Abstract
The occurrence characteristics of arsenic in matte phase are unclear, which leads to the current treatment technology not being able to remove arsenic from matte phase significantly, thus causing a large amount of arsenic to affect smelting links such as copper converting and electrolytic refining. This paper uses instrumental analysis such as XRD, SEM-EDS, MLA and chemical analysis methods such as chemical phase extraction to comprehensively analyze the occurrence characteristics of arsenic in matte phase. The results show that the occurrence states of arsenic in matte are mainly arsenic sulfide, arsenic oxide, arsenate and residual arsenic. Most of the arsenic is in residual state in matte; arsenic in matte is extremely stable and difficult to exchange with the outside world; in high-grade matte, the arsenic content is relatively high. The main occurrence state of arsenic is copper-based complex alloy compounds, in which the arsenic content is about 1-10%. Since the metal bond between Cu-As is extremely stable, it is difficult to separate arsenic from it, which is also the key to the difficulty in completely removing arsenic from matte.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-76382-1.
Keywords: Copper matte, Arsenic, Occurrence characteristics, Cu-As alloy
Subject terms: Environmental impact, Pollution remediation
Introduction
Over 85% of global copper is generated through pyrometallurgical methods. The primary techniques in copper pyrometallurgy are bath smelting and flash smelting1. Bath smelting demonstrates greater adaptability than flash smelting when handling raw materials, allowing the processing of a wide range of copper-containing smelting inputs2. Given this advantage and the backdrop of ongoing depletion of high-quality copper mines worldwide, this smelting technique has gained widespread application in recent years3. Because the bath smelting process has a strong tolerance for the raw materials entering the furnace, a large number of low-grade, high-arsenic copper concentrates4, such as arsenic copper sulfide (Cu3AsS4) and arsenic tetrahedrite (Cu12As4S13), are used in the bath smelting process5. In addition, some researchers have used the bath smelting process to dispose of high-arsenic copper smelting by-products, such as arsenic-containing gypsum slag6and high-arsenic soot produced by waste heat boilers7. The aforementioned factors lead to elevated arsenic risks in the bath smelting process compared to alternative smelting techniques.
Copper pyrometallurgy involves four primary processes: smelting, converting, refining, and electrolysis. The smelting stage stands out as the most critical segment of bath smelting. It plays a key role in the movement and transformation of elemental arsenic throughout the smelting procedure8. During the smelting operation, arsenic emanates from copper smelting feedstocks under elevated temperatures and oxygen-rich environments. The arsenic partitions into the slag, gas, and copper matte phases based on its various forms and states9. During the smelting process, arsenic is released from copper smelting raw materials under high temperatures (1100–1300 °C) and oxygen-rich conditions (40–70%). The arsenic is distributed into the slag phase (10–20%), gas phase (50–70%), and copper matte phase (1–10%) according to different forms and phases10, so the occurrence of arsenic in these two phases has received a lot of attention, there have been many research reports11,12. Relatively, the proportion of arsenic distributed in the copper matte phase is the smallest, often less than 10%13, copper matte is an intermediate product of copper smelting and has received less attention. However, copper matte is used as a raw material for subsequent copper smelting processes8, arsenic in copper matte will be carried into the subsequent copper smelting process, affecting a series of subsequent processes such as converting, refining, and electrolysis14. During the converting and refining process, some arsenic will enter the slag phase and smoke, causing continuous pollution to the environment10. Some arsenic will penetrate into the refractory bricks in the lining of the smelting furnace during the converting and refining process, destroying the refractory brick structure and reducing the service life of the furnace15. Arsenic that enters the electrolytic refining stage will have more impacts. Due to the high solubility of arsenic in copper electrolyte16,17, Arsenic in different valence states forms arsenate in the electrolyte18, which will form anode mud with other impurities in the electrolyte19, the anode mud in the electrolyte will not only exist in the electrolyte20, but also adhere to the cathode plate, which will not only cause the electrolyte circulation pipeline to be blocked, affecting production21,22, but also affect the appearance quality and chemical purity of the cathode copper23,24. Thus, finding ways to lower arsenic levels in the matte phase and minimize its effects during the smelting process is vital for the clean production of copper pyrometallurgy.
During the converting process, the iron in the matte phase enters the slag phase, while the copper is converted into crude copper and enters the next smelting stage25. In this process, only a part of the arsenic in the matte phase will be transferred to the converting slag and converting dust, and more than 60% of the arsenic enters the crude copper phase and enters the subsequent smelting process with the crude copper phase26. Although there are many studies on the separation of copper and arsenic in the crude copper phase in the subsequent refining process, such as alkali spraying slag making, the arsenic in the crude copper is removed and discharged into the slag phase in the form of arsenate, but due to the tight combination of copper and arsenic, therefore the copper-arsenic separation effect is poor, and some arsenic still enters the subsequent electrolysis stage27,28. A primary reason for this outcome stems from the lack of a thorough analysis of arsenic in the matte phase. Additionally, there is no extensive examination of the occurrence characteristics of arsenic within matte. This deficiency hinders the ability to address arsenic at the initial processing stage. Consequently, conducting mineralogical analyses of the matte phase, along with studying the occurrence characteristics of arsenic in matte, is crucial for effective source control of arsenic during copper smelting.
