Abstract
Lactic acid is an alternative treatment to hard chemicals against Varroa destructor, the parasitic mite of the Western honey bee Apis mellifera. This soft acaricide is used only for small apiaries due to its laborious administration. However, the mode of action of this honey bee medication remains unknown. Previous studies showed that a direct contact between the arolia of V. destructor and lactic acid altered their morphology and led to an impairment of grip. Yet, there is no evidence for the way of action of lactic acid in a realistic in-hive scenario, i.e. after an indirect exposure of the mite through honey bees. We investigated the nature of lactic acid activity in the hive treatment context. The local and/or systemic way of action of this honey bee treatment against V. destructor was studied through a behavioural and toxicological approach at the individual level. On one hand, we confirmed the altered morphology for the arolia of mites and studied the evolution of the process over time. On the other hand, we found that haemolymph contaminated with lactic acid did not kill the feeding parasitic mite. These findings support a local mode of action. In order to unravel the sequence of events leading to the local contact between the acid and the mite on bees, we also documented the olfactory valence of lactic acid for A. mellifera and V. destructor. This work provides a new comprehension of lactic acid activity against the parasitic mite through honey bee exposure and gives new opportunities for control strategies against V. destructor.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78371-w.
Subject terms: Animal behaviour, Entomology
Introduction
Treatments against Varroa destructor, the parasitic mite of Apis mellifera, have been studied for years. Among them, organic acids are alternative solutions to synthetic acaricides, known to promote the development of resistances from mites and the accumulation of residuals in beehive products1. Varroa destructor ranks among the top threats for honey bees along with pesticides2, pollution3, poor nutrition4, climate change5 and predators6. As a piercing-sucking mite, it transmits several viruses like the deformed wing virus7 leading to colony weakening around the world.
To defeat V. destructor and help honey bees, organic acids naturally present in honey like formic, oxalic and lactic acids are popular treatments used by beekeepers8. However, their efficacy is uneven worldwide9–11 demonstrating that key elements are still not well understood such as their mechanism of action on V. destructor. Some studies showed that formic acid interferes with the cellular respiratory chain12,13 while oxalic acid is supposed to create crystals penetrating the arolia of the mites14. Arolia are soft pads found at the end of the pretarsus structure of parasites (also named “caruncle” in certain cases)15. They can be characterised as adhesive inflated cushion-like pads essential for walking and gripping hosts. Regarding lactic acid, mites are found dead at the bottom of the hives three to seven days after spray application in colonies16,17. Lactic acid is thus considered as a miticide in the field. However, when applied directly on mites’ arolia under artificial conditions, lactic acid does not kill mites twenty-four hours post-treatment but rather impairs V. destructor grip skills18, leading to reduced biological processes like locomotion and reproduction19. Yet, the specific mechanism of action for in-hive lactic acid treatment against V. destructor might differ from this artificial exposure. Indeed, when treatments are applied to the colony by spraying, mites are likely exposed indirectly through honey bees. The spraying of honey bees does not necessarily mean that the exposure of mites happens by walking on their cuticle. Two major routes of drug activity are indeed possible: local or systemic. A local way of action indicates that the effects of the treatment does occur at the site of contact, in this case the honey bee cuticle, whereas a systemic way of action indicates that the effects of the treatment occur at a location distant from the point of contact20. More specifically, if lactic acid works through a local way of action against V. destructor, it means that lactic acid sprayed in-hive coats honey bees and the contact point could be the arolia of mites. This contact should then lead to local effects such as the ones observed in case of direct exposure to lactic acid. On the other hand, if it works through a systemic way of action against V. destructor, one plausible scenario is that lactic acid sprayed in-hive ends up in the honey bees’ organs and haemolymph from which mites fed. Following treatment application, lactic acid could indeed be ingested by honey bees, either directly or through residuals in honey8, which would result in higher concentrations in the haemolymph targeted by the mite. Lactic acid could also work through the combination of systemic and local way of action against mites, as it was recently shown for lithium chloride21–23.
Furthermore, once bees are treated, the sequence of events leading to the contact between V. destructor and lactic acid remains unknown. Lactic acid was shown to be attractive for some organisms like drosophila or was used as a reward in experiments24,25, therefore its odour valence (the perception of the odour by the organism: attractive, repulsive or neutral) for V. destructor or A. mellifera could be a key point in the contact process. Indeed, mite avoidance or attraction towards lactic acid could directly impact mite’s exposure and thus the treatment efficacy. This is especially important for lactic acid, as it was shown to be attractive for some tick species but not all of them26,27. In addition, the potential chemical confusion for the colony generated by organic acids should not be neglected28. Both honey bees and V. destructor indeed rely on diverse olfactory cues for orientation or for more complex biological processes29. The detection of chemicals by colony nestmates and mites in the close environment is thus a cornerstone in the lifecycle of both the honey bee and V. destructor. Lactic acid is likely detected as it can be naturally found in the nectar of some flowers30 as well as in honey31,32. Yet, when treatments are studied, their odour valence for V. destructor or A. mellifera is rarely explored33.
