Abstract
Background
In rural regions, atrial fibrillation (AF) management is performed predominately by local primary care professionals (PCPs). Prior work has suggested that a disparity in outcomes in AF occurs for those patients living in a rural, vs urban, location.
Methods
This post hoc analysis of the cluster randomized trial Integrated Management Program Advancing Community Treatment of Atrial Fibrillation (IMPACT-AF) compared a clinical decision support system to standard of care. Patients were classified as living in a rural (population < 10,000) or urban location. The outcomes were as follows: AF-related emergency department (ED) visits, unplanned cardiovascular (CV) hospitalizations, AF-related referrals and guideline adherence for AF treatment.
Results
A total of 1133 patients were enrolled from 2016 to 2018; 54.1% (n = 613) were classified as living in a rural location. No differences were present in age (mean, 72 ± 9.63 vs 72.5 ± 10.42 years) or Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack (CHADS2) score (mean, 2.1 ± 1.36 vs 2.16 ± 1.34). Referral rates to general internists were higher in the rural population (13.4% vs 3.9%, P < 0.001), whereas the rate of cardiology referrals was higher in the urban population (10% vs 15%, P = 0.0098). At 12 months, no difference in the composite outcome of AF-related ED visits and CV hospitalizations was seen. Fewer recurrent AF-related ED visits and CV hospitalizations occurred in the urban group (incidence rate ratio [IRR], 0.65 [95% confidence interval (0.44, 0.95), P = 0.0262). The incidence of guideline adherence was similar between the rural (IRR, 3.7 ± 1.2) and urban (IRR, 3.6 ± 1.2; P = 0.11) groups.
Conclusions
AF patients living in rural locations had higher rates of recurrent AF-related ED visits and unplanned CV hospitalizations. Further research to optimize AF-related outcomes is needed to ensure equitable delivery of care to all Canadians, irrespective of geography.
Clinical Trial Registration
Graphical abstract
Résumé
Contexte
Dans les régions rurales, la prise en charge de la fibrillation auriculaire (FA) est assurée principalement par les professionnels des soins primaires locaux. Des travaux antérieurs ont suggéré qu'une disparité dans les pronostics de la FA se manifeste pour les patients vivant en milieu rural par rapport à ceux vivant en milieu urbain.
Méthodes
Cette analyse post hoc de l'essai randomisé par grappes « Integrated Management Program Advancing Community Treatment of Atrial Fibrillation » (IMPACT-AF) a comparé un système d'aide à la décision clinique à un système de soin standard. Les patients ont été classés selon qu'ils vivaient en milieu rural (population < 10,000) ou urbain. Les résultats mesurés comprenaient les visites aux urgences (VU) liées à la FA, les hospitalisations cardiovasculaires (CV) non planifiées, l'orientation de patients liés à la FA et le respect des directives pour le traitement de la FA.
Résultats
Un total de 1 133 patients ont été recrutés de 2016 à 2018; 54,1 % (n = 613) ont été classés comme vivant dans un lieu rural. Aucune différence n'était observée dans l'âge (moyenne, 72 ± 9,63 vs 72,5 ± 10,42 ans) ou le score CHADS2 d'insuffisance cardiaque congestive, d'hypertension, d'âge, de diabète, d'accident vasculaire cérébral ou d'accident ischémique transitoire (moyenne, 2,1 ± 1,36 vs 2,16 ± 1,34). Les taux de patients orientés vers des internistes généralistes étaient plus élevés dans la population rurale (13,4 % vs 3,9 %, p < 0,001), tandis que le taux d'orientation vers des cardiologues était plus élevé dans la population urbaine (10 % vs 15 %, p = 0,0098). À 12 mois, aucune différence n'a été observée dans l'agrégat des indicateurs cliniques des VU liées à la FA et des hospitalisations pour cause de maladie CV. Moins de VU récurrentes liées à la FA et d'hospitalisations CV ont eu lieu dans le groupe urbain (rapport de taux d'incidence [RTI], 0,65; intervalle de confiance à 95 % (0,44 - 0,95), p = 0,0262). L'incidence de l'adhésion aux lignes directrices était similaire entre les groupes ruraux (RTI, 3,7 ± 1,2) et urbains (RTI, 3,6 ± 1,2; p = 0,11).