This study presents a thorough process mineralogy analysis of four copper matte samples with varying grades, utilizing techniques such as ICP, XRD, VSM, and MLA. It investigates the elemental and phase composition along with structural variations among the copper matte samples. Furthermore, it evaluates the thermal stability of these samples using roasting experiments. Additionally, it examines the arsenic content in different copper matte samples through SEM-EDS, MLA, and a stepwise chemical extraction process, analyzing the variations in arsenic occurrence across copper mattes of diverse grades. This work establishes a foundational understanding for controlling arsenic sources in the copper pyrometallurgical smelting process.
Materials and methods
Materials
Four copper matte used in the experiment were obtained from a copper smelting plant in Shandong, China. Block-shaped copper matte samples were uniformly sampled and processed to obtain copper matte powder samples with a particle size less than 74 μm, which were used for various analytical tests. Additionally, block-shaped copper matte samples were directly used for sectioning, grinding, and polishing to create 4 cm x 4 cm sections for scanning electron microscope (SEM) testing.
Methods
Mineralogical analysis
We use X-ray Diffraction (XRD) instrument to analyze the main phase composition of solid samples. The testing conditions included a copper target, tube current of 40 mA, tube voltage of 40 kV, scanning speed of 10° per minute, and a scanning range from 10° to 80°. For detecting different elements and their respective phase compositions and contents in the solid samples, we employed an Electron Microprobe Analyzer (MLA). The MLA instrument used in this experiment is the FEI MLA 650 model, manufactured by Thermo Fisher Scientific. This instrument was complemented by the FEI Quanta 650 scanning electron microscope produced by the same company, the Dual XFlash 5010 dual-probe energy dispersive spectrometer manufactured by Bruker in Germany, and the MLA3.1 analysis software from Thermo Fisher Scientific. The scanning electron microscope operated with a working distance of 10 mm and a voltage of 40 kV. It’s worth noting that all tested samples underwent resin embedding for sample preparation. After embedding, the resin samples were continuously polished on different specifications of filter paper using a metallographic grinding machine. Subsequently, they were polished on a polishing cloth until there were no visible scratches on the surface. To improve the imaging quality of the samples, gold coating was applied to the resin samples.Additionally, a Zeiss Sigma500 scanning electron microscope (SEM) was used to analyze the microstructure of the sample surfaces. An energy dispersive spectrometer (EDS) integrated with the SEM was used to analyze the elemental composition of the samples. To determine the magnetic strength and variations in iron phases within the samples, a Vibrating Sample Magnetometer (VSM) was employed. The VSM analysis was conducted at room temperature with a testing range from − 20,000 Oe to 20,000 Oe.
We used Inductively Couples Plasma-Atomic Emission Spectrometry (ICP-AES) to quantitatively analyze the main elements in copper matte.
Phase and morphological analysis analysis
Using the method of sequential extraction of element chemical phases [137], the analysis of the arsenic phase composition in the copper matte sample is carried out with the following specific steps:
Weigh 1.0000 g of powdered sample and place it in a conical flask, then add 100mL of deionized water. Heat the conical flask on a hot plate to a gentle boil and maintain it for 90 min. After cooling, filter the sample, and the arsenic dissolved in the filtrate is in the oxidized state.
Add 100 ml of 20% sulfuric acid to the residue obtained in the previous step, shake for 30 min, and then filter. The arsenic in the filtrate obtained in this step is in the form of arsenate.
Add 100 ml of 50 g/L sodium hydroxide to the residue obtained in the previous step, shake for 30 min, and then filter. The arsenic in the filtrate obtained in this step is in the sulfide state.
Add 100 ml of aqua regia to the residue obtained in the previous step. After the residue has completely dissolved in aqua regia, make up to volume and filter. The arsenic in the filtrate obtained in this step is in the residual (alloy) state.
To investigate the effective binding forms of arsenic in copper matte, the BCR sequential extraction method is used to analyze the arsenic species in copper matte.