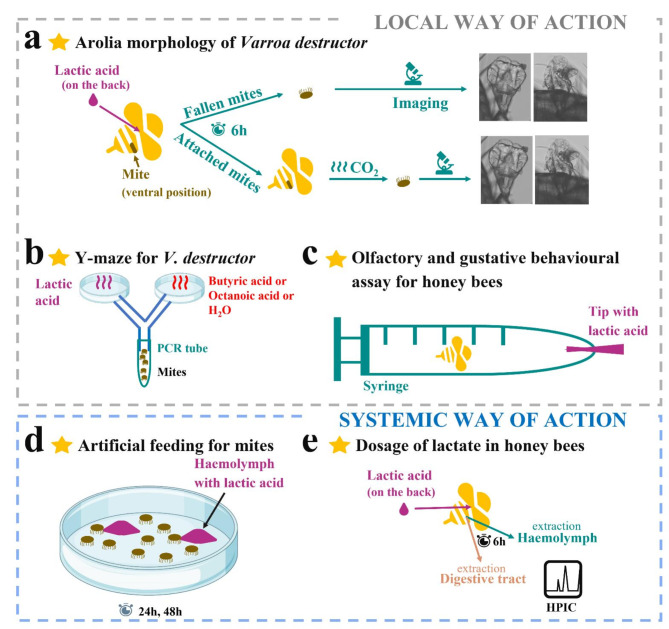
In this work, we investigated the way of action of lactic acid treatment when applied topically to infested honey bees, which is the relevant scenario in case of hive treatment. More precisely, we studied the kinetics of morphological changes in mites’ arolia when the treatment was applied only to bees to confirm the local way of action (Fig. 1A). We also checked the behaviour of the host and the parasite toward the acid under artificial conditions (Fig. 1B, C). To test the systemic way of action, we investigated if a topical application of lactic acid on bees could result in higher concentrations of lactate in honey bees’ haemolymph and digestive tract but also whether mites were affected when they were fed on honey bee larval haemolymph contaminated with a high concentration of lactic acid (Fig. 1D and E). We chose current concentrations of in-the-field treatment16 and residue in honey31 to unravel the effect of lactic acid on mite drop-off through honey bees exposure.
Fig. 1.
Schematic diagram of the experimental design to disentangle lactic acid mode of action on infested honey bees. (a) Study of the arolia morphology of mites naturally and artificially detached from their treated hosts six hours post-treatment. (b) Odour choice experiment in a Y-maze adapted to V. destructor’s size. (c) Olfactory and gustatory behavioural assay in a syringe exposing flying workers to lactic acid concentrations. (d) Artificial feeding experiment for V. destructor with bees’ haemolymph contaminated with lactic acid. (e) Study of the quantity of lactate with HPIC (High Pressure Ion Chromatography) in the haemolymph and digestive tract of honey bees after a topical application on the back of lactic acid (150 mg/mL) six hours post-treatment.
Results
Lactic acid applied on infested honey bees damages V. destructor’s arolia
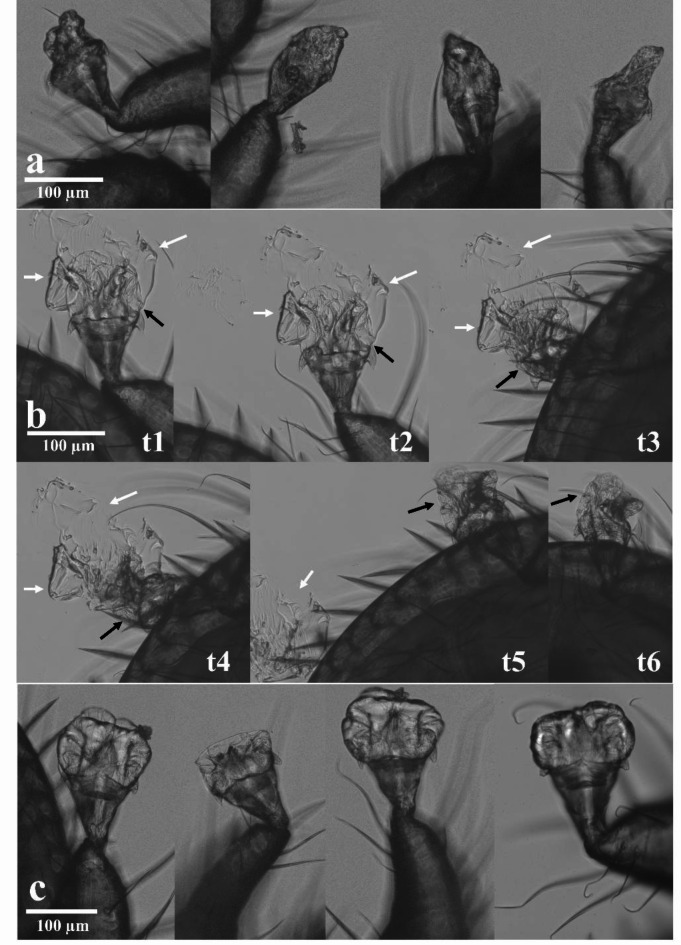
Honey bees infested with V. destructor were treated topically on the back with lactic acid (acid treated group) or demineralized water (control group) to simulate the treatment usually received in-hive. The arolia of mites were imaged (N = 30 mites from the treated honey bees group with lactic acid and N = 30 mites from the control honey bees group). Microscopy imaging showed that mites falling less than six hours post-treatment of their host presented altered and damaged arolia (Fig. 2A). In addition, the arolia of artificially detached V. destructor from treated honey bee with 150 mg/mL of lactic acid six hours post-exposure indicated a mechanism evolving over time. Indeed, the inflated arolium started to stick on the glass slide and leak with the fluid from inside the pad (Fig. 2B, from t1 to t3 & Figure S1). Once the liquid was gone, the arolium looked empty, dry, and damaged like the ones of fallen mites (Fig. 2B, from t4 to t6). On the contrary, control mites artificially detached from their control honey bee treated with demineralized water six hours post-treatment showed cushion-like inflated arolia (Fig. 2C).
Fig. 2.
Effects of lactic acid on V. destructor arolia during the first six hours after the treatment of honey bees. (a) Altered arolia from four different fallen mites between zero and six hours. (b) Kinetics showing that the fluid is drained from the arolium. The arolium from one mite artificially detached from its host showed a modified shape which cannot be filled with fluid anymore (t1-t6 stands for the images taken over time). White arrows indicate the fluid. Black arrows indicate the arolium. (c) Arolia with a normal cushion-like shape from four control parasites.
