.9-fold), IL1B (3.1-fold), NOS2 (4.5-fold), CXCL8 (12-fold), and TNFA (2.1-fold), in comparison to EG7 PTK-UR therapies. On the other hand, after the EG7 PTK-UR scaffold extracted from wounds on Day 31, showed a significant increase in the anti-inflammatory interleukins expression of such as IL-13 (2.8 times) and IL-33 (3.2 times), which promotes tissue healing in macrophages. On the other hand, the NovoSorb foams, comparable in composition and structure, did not display the same level of up-regulation of these interleukins to the same extent.
Interestingly, the results suggested that wounds treated with the EG7 PTK-UR scaffold had considerably reduced levels of Arg-1 expression compared to those treated with the NovoSorb scaffold, a marker frequently used to identify an anti-inflammatory M2 macrophage phenotype. IHC analysis of wound tissue showed a reduction in the number of Arg-1-expressing macrophages in wounds treated with EG7 PTK-UR but eliminated. Strong Arg-1 staining was found near FBGCs that surrounded the remaining NovoSorb scaffold. Arg-1 expression is high in ischemic pig wounds and hominid venous leg ulcers6, even though Arg-1 metabolism plays a crucial role in macrophage polarization. High arginase activity has been connected to fibrosis, indicating different biological implications for Arg-1-expressing macrophages7. Craig L. Duvall found that wounds treated with EG7 PTKUR had a higher ratio of TGFB3/TGFB1 expression than those treated with NovoSorb regarding the fibrosis phenotype. TGFB3 has been observed to facilitate scarless healing in mice, while increased levels of TGFB1 are linked to excessive ECM accumulation and fibrosis. Craig L. Duvall and colleagues employed a pre-optimized pig model where excisional wound models were made in stroke-raised flaps due to surgical separation from the underlying tissue and adjacent areas of skin. This model accurately depicts a clinical situation in which skin wounds are not healing properly and require treatment with a biomaterial. Doppler imaging reveals that raised areas of skin have less blood circulation than the surrounding healthy skin. This model specifically signifies the features of an ischemia-reperfusion wound, a condition that can lead to the advance of chronic wound conditions when there are pre-existing vascular insufficiencies8. Ischemia-reperfusion induces the generation of ROS, which leads to oxidative stress and cellular injury. Both EG7 PTK-UR and NovoSorb foams improved the cell infiltration, although EG7 PTK-UR scaffolds resulted in quicker wound closure and better reepithelialization at the endpoint. The variances in reepithelialization between EG7 PTK-UR and NovoSorb BTM treatments were more apparent than in the nonischemic wound model. EG7 PTK-UR treatment improved the reduction of inflammation, as observed in nonischemic wounds, by enhancing the wound healing profile. This improvement was determined using IHC analysis, which showed a decrease in MPO-positive neutrophils and CCR7-positive macrophages. Based on these findings, the improved tissue regeneration observed in healthy pigs with full-closed skin wounds can also be applied to ischemic skin wounds, which more closely resemble the chronic non-healing conditions seen in patients.