Results and discussion
Mineralogical analysis of copper matte
Elemental composition analysis
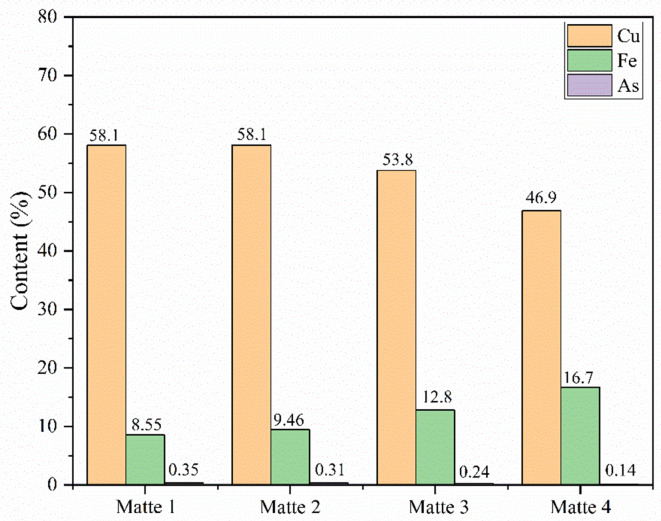
Table 1 presents the elemental composition of four copper matte samples. From the table, it is evident that the most crucial components in copper matte are Cu, Fe, and S, collectively constituting over 85% of the total elemental content of copper matte. In addition to these three elements, copper matte also contains trace amounts of heavy metal elements such as Pb, Zn, As, and Sb, along with minute quantities of noble metal elements such as Ag and Au. This is attributed to the tendency of noble metals and heavy metal elements in the copper concentrate to associate with sulfur during the smelting process, subsequently being captured by copper matte. These impurity elements are gradually removed during subsequent blister refining and electrolysis processes, while valuable metals like gold and silver are enriched and recovered in the subsequent smelting stages.
Table 1.
Composition of main elements in copper matte samples (wt%).
| Element | Sample1 | Sample 2 | Sample 3 | Sample 4 | |
|---|---|---|---|---|---|
| Main element | Cu | 58.1 | 58.1 | 53.8 | 46.9 |
| Fe | 8.55 | 9.46 | 12.8 | 16.7 | |
| S | 21.3 | 21.7 | 22.2 | 21 | |
| Impurity element | Pb | 2.38 | 2.62 | 3.33 | 1.59 |
| Zn | 1.27 | 0.62 | 1.4 | 1.6 | |
| Sb | 0.28 | 0.32 | 0.49 | 0.28 | |
| As | 0.35 | 0.31 | 0.24 | 0.14 | |
| Co | 0.037 | 0.021 | 0.021 | 0.037 | |
| K | 0.025 | 0.02 | 0.019 | 0.077 | |
| Mn | 0.017 | 0.022 | 0.016 | 0.074 | |
| Se | 0.017 | 0.029 | 0.033 | 0.016 | |
| Si | 0.018 | 0.019 | 0.02 | 0.012 | |
| Sn | 0.015 | 0.018 | 0.041 | 0.01 | |
| Ag | 0.086 | 0.1 | 0.025 | 0.08 | |
Figure 1 compares the content of As element in the four copper matte samples with the Cu and Fe content in copper matte. From the figure, it is evident that the copper content in sample 1 to sample 4 is gradually decreasing, while the iron content is increasing. This indicates a continuous decrease in the copper grade from sample 1 to sample 4. As the copper grade decreases, the arsenic content in copper matte also decreases, demonstrating a strong influence of copper grade on the occurrence of arsenic in copper matte.
Fig. 1.
Contents of Cu, Fe and As in copper matte samples.
Phase composition analysis
To ascertain the phase compositions of the four copper matte samples, X-ray diffraction (XRD) analysis was conducted on the copper matte, and the results are depicted in Fig. 2.
Fig. 2.
XRD patterns of copper matte samples.
From the figure, it is evident that the primary phases in the four copper matte samples are all chalcopyrite (Cu5FeS4). In addition, there is a minor presence of lead sulfide (PbS) in the copper matte. This can be attributed to the principles of similar solubility, where copper matte itself, as a eutectic mixture of metal sulfides, exhibits a certain degree of solubility for lead sulfide and other metal sulfides. Although the phase compositions of the four copper matte samples are relatively similar, there are some distinctions. In sample 1, besides the Cu5FeS4 phase, there is a minor amount of Cu2S phase. As the copper grade decreases, the Cu2S phase in the copper matte samples gradually diminishes. When the copper grade drops to 46.9%, there is virtually no Cu2S phase in sample 4. The solidified phase in the copper matte is primarily composed of Cu5FeS4. When the copper content in copper matte is below its stoichiometric ratio, excess FeS solid solution is generated. Conversely, when the copper grade in copper matte is too high, excess Cu2S is separated. No arsenic-containing phases were detected in the XRD spectra of the copper matte. There are two potential explanations for this: either the arsenic content is too low to be detected, or arsenic-containing phases are enveloped by other phases, rendering them unidentifiable.