No effects of contaminated haemolymph with lactic acid on mites’ survival and feeding behaviour
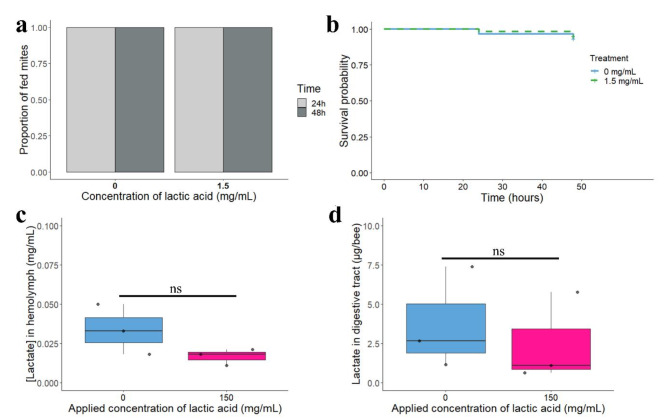
During the artificial feeding experiment, mites were confined with dyed honey bees’ haemolymph to feed or dyed honey bees’ haemolymph contaminated with 1.5 mg/mL of lactic acid. This concentration was picked prior to lactate quantification in honey bee haemolymph as a worst case scenario in which the concentration measured in honey after lactic acid treatment31 reflects the concentration ingested by the parasite when it feeds on treated honey bees. Regardless of the diet, mites did not show any inability to feed one- and two-days post-treatment as 100% of fed coloured mites were observed for both groups (Fig. 3A, Binomial GLM χ2 = 1, df = 1, p-value 1.5–24 h= 1). In addition, the probability of survival for mites was not different between contaminated and control mites during the first 48 h (Fig. 3B, Kaplan-Meier p-value = 0.69).
Fig. 3.
Nutritional state of mites when fed on lactic acid contaminated diet and concentration of lactate in honey bees after a topical application of lactic acid. (a) Proportion of fed mites on haemolymph over 48 h with or without lactic acid. (b) The survival rate of mites was not different between haemolymph or lactic acid contaminated haemolymph (N = 30 mites/concentration). (c) Concentration of lactate in honey bee haemolymph (mg/mL) six hours after topical administration of lactic acid at 150 mg/mL. Each dot represents a hive (N = 30 honey bees/concentration). (d) Quantity (µg) of lactate in honey bee digestive tract six hours after topical administration of lactic acid. Each dot represents a hive (N = 15 honey bees/concentration) and “ns” stands for not significant.
No augmentation of lactate concentration in honey bees after a topical treatment with lactic acid
Six hours after a topical treatment on the back of bees with lactic acid (150 mg/mL), the median concentration of lactate from the haemolymph of treated honey bees was 0.017 mg/mL while the median concentration measured in the haemolymph of the control group was 0.033 mg/mL (Fig. 3C, Wilcoxon rank test unpaired, W = 6, p-value = 0.25). Likewise, for the digestive tract, the median quantity of lactate when honey bees were treated was not higher with 2.14 µg/bee than the control group with 3.45 µg/bee (Fig. 3D, Wilcoxon rank test unpaired, W = 7, p-value = 0.4).
Lactic acid attracts V. destructor under artificial conditions
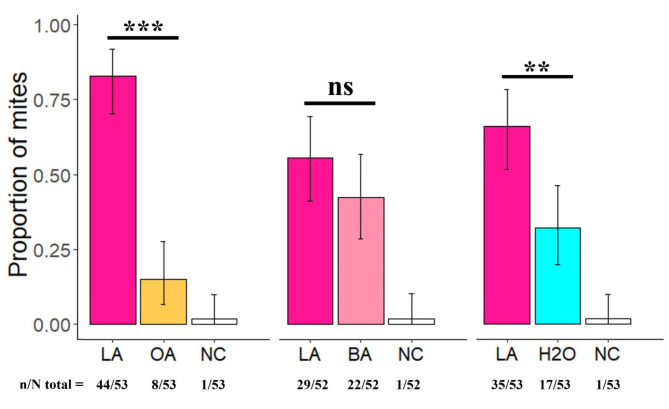
The choice of mites, at eighteen hours, showed that V. destructor significantly preferred lactic acid over octanoic acid and demineralized water (Fig. 4 | χ² = 24.92, df = 1, p-value18h-LA/OA= 5.966e-07 & | χ² = 6.23, df = 1, p-value18h-LA-H2O= 0.01). No significant preferences were recorded between lactic and butyric acids as they were not different from a random choice (Fig. 4 | χ² = 0.96, df = 1, p-value18h-LA/BA= 0.32). In this experiment it should be noted that mites needed a long period to make a choice as the no-choice proportions were high at 0.5 and 1 h (Figure S2).
Fig. 4.
Odour choice of V. destructor mites in the Y-maze. Choice between lactic and octanoic acids (LA and OA respectively), choice between lactic and butyric acids (LA and BA respectively), choice between lactic acid and demineralized water (LA and H2O respectively) 18 h post introduction. “NC” stands for no choice. Black bars represent 95% confidence intervals, ** stands for p < 0.01, *** stands for p < 0.001 and “ns” stands for not significant.
Lactic acid at in-hive treatment concentration repels honey bees under artificial conditions
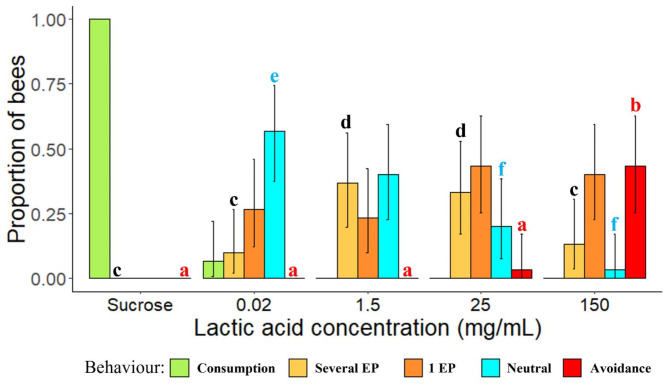
Honey bees’ behaviour toward lactic acid concentrations was measured. Bees expressed a significantly higher avoidance when 150 mg/mL of lactic acid was presented compared to 0.02, 1.5 or 25 mg/mL of lactic acid or the control group with sucrose (Fig. 5 | Fisher exact test with Bonferroni correction: p-value150 < 0.001).
Fig. 5.