Conclusions
Les patients atteints de FA vivant en milieu rural présentaient des taux plus élevés de VU récurrentes liées à la FA et d'hospitalisations non planifiées pour cause de maladie CV. D'autres recherches visant à optimiser les pronostics liés à la FA sont nécessaires pour assurer une prestation équitable des soins à tous les Canadiens, quelle que soit leur situation géographique.
Enregistrement de l'essai clinique
Atrial fibrillation (AF) is a common arrhythmia, affecting an estimated 350,000 Canadians, with the rate of incidence doubling each decade after age 55 years.1,2 In 2010, the hospital, surgical, and emergency department (ED) costs for AF management were estimated to be CAD$815 million in Canada.3 With the prevalence increasing in developed nations, the economic burden of AF is expected to rise.4 Access to care has been a focus of healthcare in Canada, with increasing recognition that disparities in care exist, among racialized groups, in rural locations, and between sexes. Rural location or remoteness may be associated with a lack of infrastructure or financial resources that results in healthcare disparities.5 Rurality has been linked to higher rates of comorbidities, leading to increased hospitalizations with ambulatory-care conditions and higher rates of in-hospital mortality.6, 7, 8 With rural patients having limited access to specialists, their management is performed predominantly by primary care professionals.9
The Integrated Management Program Advancing Community Treatment of Atrial Fibrillation (IMPACT-AF) study was a cluster randomized trial that assessed the clinical efficacy of a computerized clinical decision support (CDSS) tool designed to process data and assist in AF management in primary care settings within Nova Scotia. This study enrolled 203 primary care professionals (PCPs) and 1133 patients.10,11 No improved benefit from the clinical decision support system, compared with standard of care, was found in the primary outcome, which was a composite of unplanned cardiovascular (CV) hospitalizations and AF-related ED visits at 12 months.
This study was a post hoc analysis of the IMPACT-AF dataset, performed to determine whether any differences exist in access to care, management, and CV outcomes in AF patients between rural vs urban areas of Nova Scotia.
Methods
The IMPACT-AF study was a prospective, pragmatic, randomized, unblinded, cluster-designed trial of a computerized CDSS for AF management.10 The study was funded through an unrestricted research grant from Bayer Canada. The study design has been detailed previously.11 Briefly, the IMPACT-AF study randomized PCPs to use of a CDSS or standard of care for the management of AF. The CDSS focused on guideline adherence, rate or rhythm control, and anticoagulation management.
Primary care provider recruitment
PCPs practicing within the province of Nova Scotia (population = 969,383),12 Canada, were recruited and randomized in a 1:1 ratio to standard of care or the CDSS intervention. To avoid contamination, only PCPs who managed adults, not in a locum position and not involved with specialty work, were enrolled.
Patient selection
Patients were included if they were registered within the PCP’s practice before trial initiation, were aged ≥ 18 years, had electrocardiographically confirmed AF or supporting documentation of past AF diagnosis, had the ability to communicate in English, and were residing within Nova Scotia. Patient selection criteria were stratified by whether the PCP was in an urban population (> 10,000) or a rural population (< 10,000). The only exclusion criterion was poor likelihood of surviving 1 year.
Data collection
Baseline demographic, clinical, laboratory, and pharmaceutical management data relevant to the study were collected by trained study abstractors. Data collection included AF-related referrals, testing, and assessments. Participant baseline data were uploaded to the application, with ongoing automated data entry, such as electronic laboratory records and patient-reported data-updating management recommendations, with proactive prompting of PCPs to respond to critical alerts. Clinical data at the level of primary care were obtained through a complete review of patient charts (including both primary care and hospital-based care). Clinical data related to emergency department (ED) visits, hospitalization, or death were identified through administrative datasets corresponding to the Discharge Abstract Database, National Ambulatory Care Reporting System, review of PCP medical records, and hospital-based health systems. All clinical events were blinded to the assigned treatment arm and were reviewed independently by 2 members of an adjudication committee, with any disagreement reviewed by a third member. Distance from an urban centre was calculated using the patient’s postal code and the shortest distance to either of the 2 urban centres (Queen Elizabeth II Health Sciences Center and Cape Breton Regional Hospital). Mortality was identified through review of PCP medical records, hospital-based health systems, and via provincial Department of Health and Wellness datasets (Vital Statistics and the Medical Social Insurance client registry). Primary care physician charts were audited for any letters, discharge summaries, or other documentation pertaining to such events. Lists of patient events and reasons (most-responsible diagnosis and discharge details) for ED and hospital encounters relating to the population of ambulatory AF patients were reviewed, and those that clearly were unrelated to AF were excluded from further analysis. The patients' charts were reviewed from respective hospital sites by a trained abstractor, and relevant case report form data were extracted.