The promising research data provided here, a few significant limitations that will be considered in future work to produce products. Initially, evaluating the healing ability of the PTK-UR scaffold in fresh wounds induced on strong and fully healthy pigs provides a valuable, although restricted, understanding of its effectiveness in long-lasting human skin wounds. Chronic wounds may be linked to long-lasting inadequate blood supply, infection, and maybe increased oxidative stress, which could speed up the deterioration of EG7 PTK-UR scaffolds. To effectively treat chronic wounds, it may be necessary to adjust the mechanical properties further to counteract the accelerated degradation. Future consideration must be given to implementing a temporary, nondegradable membrane to construct a bilaminar architecture comparable to that of Integra and NovoSorb. By incorporating a detachable upper layer into EG7 PTK-UR, potential benefits include enhanced resistance to bacterial infections and moisture loss and improved mechanical properties that reduce dermal layer contraction. According to the author's experience with NovoSorb, the best time to remove the upper layer is determined by the size of the incision and the rate at which it heals. It is crucial to monitor this process carefully to prevent the upper layer from delaying reepithelialization and slowing down the closure of the wound. Reepithelialization in larger wounds can be difficult to achieve with EG7 PTK treatment alone. Larger wounds may require split-thickness skin grafts or autologous keratinocyte transplants to be successfully reepithelized with EG7 PTK therapy9. The ischemia wound model, which utilizes PTK-UR and NovoSorb matrices, is an effective method for studying the variations in wound healing when supported by PU scaffolds. This model is particularly useful for studying the healing process of wounds with limited blood supply. However, this model can provide accurate ischemia, but it fails to accurately represent the background conditions that are present in a significant number of patients who have chronic wounds. In the future, it might be beneficial to do research utilizing excisional wounds of a bigger size or diabetes animals fed a rich fat diet prior to skin injury.
To summarize, Craig L. Duvall and colleagues have designed a new type of foam dressing called EG7 PTK-UR. This dressing helps tissues and scaffolds merge together, allows for strong infiltration of cells and blood vessels, and aids in wound healing. These implants cause a mild inflammatory response that efficiently changes to a healing phenotype while also stimulating the deposition of extracellular matrix, re-growth of epithelial cells, and remodeling. EG7 PTK-UR outperformed the synthetic PE-based foam NovoSorb in swine excisional wounds, showing greater vascularization and comparable wound closing to the standard biologic substance Integra BWM.
Author contributions
Salim Ullah: Conceptualization, Writing – original draft. Hong-Tao Xu: Supervision, Writing – original draft. Jianliang Shen: Supervision, Writing – review & editing.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Hong-Tao Xu, Email: xht0071@sina.com.
Jianliang Shen, Email: shenjl@wiucas.ac.cn, sjl1@wmu.edu.cn.
References
- 1.Patil P., Russo K.A., McCune J.T., Pollins A.C., Cottam M.A., Dollinger B.R., et al. Reactive oxygen species—degradable polythioketal urethane foam dressings to promote porcine skin wound repair. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abm6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues M., Kosaric N., Bonham C.A., Gurtner G.C. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansoub N.H. The role of keratinocyte function on the defected diabetic wound healing. IJBT. 2021;11:430. [PMC free article] [PubMed] [Google Scholar]
- 4.Weigert R., Leclere F.M., Delia G., De Luca L., Al Mutairi K., Casoli V. Long-term patient-reported functional and cosmetic outcomes following severe traumatic foot and ankle wound reconstruction with acellular dermal matrix. J Cosmet Laser Ther. 2015;17:321–329. doi: 10.3109/14764172.2015.1027231. [DOI] [PubMed] [Google Scholar]
- 5.Keselowsky B.G., Lewis J.S. Dendritic cells in the host response to implanted materials. Semin Immunol. 2017;29:33–40. doi: 10.1016/j.smim.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy S., Biswas S., Khanna S., Gordillo G., Bergdall V., Green J., et al. Characterization of a preclinical model of chronic ischemic wound. Physiol Genomics. 2009;37:211–224. doi: 10.1152/physiolgenomics.90362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adhyatmika A., Putri K.S., Beljaars L., Melgert B.N. The elusive antifibrotic macrophage. Front Med. 2015;2:81. doi: 10.3389/fmed.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waller J.M., Maibach H.I. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol. 2005;11:221–235. doi: 10.1111/j.0909-725X.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 9.Larson K.W., Austin C.L., Thompson S.J. Treatment of a full-thickness burn injury with NovoSorb biodegradable temporizing matrix and RECELL autologous skin cell suspension: a case series. JBCR. 2020;41:215–219. doi: 10.1093/jbcr/irz179. [DOI] [PMC free article] [PubMed] [Google Scholar]