Behaviour and proboscis extension of flying workers when lactic acid concentrations or sucrose were offered. EP stands for Extension of the Proboscis. Letters indicate statistical differences: a and b showed a significantly higher proportion of bees expressing avoidance toward lactic acid at 150 mg/mL; c and d showed a significantly higher proportion of bees expressing several extensions of their proboscis toward lactic acid at 1.5 and 25 mg/mL; e and f showed a significantly lower proportion of bees expressing a neutral behaviour toward lactic acid at 25 and 150 mg/mL. Black bars represent 95% confidence intervals (N = 30 honey bees/concentration except for sucrose N = 51 honey bees).
In addition, the proportion of honey bees which showed several extensions of their proboscis was significantly higher at 1.5 and 25 mg/mL compared to control (Fig. 5 | Fisher exact test with Bonferroni correction: p-value1.5= 4.506e-05 & p-value25 = 7.998e-05). Lastly, the proportion of bees expressing a neutral behaviour toward lactic acid was significantly higher for 0.02 mg/mL compared to 150 or 25 mg/mL of lactic acid (Fig. 5 | Fisher exact test with Bonferroni correction: p-value150 = 2.652e-05 & p-value25 = 0.012).
Discussion
To our knowledge, this is the first study simulating the treatment in-hive to disentangle the mode of action of lactic acid on V. destructor when administered to infested honey bees. Previous studies showed that once mites have walked directly on a paper impregnated with lactic acid, their grip skills were impaired and the shape of their arolia was modified19. Although interesting, these studies did not reflect the realistic in-hive scenario and the topical exposure of infested honey bees could result in a different mechanism of action of lactic acid towards V. destructor. In the present study, we thus investigated whether lactic acid acted as a systemic or local drug against mites when administered to honey bees. To disentangle the sequence of events leading to mite exposure, we also documented the behaviour of V. destructor and A. mellifera workers toward lactic acid. When administered to infested honey bees, we found that lactic acid damaged V. destructor females’ arolia overtime. During the first six hours, grip deficient mites showed altered arolia like the ones imaged twenty-four hours after direct exposure19, demonstrating the local way of action of lactic acid after treatment applied to honey bees. Interestingly, even mites that stayed attached to the treated bees for six hours showed in-between shapes for their arolia with a visible leak of fluid driving the arolia from inflated-like to altered-like. It could support a kinetic where lactic acid dried the membrane34,35, thus compromising the integrity of the arolium, and resulting in the fluid drained outside. From our observations, altered arolia could not recover and became unusable by the mite to walk and grip. It was suggested that arolia are inflated with haemolymph pressure36. However, Dirks & Federle (2011)37 described the mechanism of fluid production in smooth adhesive pads in insects. They showed that a storage volume of liquid, once empty, can be refilled in fifteen minutes. We do not know if there is fluid storage for V. destructor, but this mechanism seems consistent with our findings as once the arolium was empty of fluid, parasites were not completely drained and dead-dry from the inside. It also indicates that the normal functioning of the arolium was compromised inside by lactic acid because the arolium looked dried and never inflated again. To better understand the local action of lactic acid on the arolia and the fluid, future investigations should focus on a cryo-SEM and mass spectrometry analysis.
To challenge another delivery route that could be associated with a systemic mode of action, we chose to test the worst scenario for mites with the concentration of lactic acid measured in honey (1.5 mg/mL)31 and thus eaten by bees after in hive treatment was applied (150 mg/mL)16. We artificially fed V. destructor with lactic acid contaminated haemolymph (1.5 mg/mL) and mites did not die during the first forty-eight hours. The absence of mite susceptibility to lactic acid is even more remarkable considering that due to dilution effects, the concentration of lactate we measured in the haemolymph of the bees was extremely lower (0.017 mg/mL) than the concentration fed to the bees38. In addition, when lactic acid was topically administered on the back of bees, simulating the treatment in the hive, there were no significant differences in the median concentration of lactate measured in the haemolymph or digestive tract six hours post-treatment. It suggests that the risk of acidosis is very low but also that lactic acid if penetrating the cuticle seems regulated at very short term. These findings could be consistent with only a local mode of action rather than a systemic one or both21,22. However, honey bee haemolymph is not the only tissue that can be ingested by V. destructor as fat body is also a source of food for mites39. Further investigation about the concentration of lactate in worker fat body could bring complementary results. Furthermore, additional experiments should be conducted with a wider range of time points but also at the colony level and in different areas of the world to check the universality of this mechanism.