Outcomes
Outcome measures included a composite of AF-related ED visits and unplanned CV hospitalizations at 12 months. AF-related ED visits were defined as any presentation with heart failure, presyncope or syncope, transient ischemic attack or stroke, acute coronary syndrome, palpitations or rapid heart rate, or hemodynamic instability managed with cardioversion or rate control.
Unplanned CV hospitalizations were defined as any ≥ 1 overnight admission for the management of: acute coronary syndrome, presyncope or syncope, transient ischemic attack or stroke, symptomatic AF or atrial flutter, deep vein thrombosis or systemic embolism, and management of decompensated heart failure.
Guideline adherence was scored from 0 to 6, based on the number of guideline recommendations that were followed. These were based on the Canadian Cardiovascular Society AF guidelines.13 The following guideline recommendations were considered: (i) use of appropriate oral anticoagulation, defined as being aged > 65 years or having a Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack (CHADS2) score ≥ 1, use of aspirin only in those with coronary or vascular disease, and a CHADS2 score = 0, and no anticoagulant and/or antiplatelet in the setting of a CHADS2 score = 0 without coronary and/or vascular disease 13; (ii) thyroid-stimulating hormone measurement performed at the time of or since the AF diagnosis; (iii) echocardiogram; (iv) blood pressure control, defined as readings < 140/90 mm Hg, or < 130/85 mm Hg if the patient had a confirmed diagnosis of diabetes; (V) obstructive sleep apnea (OSA) assessment, defined as having a sleep study or overnight oximetry; and (vi) an appropriate level of alcohol consumption, defined as no clinical notation of alcohol dependency, and < 11 drinks per week or < 3 per day in female patients, and < 16 drinks per week, or < 4 per day in male patients.
Statistical analysis
Descriptive statistics were reported as counts and percentages for categorical variables, means and standard deviations for normally distributed continuous variables, and medians and interquartile ranges for non-normally distributed continuous variables. The χ2 test was utilized for comparison of categorical variables between the urban and rural groups. Rates of incidence reoccurrence were analyzed via a negative binomial model. Multivariable regression analysis for outcome measures was performed adjusting for sex, CHADS2 score, age, OSA, and urban vs rural as the variables of interest.
Results
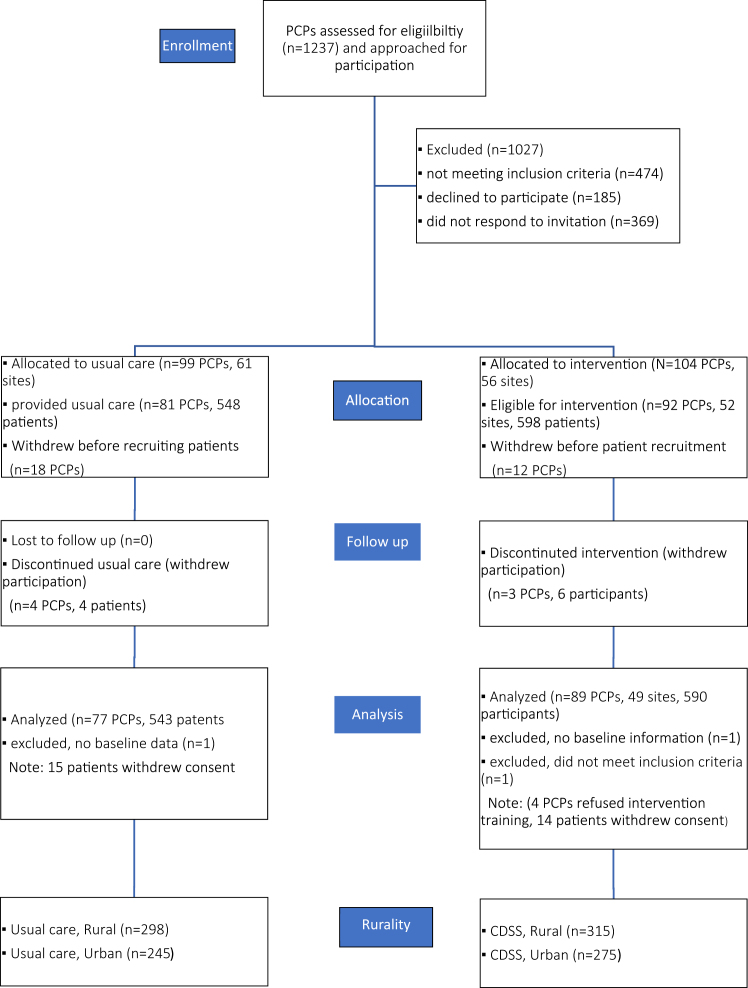