Micro structure analysis
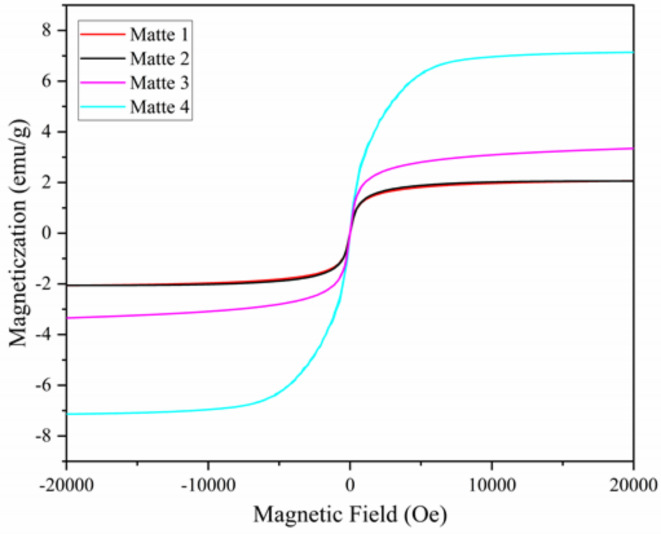
To examine the differences in magnetic intensity among copper matte samples, magnetic hysteresis loop analysis was conducted using a vibrating sample magnetometer, and the results are presented in Fig. 3.
Fig. 3.
Hysteresis loop diagram of copper matte samples.
From the graph, it can be observed that all four copper matte samples exhibit certain magnetism, and there is considerable variation in magnetic performance among the samples, displaying evident regularities.Numerically, at a magnetic field intensity of 20,000 Oe, the magnetization intensity difference between sample 1 and sample 2 is relatively small, both around 2 emu/g. sample 3, under a magnetic field intensity of 20,000 Oe, achieves a magnetization intensity of approximately 3.3 emu/g, while sample 4 reaches around 7.1 emu/g. This trend corresponds to the trend in the copper matte grade, indicating that lower copper matte grades exhibit stronger magnetic properties.The reason copper matte exhibits magnetism is due to the introduction of a large amount of oxygen through an oxygen lance during the smelting process. While the majority of oxygen molecules enter the smelting slag and dust, about 2 wt% to 4 wt% of oxygen dissolves into the copper matte phase. Literature suggests29that the dissolved oxygen content in copper matte mainly depends on the FeS content in the copper matte. Due to copper’s affinity for sulfur, oxygen hardly dissolves in the Cu2S phase, while FeS can dissolve up to approximately 10% oxygen. These oxygen molecules dissolved in the copper matte combine with iron sulfide to form ferric oxide (Fe3O4), resulting in the copper matte exhibiting weak magnetism. Therefore, the gradual increase in magnetic properties from sample 1 to sample 4 may be attributed to the increasing Fe3O4 content in the copper matte samples. This also indirectly indicates that as the iron content in copper matte increases, the oxygen content in copper matte also increases. This is one of the reasons for the low arsenic content in low-grade copper matte.
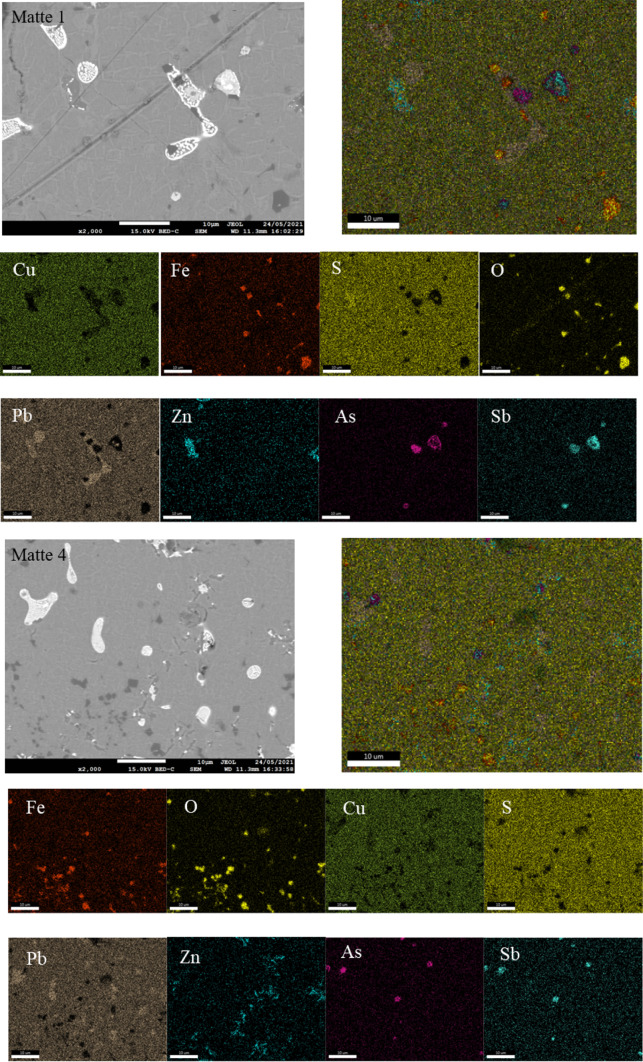
To investigate the microstructure and elemental distribution characteristics of copper matte samples, SEM-EDS analysis was conducted on sample 1, which has the highest arsenic content, and sample 4, which has the lowest arsenic content among the four copper matte samples. The microscopic morphology and elemental distribution characteristics are shown in Fig. 4.