Finally, to understand how lactic acid treatment on infested honey bees reached V. destructor initially on the ventral side of bees, a behavioural study was conducted. We tested the valence of lactic acid treatment for the parasite and the host. We found that for the same concentration applied in hive (150 mg/mL), V. destructor seemed attracted and A. mellifera seemed repulsed as half of them avoided it and the other half tasted it only once. For mites, we tested a known repellent, octanoic acid as it is a component of the royal jelly40 and an attractant, butyric acid41 as it is part of the cuticle of L5 larvae. We discovered that lactic acid did not repulse like octanoic acid when given the choice. However, there were no differences in the proportion of mites which chose between butyric and lactic acid, thus a random choice was observed. Interestingly, for host-odour recognition of blood sucking animals like mosquitoes, lactic acid is known to attract when combined with other olfactory cues42. On the other hand, for some ticks when given the choice between distilled water and lactic acid, they did not choose lactic acid26,27 whereas in our study a majority of mites did. Even if human host odorant signatures are far from honey bees, these studies showed the significance of lactic acid across taxa, thus its valence could not be overlooked. A repulsion or attraction of mites towards lactic acid could indeed have a great influence on the efficacy of such treatment in colonies. Further investigations should check if V. destructor are able to discriminate between untreated and treated honey bees with lactic acid. The impact of lactic acid on the cuticle of bees, its blending with cuticular hydrocarbons and its kinetics of evaporation/absorption throughout time indeed remains unknown and could impact its valence for V. destructor. For honey bees, we measured their individual behaviour when one concentration of lactic acid was proposed. From 0.02 mg/mL that they can find in nectar of flowers30, to 1.5 mg/mL that they can taste in honey after a treatment31 and to 150 mg/mL when they are treated by spraying16. Note that we took flying honey bees without specifying if they were specialized in nectar or pollen collection43, thus further experiments could check the universality of these results among specialized foragers. The experiments were performed on the most odour sensitive workers in the colony to be able to conclude on the behavioural response of honey bees towards lactic acid. The same analyses conducted on less sensitive nurses could nevertheless confirm the general aversion of worker bees for lactic acid. For the field relevant concentration, their lack of attraction can be a strength for hive treatment as they will not eat large quantity and limit the risk of acidosis by ingestion. It can also represent a drawback as bees could fly away from the hive. However, it was not mentioned in previous field experiments16,17,44 but the study of sub-lethal effects of lactic acid on honey bees needs further investigations. From these results we can hypothesize that honey bees, once sprayed in-hive, do not really ingest pure lactic acid at 150 mg/mL as they do not seem to like it. On the contrary, they were observed doing a lot of allo- and auto-grooming, spilling lactic acid all over their bodies. Combined to the known attraction of V. destructor to lactic acid, this could partially explain the efficacy of this molecule in dislodging mites. It would be interesting to test this hypothesis and quantify auto- and allo-grooming during the experiment, which could potentially displace mites from their ventral position. Bees’ hygienic behaviour45 should definitely be investigated in the future to understand the precise role of honey bees in the contact-making between lactic acid and mites. In addition, to better characterise the valence of lactic acid for honey bees compared to known odours, a behavioural assay in a Y-maze would be important. Likewise, an experiment with a stained lactic acid conducted in hive would be the next step to understand the mechanism by which lactic acid reaches mites through bees as the olfactory environment in the hive is complex and we do not know the place of lactic acid among other odours for V. destructor.
To conclude, this study is the first to describe one of the ways of action of lactic acid treatment on V. destructor through A. mellifera exposure in the context of control strategies. Lactic acid treatment on infested honey bees works locally by damaging mites’ arolia. This contact could be mediated by honey bees’ and mites’ behaviours. Our results, even under artificial conditions, bring a deeper understanding on the way of action of lactic acid and can help the development of different methods for in-hive applications.
Methods
Mites and honey bees sampling
Varroa destructor females were collected with a soft paint-brush from brood cells and were taken to the laboratory following standard procedure46. They were stored in a Petri dish (5 cm diam) for two days to homogenize their state. In the Petri dish, they were gathered by ten on honey bee pupae to feed ad libitum in an incubator (34.5 °C, 70% Relative Humidity). Each experiment was conducted on three different colonies (infestation rate: 1%). Sample sizes for each experiment are detailed in Table 1.
Table 1.
Overview of the sample size for each experiment.
| Experiments | Conditions | N / hive | Total N |
|---|---|---|---|
| Arolia morphology | Treated with lactic acid | 10 mites from 10 honey bees | 30 mites from 30 honey bees |
| Control | 10 mites from 10 honey bees | 30 mites from 30 honey bees | |
| Artificial feeding | Treated with lactic acid | 10 mites | 30 mites |
| Control | 10 mites | 30 mites | |
|
Dosage HPIC digestive tract |
Treated with lactic acid | 5 honey bees | 15 honey bees |
| Control | 5 honey bees | 15 honey bees | |
| Dosage HPIC haemolymph | Treated with lactic acid | 10 honey bees | 30 honey bees |
| Control | 10 honey bees | 30 honey bees | |
| Y-Maze Varroas | Choice LA/OA | 18, 18 and 17 mites | 53 mites |
| Choice LA/BA | 18, 17 and 17 mites | 52 mites | |
| Choice LA/H2O | 18, 18 and 17 mites | 53 mites | |
| Olfactory gustatory assay honey bees | Control (sucrose) | 17 honey bees | 51 honey bees |
| 0.02 mg/mL of lactic acid | 10 honey bees | 30 honey bees | |
| 1.5 mg/mL of lactic acid | 10 honey bees | 30 honey bees | |
| 25 mg/mL of lactic acid | 10 honey bees | 30 honey bees | |
| 150 mg/mL of lactic acid | 10 honey bees | 30 honey bees |
Workers were collected in the morning while flying to leave their hives and kept for the experiment. They were stored according to standard procedure47. Briefly, workers were kept 2 h maximum in experimental cages (Pain48 type: 10.5 × 7.5 × 11.5 cm) in an incubator (28 °C, 60% RH) before the start of the experiment. They were fed ad libitum with a gravity feeder delivering sucrose 50% (w/v). Each experiment was led on three different colonies.
Acid preparation
Acids were purchased from Thermoscientific, USA. Dilutions of lactic acid (90%) (CAS no. 50-21-5), butyric acid (99%) (CAS no. 107-92-6) and octanoic acid (99%) (CAS no. 124-07-2) were made with demineralized water. All stock solutions were kept at 4° C. Final concentrations were 0.02, 1.5, 25 and 150 mg/mL for lactic acid according to Vilarem et al., 202318 and 1 mg/mL for butyric and octanoic acids according to Light et al., 202049. Note that 0.02 mg/mL was chosen according to the natural concentration in the nectar of some flowers30. In addition, 1.5 mg/mL was selected as it was measured after spray application of lactic acid in-hive31.
Microscopy imaging - arolia morphology of mites after treatment of honey bees
Acid administration - Two days prior to the experiment, mites were collected in brood cells and put on worker bees to simulate and standardize the dispersal phase in laboratory conditions. Hosts and parasites were kept in experimental cages (Pain type48) at 28 °C and 60–65% RH. Each bee carrying one or two mites received 5 µL of lactic acid at 150 mg/mL or demineralized water (control)18,50 on the back, from the thorax to the abdomen (5 × 1 µl drops). Honey bees were kept 5 min to let the solution dry on the back.