A total of 1133 patients were included in the study, estimated to represent about 12% of patients with AF in the province. Of these, 54.1% (n = 613) were from a rural region, in keeping with the actual geographic population distribution in Nova Scotia (Fig. 1). The mean age was similar between the groups (rural, 72.1 ± 9.6 years; urban, 72.6 ± 10.4 years); 37.4% of those in the rural group were women, and 39% of those in the urban group. The mean CHADS2 scores were 2.1 ± 1.4 and 2.2 ± 1.3, respectively. More patients had OSA (22.2% vs 16.5%), pulmonary disease (38% vs 29%), and former smoking (43.4% vs 36%) in the rural group, compared to the urban group (Table 1).
Figure 1.
Patient allocation. CDSS, clinical decision support system; PCP, primary care professional.
Table 1.
Baseline characteristics
| Characteristic | Rural (n = 613) | Urban (n = 520) |
|---|---|---|
| Age, y | 72.1 (9.6) | 72.6 (10.4) |
| Male sex | 384 (62.6) | 317 (61) |
| Systolic blood pressure, mm Hg, mean (SD) | 129.72 (16.32) | 127.13 (16.56) |
| Diastolic blood pressure, mm Hg, mean (SD) | 74.06 (9.5) | 73.77 (9.7) |
| Type of atrial fibrillation | ||
| First episode | 39 (6.4) | 28 (5.4) |
| Not documented | 241 (39.3) | 267 (51.4) |
| Paroxysmal | 170 (27.7) | 134 (25.8) |
| Persistent / chronic | 163 (26.6) | 91 (17.5) |
| CHADS2 score, mean (SD) | 2.1 (1.36) | 2.16 (1.34) |
| Previous stroke, systemic embolism, or transient ischemic attack | 104 (17) | 105 (20.2) |
| Congestive heart failure | 146 (23.8) | 145 (27.9) |
| Hypertension | 490 (79.9) | 401 (77.1) |
| Diabetes mellitus | 189 (30.8) | 138 (26.5) |
| Previous myocardial infarction | 82 (13.4) | 75 (14.4) |
| Vascular disease | 206 (33.6) | 192 (36.9) |
| Congenital heart disease | 13 (2.1) | 15 (2.9) |
| Obstructive sleep apnea | 136 (22.2) | 86 (16.5)∗ |
| Tobacco use | ||
| Current | 38 (6.2) | 27 (5.2) |
| Former | 266 (43.4) | 187 (36) |
| Alcohol abuse | 44 (7.2) | 42 (8.1) |
| Pulmonary disease | 233 (38) | 151 (29) |
| CABG or PCI—combined group | 83 (13.5) | 78 (15) |
| Prior ablation (AF or atrial flutter) | 39 (6.4) | 37 (7.1) |
| Pacemaker / ICD | 82 (13.4) | 66 (12.7) |
| Any bleeding | 200 (32.6) | 154 (29.6) |
| Rate-control medications | 431 (70.3) | 340 (65.4) |
| Antiarrhythmic medications | 59 (9.6) | 40 (7.7) |
Values are n (%), unless otherwise indicated.
AF, atrial fibrillation; CABG, coronary artery bypass grafting; CHADS2, score denoted by C = congestive heart failure (1), H = hypertension (1), A = age ≥ 75 years (1), D = diabetes (1), S = prior stroke or transient ischemic attack (2); ICD, implantable cardioverter defibrillator; PCI, percutaneous coronary intervention; SD, standard deviation.
P < 0.05 between the 2 groups.