Fig. 4.
Element distributions in matte 1 and matte 4.
From the SEM-EDS image of sample 1, it can be observed that the morphology of copper matte samples is smooth and dense. Based on the brightness of the samples, copper matte samples can be divided into three regions: a light gray region at the base, a dark gray region locally, and a bright region. This image was captured in the backscattered electron mode of the scanning electron microscope, where elements with higher atomic mass appear brighter. Therefore, the darkest region in the sample is the iron compound zone, the substrate phase is the copper compound zone, and the brightest region may be the area where lead, zinc, and arsenic are distributed. According to the elemental content analysis and spectrum analysis of copper matte samples mentioned earlier, it can be inferred that the darkest region in the sample is mainly composed of iron and oxygen elements. The areas of enrichment of these two elements highly overlap, and combined with the previous magnetic hysteresis loop analysis, this region corresponds to the magnetite (Fe3O4) phase. The substrate region is centrally colored, has the largest area, and contains the highest amounts of copper, iron, and sulfur, suggesting it is the chalcopyrite phase with the highest content in copper matte. The bright region exhibits some variations, with an area where lead overlaps with sulfur, representing the galena phase, and an area where zinc highly overlaps with sulfur, representing the sphalerite phase. It can be seen that lead and zinc in copper matte are mainly combined with sulfur to form metal sulfides. Arsenic and antimony, on the other hand, do not form relevant compounds with sulfur or oxygen elements. This is because arsenic and antimony sulfides and oxides are easily converted to gaseous phases at the high temperatures of smelting. As the smelting fumes volatilize, it is evident that arsenic and antimony, aside from being scattered uniformly throughout the copper matte sample, are highly overlapping in areas of enrichment. According to literature30, arsenic and antimony, due to their similar physicochemical properties, have unlimited mutual solubility characteristics and readily form arsenic-antimony alloys in high-temperature melts.
The overall morphological features and elemental distribution of sample 4 are very similar to those of sample 1, both consisting of a chalcopyrite base and dissolved phases of lead sulfide, zinc sulfide, magnetite, and arsenic-antimony alloy. The differences between sample 4 and sample 1 lie in the significantly greater presence of dissolved iron oxides in the structure of sample 4, and the noticeably lower content of arsenic-antimony alloy compared to sample 1. Combining the elemental composition analysis of copper matte samples mentioned earlier, sample 4 contains a higher content of iron, which is the primary carrier of oxygen in copper matte. The increased presence of iron leads to the dissolution of more oxygen in sample 4, forming magnetite. This is the reason why the arsenic content in sample 4 is lower than that in sample 1. As more oxygen is dissolved into sample 4, oxygen combines with arsenic in copper matte, resulting in the formation of gaseous arsenic oxide, leading to a reduction in the arsenic content in copper matte. This inference requires further confirmation in subsequent studies.
Occurrence characteristics of arsenic in matte
The morphological composition of arsenic in matte
The chemical forms of arsenic in four sets of copper matte samples were examined through a step-by-step extraction method, and the results are presented in Table 2.
Table 2.
Phase composition of arsenic in copper matte samples.
| Physical phase | Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|---|
| Oxidized arsenic | 1% | 0.59% | 0.7% | 2% |
| Sulfide arsenic | 2.25% | 0.59% | 2.7% | 4% |
| Arsenate | 22.5% | 25.3% | 27.3% | 27.3% |
| Residual arsenic | 75% | 73.5% | 69.2% | 66.7% |
| Total arsenic | 100% | 100% | 100% | 100% |
From Table 2, it can be observed that arsenic in copper matte mainly exists in the form of arsenate and residual arsenic. In addition, there are small amounts of oxidized arsenic and sulfide arsenic. Even after three rounds of extraction, around 70% of arsenic still remains in the residue. This indirectly indicates the considerable stability of arsenic in copper matte, making it resistant to separation and extraction.
In sample 1, the content of oxidized arsenic and sulfide arsenic is only 1% and 2.25%, respectively, while the arsenate content reaches 22.5%, with the remaining residual arsenic being as high as 75%. This is because oxidized and sulfide forms of arsenic tend to nucleate into the gas phase at the temperature of copper smelting, converting into gaseous arsenic that evaporates with the smelting flue gas. On the other hand, arsenate and residual arsenic exhibit strong stability at high temperatures, making them difficult to decompose during the copper smelting process and, therefore, remaining in the copper matte phase.
Furthermore, although the overall arsenic phase composition is similar from sample 1 to sample 4, there are proportional differences in the composition of arsenic phases between them. As the copper matte grade gradually decreases, the arsenate content in sample 1 to sample 4 gradually increases. According to the previous analysis of elemental content and hysteresis loop, this is due to the gradual increase in oxygen and iron content in sample 1 to sample 4. Arsenic in copper matte reacts with iron oxide to form arsenic iron oxide, which is difficult to decompose at the temperature of copper smelting. This leads to the increase in the arsenate phase in sample 1 to sample 4.