Set up – Once lactic acid or demineralized water were administered to infested honey bees, groups of 5 treated individuals were placed in pierced plastic glasses (7 cm diameter, 9.5 cm height) on Petri dishes (90 mm diameter) covert with Whatman paper51. Honey bees were fed ad libitum with sucrose 50% (w/v) and stored in an incubator (28 °C, 60% RH). The alive mites fallen during the first six hours post-treatment were collected and their arolia were imaged with an AMG EVOS FL Digital Inverted Microscope with transmitted light imaging to take pictures with magnification between x100 and x600. Six hours post-exposure, a CO2 treatment was applied on bees (from treated and control groups) to collect mites still onto their hosts and their arolia were also imaged. Ten V. destructor females were collected from three different hives (N = 30 mites/condition).
Artificial feeding experiment for mites
The collection of mites is described in the section “mites sampling”. Ten parasites were transferred into a Petri dish (35 mm diameter) with artificial feeding at 34.5 °C (70% RH) in an incubator. The artificial feeding set up was made according to Piou et al., (2023)52,53. Briefly, a full Parafilm™ covered Petri dish with spinning larva haemolymph encapsulated in an extra stretched Parafilm was created. Haemolymph was heated at 65 °C for 7 min. Blue dye 1% (Brillant Blue FCF, Vahiné, France) and lactic acid with a final concentration of 1.5 mg/mL were added to the food or demineralized water for control group (a volume of 6% was added to the haemolymph). Feeding status and survival were checked according to the colour of the mite (blue or not) every 24 h for 48 h. For survival, mites were considered dead once their internal body fluids were not moving anymore and they did not move or vibrate when touch by the paint brush. Ten parasites were collected from each of the three different hives (N = 30 mites/condition).
Treatment and honey bee organ dosage of lactate with HPIC (high pressure ion chromatography)
Acid administration – Worker bees received 5 µL of lactic acid at 150 mg/mL or demineralized water (control)18,50 on the back, from the thorax to the abdomen (5 × 1 µl drops). Honey bees were kept 5 min to let the solution dry on the back. Each bee was stored alone in a Petri dish with sucrose 50% (w/v) ad libitum for six hours in an incubator (28 °C, 60% RH).
Dissection and purification – Honey bees were cooled at 4 °C for 30 min and dissected on ice. Haemolymph from ten bees was collected through the antenna and pooled in a tube. The digestive tract (including midgut and hindgut) was pulled out and each collection tube kept on ice received five organs in addition to 1 mL of ultrapure water. Tissue disruption was led with a TissueLyser II (Qiagen, Germany) and three iron beads (3 mm) for 1 min at 30 Hz. A filtration step (0.2 μm) was followed, and each tube was stored at -20 °C until further analysis.
HPIC analysis – Each sample was unfrozen, centrifugated and filtered (0.22 μm PTFE syringe filter) prior to analysis of lactate concentrations by high-pressure ion chromatography with AS11-HC-4 μm column, solvent: KOH, gradient from 1mM to 44 mM (Dionex Ics-5000+, Thermo Fisher Scientific Inc., Waltham, MA, USA) following standard procedures with conductometric cell and UV absorption at 194 nm.
Y-maze experiment for V. destructor
Acid administration – 10 µL of diluted lactic (150 mg/mL), butyric, octanoic acids or demineralized water were deposited in the middle of a filter paper (Whatman, 30 mm diameter) inside a Petri dish. A two-minute delay with open lids plus a five-minute delay with sealed lids were observed before the start of the choice experiment.
Set up – The Y-maze adapted to the mite size is composed of a PCR tube (0.2 mL, VWR), a Y connector (Amzlab GmbH, size of each branch inner dimensions: 1.5 cm long and 0.3 cm diameter, at 120° from each other) and two Petri dishes (35 mm diameter) coated with a filter paper impregnated with acids or demineralized water. Varroa destructor females were collected from six different hives and randomized (N = 50/choice). The collection of mites is described in the section “mites sampling”. At the start of the experiment, five parasites were inserted in the PCR tube connected to the Y-maze and were able to walk freely towards their preferred odour. Y-mazes were kept in an incubator during the whole experiment (dark, 34.5 °C, 70% RH). The choice of mites was checked 0.5, 1 and 18 h post insertion in the Y-maze. Odour side was randomized across replicates. Bias of lateralization was checked, and none was found (Figure S3).
Olfactory and gustatory behavioural assay for honey bees
Workers were collected from three different hives according to the “honey bee sampling” section. Fifteen workers were collected and transferred in an experimental cage with sucrose 50% ad libitum. Syringes of 10 mL (Fisherbrand, England) were pierced with 6 holes on the sides for breathing. In each syringe one bee was stored one hour for habituation and starvation into the incubator at 28.5 °C and 60% RH. The end of the syringe allowed the insertion of a 10 µL cut tip with 5 µL loaded of diluted lactic acid with demineralized water (0.02, 1.5, 25 or 150 mg/mL) or sucrose 50%. When the bee was the furthest at the end of the syringe and her back turned away, the tip was inserted. The behaviour of the bee was classified as follows: “consumption” (the bee eats), “Several EP” (Extension of the Proboscis: the bee tastes with an extension of her proboscis, leaves, tastes again), “1 EP” (the bee tastes once), “Neutral” (the bee walks back and forth into the syringe with no extension of her proboscis), “Avoidance” (the bee stays the furthest from the tip). The observations were conducted for one minute. Then the tip was replaced by one filled with sucrose to check the ability of the bee to still extend her proboscis (avoid mechanic or fatigue bias). Only “able” bees were kept for the analysis of the experiment (N = 30 honey bees/concentration).
Statistical analyses
The statistical analysis of data was carried out on R (version 4.0.5) and RStudio (version 1.3.1093) with dplyr and FSA packages. Ggplot2 package was used for data visualization. For nutrition assay, results were presented as proportions with 95% confidence intervals and a Binomial GLM (Generalized linear model) was used to examine the correlation between different parameters: hives, time. Survival probability was analysed over two days through a Kaplan-Meier method with Survival and Survminer packages54,55. In addition, median concentrations of lactate measured in the haemolymph and the digestive tract of bees were compared between treated and control groups using a Wilcoxon rank test for unpaired data. Results for the Y-maze experiments are presented as proportions with confidence intervals of 95%. Conformity Chi-square tests were conducted with two choices. In addition, results for behavioural assays are presented as proportions with confidence intervals of 95%. A Fisher exact test for binary variables with a Bonferroni correction to further analyse the pairwise comparison between conditions and with the control group was conducted (packages car and RVAideMemoire56).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank B. Hemart and A. Rivel for their help in the behavioural assays. We would like to thank our funders M2i Biocontrol and the Région Occitanie (ADEME grant n°2082C0061). M2I Biocontrol did not influence the analysis and publication.