At 12 months, no difference was present in the outcome of a composite of AF-related ED visits or unplanned CV hospitalizations (Table 2). CDSS management did not change the occurrence of these outcomes in urban or rural groups. A significant difference was found in the incidence of recurrent events for the composite outcome (incidence rate ratio for urban vs rural (1.65 [95% confidence interval [CI] 1.05, 2.27), P = 0.0262; Table 3). This difference persisted in a multivariable model, after adjustment for age, sex, CHADS2 score, and sleep apnea, with the greatest difference in unplanned CV hospitalizations, but a trend toward an effect on AF-related ED visits alone (Table 4, Table 5, Table 6). When distance from an urban centre was considered, no statistically significant effect on the outcome occurred.
Table 2.
Outcomes
| Outcome | Rural (n = 613), n (%) | Urban (n = 520), n (%) | P |
|---|---|---|---|
| Composite of any AF-related emergency department visit or unplanned CV hospitalization | 89 (14.5) | 58 (11.2) | 0.093 |
| Major bleeding per ISTH criteria | 2 (0.3) | 1 (0.2) | 1 |
| AF-related emergency department visits | 61 (10) | 39 (7.5) | 0.1473 |
| Unplanned CV hospitalization | 35 (5.7) | 22 (4.2) | 0.2564 |
AF, atrial fibrillation; CV, cardiovascular; ISTH, International Society of Thrombosis and Hemostasis.
Table 3.
Recurrent events
| Outcome | Rural (n = 613), number of events | Urban (n = 520), number of events | IRR urban vs rural (95% CI) | P |
|---|---|---|---|---|
| Composite of any AF-related emergency department visit or unplanned CV hospitalization | 159 | 87 | 0.65 (0.44, 0.95) | 0.0262 |
| AF-related emergency department visits | 110 | 63 | 0.68 (0.42, 1.09) | 0.1106 |
| Heart failure | 12 | 5 | — | |
| Syncope / presyncope | 5 | 5 | — | |
| TIA / stroke | 3 | 3 | — | |
| ACS | 6 | 4 | — | |
| Rate / rhythm | 88 | 50 | — | |
| Unplanned CV hospitalization | 49 | 24 | 0.58 (0.32, 1.04) | 0.0667 |
| Heart failure (including pulmonary edema or dyspnea of cardiac origin) | 22 | 13 | — | |
| Syncope / presyncope | 2 | 0 | — | |
| TIA / stroke / SE | 10 | 4 | — | |
| ACS | 11 | 4 | — | |
| Rate / rhythm | 21 | 13 | — |
ACS, acute coronary syndrome; AF, atrial fibrillation; CI, confidence interval; CV, cardiovascular; IRR, incidence rate ratio; SE, systemic embolism; TIA, transient ischemic attack.
Table 4.
Multivariable model for the composite outcome of any atrial fibrillation–related emergency department (ED) visits or unplanned cardiovascular (CV) hospitalizations and the individual components
| Variable | Composite outcome |
P | AF-related ED visits |
P | Unplanned CV hospitalizations |
P |
|---|---|---|---|---|---|---|
| IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | ||||
| Location: urban vs rural | 0.62 (0.42, 0.91) | 0.0149 | 0.64 (0.4, 1.04) | 0.0689 | 0.53 (0.3, 0.96) | 0.0349 |
| Age (1-y increase) | 0.97 (0.95, 0.99) | 0.0036 | 0.97 (0.94, 0.99) | 0.0142 | 0.98 (0.95, 1.01) | 0.2056 |
| Sex: female vs male | 1.45 (0.98, 2.15) | 0.0637 | 1.56 (0.95, 2.55) | 0.078 | 1.25 (0.71, 2.22) | 0.4409 |
| CHADS2 (1-unit increase) | 1.15 (0.99, 1.34) | 0.0704 | 0.98 (0.8, 1.2) | 0.8613 | 1.57 (1.28, 1.94) | <.0001 |
| Obstructive sleep apnea | 1.37 (0.86, 2.18) | 0.1799 | 1.58 (0.9, 2.78) | 0.1144 | 0.79 (0.38, 1.66) | 0.5365 |
CHADS2, Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack; CI, confidence interval; IRR, incidence rate ratio.
Table 5.