Moreover, the residual arsenic in sample 1 to sample 4 is gradually decreasing. This indicates that the higher the copper matte grade, the easier it is for arsenic to form residual arsenic in copper matte. According to literature31, metallic copper has a strong affinity for arsenic. In the smelting furnace, metallic copper combines with arsenic to produce copper arsenide, which is also a difficult-to-decompose arsenic compound. Therefore, as the copper grade in sample 1 to sample 4 gradually decreases, the theoretical copper content is also gradually decreasing. This results in the inability to provide enough metallic copper to react with arsenic, leading to a gradual reduction in the trend of residual arsenic.
The mineral phases composition of arsenic in matte
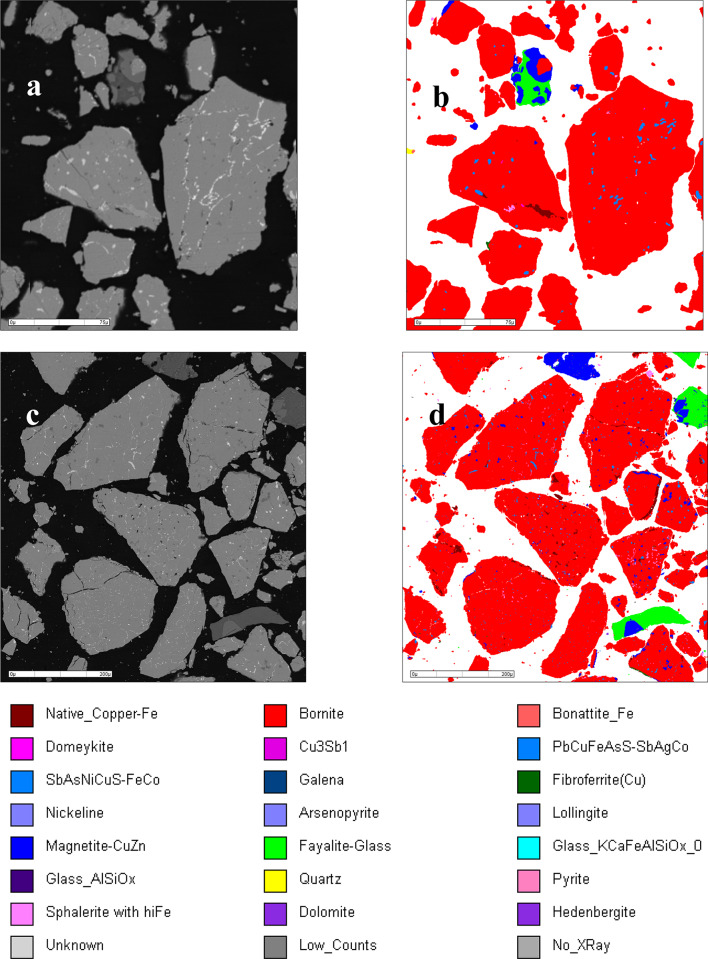
Utilizing the MLA automatic analysis and detection system, the mineral composition and distribution characteristics of arsenic in samples 1 and 4 were examined, and the results are shown in Fig. 5. Additionally, the distribution of arsenic in different mineral phases in copper matte was analyzed, and the results are presented in Table 3. From Fig. 5; Table 3, it can be observed that the mineral compositions of samples 1 and 4 are similar, with the main phase being chalcopyrite, consistent with the earlier XRD detection results. Both sets of copper matte samples also contain small amounts of glass phase and magnetite phase. The glass phase is caused by the admixture of a small amount of copper slag, while the magnetite phase, as per our previous hysteresis loop analysis, results from the combination of dissolved oxygen in copper matte with pyrrhotite in copper matte.
Fig. 5.
Mineral composition and arsenic distribution characteristics in sample 1 (a and b) and sample 4 (c and d).
Table 3.
Mineral composition and the arsenic content in mineral of sample 1 and sample 4.