Author contributions
Experiments and analysis: C.V., except for: Nutrition: C.V., V.P; Y-maze: C.V., S.B; HPIC: F.J.; Conception: C.V., S.B., V.P.; Writing—original draft preparation, C.V.; writing—review and editing, C.V., S.B, F.J., A.V., and V.P.; supervision, A.V and V.P.; funding acquisition, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
M2i Biocontrol and the Région Occitanie (ADEME grant n°2082C0061).
Data availability
Datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Experiments and analysis compliance with ethical standards
Ethical standards were respected.
Patents
Results from this article may be part of a patent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Angélique Vétillard and Vincent Piou contributed equally to this work.
Contributor Information
Angélique Vétillard, Email: angelique.vetillard@lecnam.net.
Vincent Piou, Email: vincent.piou@univ-tlse3.fr.
References
- 1.Mitton, G. et al. More than sixty years living with Varroa destructor: a review of acaricide resistance. Int. J. Pest Manage.1–1810.1080/09670874.2022.2094489 (2022).
- 2.Colin, T., Monchanin, C., Lihoreau, M. & Barron, A. Pesticide dosing must be guided by ecological principles. Nat. Ecol. Evol.10.1038/s41559-020-01302-1 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Monchanin, C. et al. Chronic exposure to trace lead impairs honey bee learning. Ecotoxicol. Environ. Saf.212, 112008 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Goulson, D., Nicholls, E., Botias, C. & Rotheray, E. L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 347, 1255957–1255957 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Le Conte, Y. & Navajas, M. Climate change: impact on honey bee populations and diseases. Rev. Sci. Tech.27, 485–497 (2008). [PubMed] [Google Scholar]
- 6.Cappa, F., Cini, A., Bortolotti, L., Poidatz, J. & Cervo, R. Hornets and honey bees: a coevolutionary arms race between ancient adaptations and New Invasive threats. Insects. 12, 1037 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dainat, B., Evans, J., Chen, Y., Gauthier, L. & Neumann, P. Dead or alive: deformed Wing Virus and Varroa destructor reduce the life span of Winter Honeybees. Appl. Environ. Microbiol.78, 981–987 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilarem, C., Piou, V., Vogelweith, F. & Vétillard, A. Varroa destructor from the Laboratory tfield Fcontrolontrol, Biocontrol and IPM PerspectireviewReview. Insects. 12, 800 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elzen, P., Westervelt, D. & Lucas, R. Formic acid treatment for control of Varroa destructor (Mesostigmata: Varroidae) and safety to Apis mellifera (Hymenoptera: Apidae) under Southern United States conditions. Ec. 97, 1509–1512 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Eguaras, M., Del Hoyo, M., Palacio, M., Ruffinengo, S. & Bedascarrasbure, E. A New Product with Formic Acid for Varroa jacobsoni Oud. Control in Argentina. I. Efficacy: Efficacy of New Product for V. jacobsoni Control. J. Veterinary Med. Ser. B. 48, 11–14 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Maggi, M. et al. A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie. 47, 596–605 (2016). [Google Scholar]
- 12.Genath, A., Sharbati, S., Buer, B., Nauen, R. & Einspanier, R. Comparative transcriptomics indicates endogenous differences in detoxification capacity after formic acid treatment between honey bees and varroa mites. Sci. Rep.10, 21943 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genath, A., Petruschke, H., von Bergen, M. & Einspanier, R. Influence of formic acid treatment on the proteome of the ectoparasite Varroa destructor. PLoS ONE. 16, e0258845 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papežíková, I. et al. Effect of oxalic acid on the mite Varroa destructor and its host the honey bee Apis mellifera. J. Apic. Res.56, 400–408 (2017). [Google Scholar]
- 15.Wolff, J. O. & Gorb, S. N. Attachment Structures and Adhesive Secretions in Arachnids Vol. 7 (Springer International Publishing, 2016).
- 16.Kraus, B. & Berg, S. Effect of a lactic acid treatment during winter in temperate climate upon Varroa jacobsoni Oud. and the bee (Apis mellifera L.) colony. Exp. Appl. Acarol. 18, 459–468 (1994). [Google Scholar]
- 17.Schultermandl, F. & Imdorf, A. Milchsäure-Aerosol Zur Bekämpfung Von Varroa destructor. Schweizerisches Zentrum für Bienenforschung (2002).
- 18.Vilarem, C., Piou, V., Blanchard, S., Vogelweith, F. & Vétillard, A. Lose your grip: challenging Varroa destructor host attachment with Tartaric, Lactic, Formic, and citric acids. Appl. Sci.13, 9085 (2023). [Google Scholar]
- 19.Vilarem, C. et al. Lactic acid impairs Varroa destructor grip skill: fitness costs and effects on behaviour under artificial conditions. Entomol. Generalis. 10.1127/entomologia/2023/1975 (2023). [Google Scholar]
- 20.Katzung, B., Masters, S. & Trevor, A. Basic & Clinical Pharmacology (McGraw-Hill Medical; McGraw-Hill, 2012).