Multivariable model for atrial fibrillation–related emergency department visits
| Variable | IRR (95% CI) | P |
|---|---|---|
| Location: urban vs rural | 0.64 (0.4, 1.04) | 0.0689 |
| Age (1-y increase) | 0.97 (0.94, 0.99) | 0.0142 |
| Sex: female vs male | 1.56 (0.95, 2.55) | 0.078 |
| CHADS2 (1-unit increase) | 0.98 (0.8, 1.2) | 0.8613 |
| Obstructive sleep apnea | 1.58 (0.9, 2.78) | 0.1144 |
CHADS2, Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack; CI, confidence interval; IRR, incidence rate ratio.
Table 6.
Multivariable model for unplanned cardiovascular hospitalizations
| Variable | IRR (95% CI) | P- |
|---|---|---|
| Location: urban vs rural | 0.53 (0.3, 0.96) | 0.0349 |
| Age (1-y increase) | 0.98 (0.95, 1.01) | 0.2056 |
| Sex: female vs male | 1.25 (0.71, 2.22) | 0.4409 |
| CHADS2 score (1-unit increase) | 1.57 (1.28, 1.94) | <.0001 |
| Obstructive sleep apnea | 0.79 (0.38, 1.66) | 0.5365 |
CHADS2, Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack; CI, confidence interval; IRR, incidence rate ratio.
Referral rates to specialists were no different in the rural population (23.9% vs 20.1%). However, the referral rate to general internists was significantly higher in the rural population, compared to the rate to cardiologists (13.4% vs 3.9%, P < 0.001), whereas, in the urban cohort, referrals were made more frequently to cardiologists than to general internists (15% vs 10%, P = 0.0098). Referral rates to electrophysiologists or AF clinics were minimal within both groups. The rates of use of cardioversions (3.4% in the rural group, 4.0% in the urban group), catheter ablations (6.4% in the rural group, 7.1% in the urban group), and cardiac implantable electronic device implantation (13.4% in the rural group, 12.7% in the urban group) did not differ between the 2 groups.
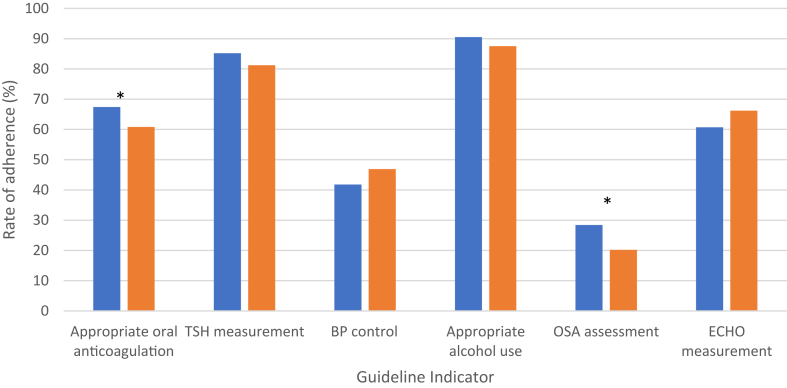
Guideline adherence was similar for thyroid-stimulating hormone measurement, blood pressure control, an appropriate level of alcohol use, and use of echocardiograms (Fig. 2). Guideline adherence for appropriate anticoagulant use (67.3% vs 60.8%; P = 0.02) and OSA assessment (28.4% vs 20.2%; P = 0.001) was higher in the rural vs the urban population, respectively. Of patients who were aged ≥ 65 years or had a CHADS2 score ≥ 1 on oral anticoagulation, rural participants (n = 401) were more often on warfarin (58.9% vs 46.7%) than on direct oral anticoagulants (41.1% vs 53.3%), as compared to their urban counterparts (n = 315). Overall, guideline adherence rates were similar between study cohorts (mean 3.74 vs 3.63, P = 0.11; Fig. 3).
Figure 2.
This chart demonstrates the rate of adherence to each guideline measure. The asterisk indicates a P value < 0.05. The blue bars represent the rural cohort; the orange bars represent the urban cohort. BP, blood pressure; ECHO, echocardiographic; OSA, obstructive sleep apnea; TSH, thyroid-stimulating hormone.
Figure 3.
Cumulative guideline adherence rate. The blue bars represent the rural cohort; the orange bars represent the urban cohort.
Discussion
This study examined differences in AF management and outcomes in patients in urban, compared to rural, regions in the province of Nova Scotia. The study demonstrated that no difference occurred in the incidence of AF-related ED visits or unplanned CV hospitalizations. However, the rate of recurrent events was higher in the rural region. Guideline adherence was similar between the 2 groups. A trend toward a higher level of use of echocardiograms was found in the urban population, but a higher incidence of appropriate oral anticoagulation use was found in the rural population.