| Mineral composition and content of sample 1 (wt%) | As content in mineral (wt%) | Mineral composition and content of sample 4 (wt%) | As content in minerals (wt%) | ||
|---|---|---|---|---|---|
| Bornite | 93.06 | 0 | Bornite | 81.71 | 0 |
| Cu-Pb-Fe-As-S allay | 4.89 | 31.02 | Fayalite-Glass | 6.29 | 56.1 |
| Magnetite | 5.11 | 0 | |||
| Native copper | 0.75 | 0 | Pb-Cu-Fe-S-Ag allay | 2.61 | 0 |
| Cu-Ni-Sb-As-S allay | 0.61 | 60.54 | |||
| Native copper | 2.19 | 0 | |||
| Magnetite | 0.39 | 0 | Cu-Ni-Sb-As-S allay | 0.69 | 27.59 |
| Sphalerite | 0.09 | 0 | |||
| Fibroferrite | 0.05 | 0 | Cu-Pb-Fe-As-S allay | 0.66 | 14.62 |
| Quartz | 0.03 | 0 | |||
| Glass | 0.03 | 0 | Sphalerite | 0.39 | 0 |
| Fayalite-Glass | 0.02 | 0.07 | Bonattite | 0.13 | 1.04 |
| Domeykite | 0.02 | 2.99 | Fibroferrite | 0.08 | 0 |
| Nickeline | 0.02 | 4.29 | Dyscrasite | 0.04 | 0 |
| Arsenopyrite | 0.01 | 0.33 | Native Iron | 0.03 | 0 |
| Lollingite | 0.01 | 0.34 | Glass | 0.01 | 0.05 |
| Arsenopyrite | 0.01 | 0.39 | |||
| Lollingite | 0.01 | 0.18 | |||
| Domeykite | 0.01 | 0.03 | |||
| Total | 99.98 | 99.58 | Total | 99.99 | 100 |
As analyzed earlier, the iron content in sample 4 is significantly higher than in sample 1. As seen from the graph, the content of magnetite phase and iron silicate glass phase in sample 4 is higher than in sample 1. Therefore, this excess iron is likely present in these two phases. Additionally, the interstitial gaps between the main phases in both sample sets contain many small alloy phases. These alloy phases have complex compositions, dissolving various precious metal elements such as As, Sb, Au, Ag, Pb, etc. Comparing the proportions of alloy phases in the two copper matte, it can be observed that the alloy phase in sample 1 is much higher than in sample 4, which may be the reason for the higher arsenic content in sample 1.
Table 3 lists the distribution ratios of arsenic in various mineral phases in copper matte. From the table, it can be seen that there is a certain difference in the distribution of arsenic in the two copper matte. In sample 1, arsenic is mainly distributed in copper-based complex alloys, with a relatively small proportion in other phases. In sample 4, although arsenic is also distributed in alloy phases, the content is lower, as sample 4 itself has fewer alloy phases. Most of the arsenic in sample 4 is distributed in the glass phase. According to literature32, the glass phase, due to its network structure, has a good solidifying effect on arsenic, causing it to remain in copper matte. However, this part of arsenic has little impact, as the glass phase in copper matte is easily combined with smelting slag, carrying the arsenic into the slag phase, without affecting subsequent copper smelting. Additionally, magnetite, as an iron oxide, may react with arsenic-containing metal sulfides and alloy phases, causing the dissolved arsenic to evaporate, resulting in a decrease in arsenic content in copper matte.
The morphological composition of arsenic in copper matte
In order to further investigate the chemical speciation of arsenic in copper matte, a BCR sequential extraction was performed on the four groups of copper matte, and the results are shown in Fig. 6. From the figure, it can be observed that the chemical forms of arsenic in Sample 1 are as follows: Residual arsenic (70.13%) > Oxidizable arsenic (19.36%) > Acid soluble arsenic (7.70%) > Reducible arsenic (2.81%). For Sample 2, the chemical forms of arsenic are: Residual arsenic (72.43%) > Oxidizable arsenic (17.23%) > Acid soluble arsenic (5.85%) > Reducible arsenic (4.49%). In Sample 3, the chemical forms of arsenic are: Residual arsenic (73.63%) > Oxidizable arsenic (10.64%) > Reducible arsenic (8.82%) > Acid soluble arsenic (6.9%). Lastly, in Sample 4, the chemical forms of arsenic are: Residual arsenic (73.36%) > Oxidizable arsenic (12.53%) > Acid soluble arsenic (7.22%) > Reducible arsenic (6.89%).
Fig. 6.
Chemical species analysis of arsenic in copper matte samples.
Analyzing the proportions of arsenic in different chemical forms in each copper matte sample, it can be concluded that over 70% of arsenic in copper matte exists in the residual form, indicating a strong chemical stability of arsenic in copper matte, making it difficult to exchange with the external environment. From an environmental perspective, the potential harm of arsenic in copper matte to the environment is low. Additionally, the trend of decreasing oxidizable arsenic and increasing reducible arsenic from Sample 1 to Sample 4 may be attributed to the gradual increase in iron content in copper matte with decreasing ore grade. This increase in iron leads to a gradual rise in dissolved oxygen content, causing the transformation of other forms of arsenic into oxidizable arsenic, ultimately resulting in an increasing trend of reducible arsenic content in low-grade copper matte.