- 21.Ziegelmann, B. et al. Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action. Sci. Rep.8, 683 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolics, E., Mátyás, K., Taller, J., Specziár, A. & Kolics, B. Contact effect contribution to the high efficiency of lithium chloride against the mite parasite of the honey bee. Insects. 11, 333 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rein, C., Blumenschein, M., Traynor, K. & Rosenkranz, P. Lithium chloride treatments in free flying honey bee colonies: efficacy, brood survival, and within-colony distribution. Parasitol. Res.123, 67 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rimal, S. et al. Mechanism of acetic acid gustatory repulsion in Drosophila. Cell. Rep.26, 1432–1442e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanley, M., Ghosh, B., Weiss, Z., Christiaanse, J. & Gordon, M. Mechanisms of lactic acid gustatory attraction in Drosophila. Curr. Biol.31, 3525–3537e6 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Carr, A. et al. Responses of Amblyomma americanum and Dermacentor variabilis to odorants that attract haematophagous insects. Med. Vet. Entomol.27, 86–95 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Faraone, N., Light, M., Scott, C., MacPherson, S. & Hillier, N. Chemosensory and behavioural responses of Ixodes scapularis to natural products: role of chemosensory organs in volatile detection. Insects. 11, 502 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bortolotti, L. & Costa, C. Chemical Communication in the Honey Bee Society. in Neurobiology of Chemical Communication (CRC Press/Taylor & Francis, Boca Raton (FL), (2014). [PubMed] [Google Scholar]
- 29.Soroker, V., Singh, N., Eliash, N. & Plettner, E. Olfaction as a target for Control of Honeybee Parasite Mite Varroa destructor. in Olfactory Concepts of Insect Control - Alternative to Insecticides 117–134 (Springer International Publishing, Cham, doi:10.1007/978-3-030-05060-3_6. (2019). [Google Scholar]
- 30.Hölscher, D., Brand, S., Wenzler, M. & Schneider, B. NMR-based metabolic profiling of Anigozanthos floral nectar. J. Nat. Prod.71, 251–257 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Bogdanov, S., Kilchenmann, V., Fluri, P., Bühler, U. & Lavanchy, P. Influence des acides organiques et des composants d’huiles essentielles sur le goût du miel. Revue Suisse d’Apiculture. 95, 352–358 (1998). [Google Scholar]
- 32.Mato, I., Huidobro, J. F., Simal-Lozano, J. & Sancho, M. T. Analytical methods for the determination of organic acids in honey. Crit. Rev. Anal. Chem.36, 3–11 (2006). [Google Scholar]
- 33.Schlüns, H., Welling, H., Federici, J. & Lewejohann, L. The glass is not yet half empty: agitation but not Varroa treatment causes cognitive bias in honey bees. Anim. Cogn.20, 233–241 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Hasan, M. Z. et al. Transcriptional profiling of lactic acid treated reconstructed human epidermis reveals pathways underlying stinging and itch. Toxicol. In Vitro. 57, 164–173 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Issachar, N., Gall, Y., Borfll, M. & Poelman, M. pH measurements during lactic acid stinging test in normal and sensitive skin. Contact Dermat.36, 152–155 (1997). [DOI] [PubMed] [Google Scholar]
- 36.Baker, G. T. The pulvillus: cuticular structure and function (Acarina: Ixodida). J. Acarol Soc. Jpn. 6, 25–31 (1997). [Google Scholar]
- 37.Dirks, J. & Federle, W. Mechanisms of fluid production in smooth adhesive pads of insects. J. R Soc. Interface. 8, 952–960 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strachecka, A. et al. Comparison of lactate dehydrogenase activity in hive and forager honeybees may indicate delayed onset muscle soreness – preliminary studies. Biochem. Mosc.84, 435–440 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Ramsey, S. D. et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA. 116, 1792–1801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nazzi, F. et al. Octanoic acid confers to royal jelly varroa-repellent properties. Naturwissenschaften. 96, 309–314 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Teal, P., Duehl, A. & Carroll, M. Methods for attracting honey bee parasitic mites.
- 42.Dormont, L., Mulatier, M., Carrasco, D. & Cohuet, A. Mosquito attractants. J. Chem. Ecol.47, 351–393 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Moreno, E., José Corriale, M. & Arenas, A. Differences in olfactory sensitivity and odor detection correlate with foraging task specialization in honeybees Apis mellifera. J. Insect. Physiol.141, 104416 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Imdorf, A. Varroabekämpfung Mit Milchsäiur. Sehweizerische Bienen-Zeitung. 112, 449–452 (1989). [Google Scholar]
- 45.Pritchard, D. J. Grooming by honey bees as a component of varroa resistant behavior. J. Apic. Res.55, 38–48 (2016). [Google Scholar]
- 46.Dietemann, V. et al. Standard methods for varroa research. J. Apic. Res.52, 1–54 (2013). [Google Scholar]
- 47.Williams, G. et al. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res.52, 1–36 (2013). [Google Scholar]
- 48.PainJ. Note technique nouveau modèle de cagettes expérimentales pour le maintien d’abeilles en captivité. Les Ann. De L’abeille. hal-00890230 (1966).
- 49.Light, M., Shutler, D., Cutler, G. & Hillier, N. Electrotarsogram responses to synthetic odorants by Varroa destructor, a primary parasite of western honey bees (Apis mellifera). Exp. Appl. Acarol. 81, 515–530 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Gashout, H. & Guzmán-Novoa, E. Acute toxicity of essential oils and other natural compounds to the parasitic mite, Varroa destructor, and to larval and adult worker honey bees (Apis mellifera L). J. Apic. Res.48, 263–269 (2009). [Google Scholar]
- 51.Evans, J., Chen, Y., di Prisco, G., Pettis, J. & Williams, V. Bee cups:single-use cages for honey bee experiments. J. Api Res. 300–302. 10.3896/IBRA.1.48.4.13 (2009).
- 52.Piou, V. et al. Honey bee larval hemolymph as a source of key nutrients and proteins offers a promising medium for varroa destructor artificial rearing. IJMS. 24, 12443 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piou, V. et al. Varroa destructor relies on physical cues to feed in artificial conditions. Parasite. 30, 49 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alboukadel, K., Marcin, K., Przemyslaw, B. & Scheipl, F. survminer: Drawing Survival Curves using ‘ggplot2’. (2022).
- 55.Therneau, T. survival: Survival Analysis. (2023).
- 56.Hervé, M. RVAideMemoire Testing and Plotting Procedures for Biostatistics. (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated and analysed during the current study are available from the corresponding author on reasonable request.