Our study found comparable rates of overall anticoagulation use between our study populations, but a higher level of warfarin use was found in the rural cohort. Within Canada, use of direct oral anticoagulants has increased as barriers to access have lessened over time, but public-payer restrictions on reimbursement were in place during the time the IMPACT-AF study was ongoing.14 Possibly, the level of access to third-party insurance, which is otherwise the default source of drug coverage, is lower in the rural cohort, and the higher use of warfarin may be due to inability to pay for the drug. The Canadian Cardiovascular Society has recommended use of direct oral anticoagulants over use of warfarin, largely due to their having a similar efficacy in stroke prevention with a lower risk of bleeding.13
The divide in rural vs urban access to medical care and specialists has been well documented.15, 16, 17 Some of the differences in access may be due to the lack of availability of services, such as walk-in clinics, specialty clinics, or extended hours at a primary care clinic in a rural region. In addition, many studies examining geographic differences show large discrepancies in baseline CV health, and the difference in the rate of comorbidities between rural vs urban populations in North America.6,18 In this study, the rural cohorts had higher rates of sleep apnea, pulmonary disease, and prior smoking. A previous Canadian study also has found this disparity.18 Our data showed that rates of referral for AF management were higher in the rural population. This finding is different from results in prior studies showing that rurality is associated with a reduced 30-day follow-up for AF diagnosis when the diagnosis occurs in an ED.19 Atzema et al.19 found that having a PCP is the biggest factor for undergoing follow-up; this finding is universal within our dataset, as being followed by a PCP before study onset was required for inclusion. In Nova Scotia, the shortage of PCPs is well documented. The Nova Scotia PCP waiting list has increased steadily over time, with roughly 11% of the population awaiting PCP access, with nearly 60% of the waitlist living outside the urban central zone.20 The higher rate of recurrent events in the rural population, however, could be linked to a perceived lack of access to more-advanced therapies that may be offered through tertiary cardiac care, which is easier to access for the urban cohort.
The paucity of referrals for advanced arrhythmia care in both the urban and rural cohorts is apparent. The recent Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST AFNet-4; n = 2789) was a study examining rate vs rhythm control (either ablation or antiarrhythmic drugs) in patients with AF diagnosed in the prior 1 year, and it found a benefit to early rhythm control in reduction of CV death, stroke, heart failure, or acute coronary syndrome (hazard ratio 0.79; 96% confidence interval, 0.66 to 0.94; P = 0.005).21 Given the findings of this study, one possibility is that patients in both urban and rural regions could benefit from earlier access to advanced therapies for AF.
Limitations
Although the study purposefully sought to recruit participants so as to reflect Nova Scotia’s urban-rural population split, place of residence was not a feature of participant randomization. Given this, some confounding variables that might influence the results may not have been captured in the study. Some variables that were not captured include socioeconomic status, presence of obesity, and ethnic or racial differences. These observations do not imply causation, but rather association. Our inclusion criteria could mask true rural vs urban discrepancies in follow-up, based on all patients included having a PCP.
Conclusion
AF patients in rural locations have a higher rate of recurrent AF-related ED visits and unplanned CV hospitalizations compared to those in urban regions. Further research in rural regions to optimize AF-related outcomes is needed to ensure equitable delivery of care to all Canadians, irrespective of geography.
Acknowledgments
Ethics Statement
This study was approved the Nova Scotia Health Authority Research Ethics board and is reported in accoradance with the releveant ethical standards and guidelines.
Patient Consent
All patients provided consent prior to inclusion in the IMPACT-AF study. This post hoc analysis did not require further consent, as it was a secondary analysis of data that had already been collected.
Funding Sources
Funding for this work was provided by Bayer Inc.
Disclosures
J.C. received an unrestricted research grant to conduct the IMPACT-AF study from Bayer Inc. R.P. has research grants from Medtronic and Abbott, and acts as a consultant for Medtronic, Inc. The other authors have no conflicts of interest to disclose.
Footnotes
See page 1168 for disclosure information.
References
- 1.Heart and Stroke Foundation of Canada Atrial fibrillation. www.heartandstroke.ca/heart-disease/conditions Available at:
- 2.Heeringa J., van der Kuip D.A., Hofman A., et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 3.O'Reilly D.J., Hopkins R.B., Healey J.S., et al. The burden of atrial fibrillation on the hospital sector in Canada. Can J Cardiol. 2013;29:229–235. doi: 10.1016/j.cjca.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Ball J., Carrington M.J., McMurray J.J., Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013;167:1807–1824. doi: 10.1016/j.ijcard.2012.12.093. [DOI] [PubMed] [Google Scholar]
- 5.Patrick K., Laupacis A. A focus on access to health care in Canada. Can Med Assoc J. 2023;195:E123–E124. doi: 10.1503/cmaj.230040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Neal W.T., Sandesara P.B., Kelli H.M., Venkatesh S., Soliman E.Z. Urban-rural differences in mortality for atrial fibrillation hospitalizations in the United States. Heart Rhythm. 2018;15:175–179. doi: 10.1016/j.hrthm.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laditka J.N., Laditka S.B., Probst J.C. Health care access in rural areas: evidence that hospitalization for ambulatory care-sensitive conditions in the United States may increase with the level of rurality. Health & Place. 2009;15:761–770. doi: 10.1016/j.healthplace.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Deering T.F., Bhimani A.A. Atrial fibrillation: location, location, location—does it matter? Heart Rhythm. 2018;15:180–181. doi: 10.1016/j.hrthm.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Rush K.L., Burton L., Van Der Merwe F., Hatt L., Galloway C. Atrial fibrillation care in rural communities: a mixed methods study of physician and patient perspectives. BMC Fam Pract. 2019;20:144. doi: 10.1186/s12875-019-1029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox J.L., Parkash R., Abidi S.S., et al. Optimizing primary care management of atrial fibrillation: the rationale and methods of the Integrated Management Program Advancing Community Treatment of Atrial Fibrillation (IMPACT-AF) study. Am Heart J. 2018;201:149–157. doi: 10.1016/j.ahj.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Cox J.L., Parkash R., Foster G.A., et al. Integrated Management Program Advancing Community Treatment of Atrial Fibrillation (IMPACT-AF): a cluster randomized trial of a computerized clinical decision support tool. Am Heart J. 2020;224:35–46. doi: 10.1016/j.ahj.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Canada S. Census profile. 2021 Census of Population. Catalogue no 98-316-X2021001. 2023. https://www12.statcan.gc.ca/census-recensement/2021/dp-pd/index-eng.cfm
- 13.Andrade J.G., Aguilar M., Atzema C., et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–1948. doi: 10.1016/j.cjca.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Weitz J.I., Semchuk W., Turpie A.G., et al. Trends in prescribing oral anticoagulants in Canada, 2008-2014. Clin Ther. 2015;37:2506–2514.e4. doi: 10.1016/j.clinthera.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Subedi R., Greenberg T.L., Roshanafshar S. Does geography matter in mortality? An analysis of potentially avoidable mortality by remoteness index in Canada. Health Rep. 2019;30:3–15. doi: 10.25318/82-003-x201900500001-eng. [DOI] [PubMed] [Google Scholar]
- 16.Fleet R., Bussières S., Tounkara F.K., et al. Rural versus urban academic hospital mortality following stroke in Canada. PLoS One. 2018;13 doi: 10.1371/journal.pone.0191151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly M.S., Goldstein J.P., Currie M., et al. Urban-rural differences in cardiac arrest outcomes: a retrospective population-based cohort study. CJC Open. 2022;4:383–389. doi: 10.1016/j.cjco.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C., McMurtry M.S., Sandhu R.K., et al. Impact of rural residence on warfarin use and clinical events in patients with non-valvular atrial fibrillation: a Canadian population based study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atzema C.L., Yu B., Ivers N.M., et al. Predictors of obtaining follow-up care in the province of Ontario, Canada, following a new diagnosis of atrial fibrillation, heart failure, and hypertension in the emergency department. Can J Emerg Med. 2018;20:377–391. doi: 10.1017/cem.2017.371. [DOI] [PubMed] [Google Scholar]
- 20.N.S.H. Authority Reports, statistics and accountability. https://www.nshealth.ca/sites/default/files/documents/Finding%20a%20Primary%20Care%20Provider%20in%20Nova%20Scotia%20December%202022.pdf
- 21.Kirchhof P., Camm A.J., Goette A., et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]