The thermal stability of arsenic in copper matte
To study the thermal stability of arsenic in copper matte, the tube furnace was heated to the reaction temperature. Then, the copper matte samples were placed in the furnace under a protective argon gas atmosphere and roasted for 60 min. After roasting, the samples were quickly cooled, and the overall mass loss of the copper matte samples and the change in arsenic content in the high-temperature roasted products were measured. The results are shown in Fig. 7. As seen in Fig. 7-a, with the increase in roasting temperature, the overall mass loss of copper matte also increased. However, when the temperature reached 1200℃, the overall mass loss of copper matte did not exceed 1%, indicating that copper matte has a very strong stability and is not easily decomposed. Specifically, at 1200℃, Sample 1 had the smallest mass loss of only 0.58 g. As the grade of the copper matte decreased, the mass loss at this temperature showed a gradually increasing trend. For Sample 4 at this temperature, the mass loss reached 0.89 g, indicating that high-grade copper matte has stronger thermal stability compared to low-grade copper matte. This may be because the content of metallic copper is higher in high-grade copper matte. Metallic copper can react with other easily decomposable substances to form copper compounds that are stable at high temperatures, thereby reducing the mass loss of copper matte at high temperatures.
Fig. 7.
Thermal decomposition mass loss (a) and residual arsenic (b) of copper matte samples.
Figure 7-b shows the residual arsenic content in four groups of copper matte samples after roasting at different temperatures for 60 min. It can be seen that all four groups of copper matte samples exhibit a trend: when the roasting temperature is below 800℃, the arsenic content in copper matte decreases with the increase in roasting temperature; however, as the temperature continues to rise, the arsenic content in copper matte does not decrease but increases. In Sample 1, the mass ratio of arsenic in the original sample was 0.35 wt%. After roasting at 600℃ for an hour, the mass ratio of arsenic in the sample decreased to 0.23 wt%. At 800℃, this value sharply decreased to 0.024 wt%, indicating that over 90% of the arsenic in copper matte was lost at 800℃. However, as the temperature increased to 100℃, this trend did not continue but instead, the proportion of arsenic in copper matte slightly increased to 0.027 wt%. When the roasting temperature was further increased to 1200℃, the mass ratio of arsenic in Sample 1 after roasting increased again to 0.094 wt%. The other three groups of copper matte samples showed the same trend. This may be because, under low-temperature roasting conditions, some of the volatile arsenic phases in copper matte can evaporate at these temperatures. However, as the temperature rises, the arsenic in copper matte reacts with the metallic copper in copper matte to form a copper-arsenic alloy. This part of arsenic is difficult to evaporate at high temperatures, leading to an increase in the residual arsenic in copper matte.
The distribution characteristics of arsenic in copper matte
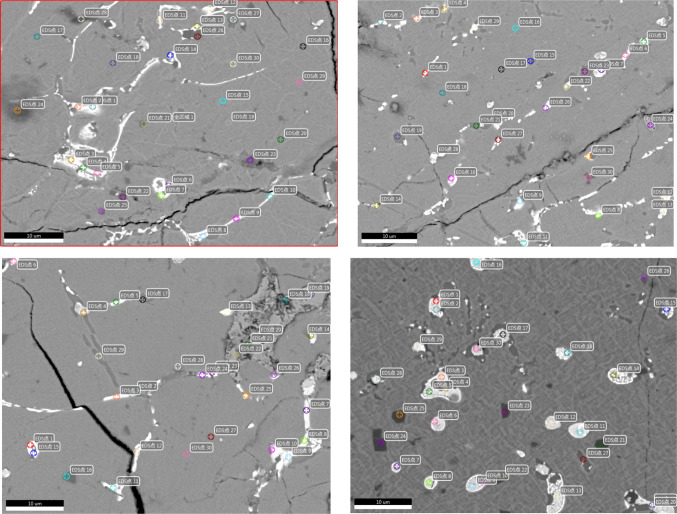
As deduced from previous analyses, arsenic in copper matte exhibits a characteristic of “ubiquitous distribution with localized enrichment. To delve deeper into the distribution characteristics of arsenic within copper matte, Field Emission Scanning Electron Microscopy (FESEM) combined with an Energy Dispersive Spectrometer (EDS) was employed to analyze the distribution of arsenic in four copper matte samples. The findings are presented in Supplementary File.
Based on the electron microscopy images of copper matte under the backscattered mode in Fig. 8, and the EDS data in Supplementary File, it is evident that copper matte primarily consists of four distinct phase regions.
Fig. 8.
SEM-EDS for sample 4.
Chalcopyrite phase
The chalcopyrite phase is a fundamental phase of copper matte, primarily constituted of Cu, Fe, and S, which together account for over 70% of its mass composition. Its most significant chemical component is Cu5FeS4. In the chalcopyrite phase, apart from Cu, Fe, and S, there are considerable amounts of Pb and Zn. This is because, following the principle of similar phase solubility, copper matte captures numerous other metal sulfides such as lead sulfide (PbS) and zinc sulfide (ZnS). Additionally, it is noted that this phase region does contain arsenic, yet in very low concentrations, generally below 0.1%. This is attributed to the chalcopyrite phase being a metal sulfide, which has a good solubility for other sulfides, whereas arsenic sulfides typically vaporize into the flue gas in gaseous form at smelting temperatures. Other non-sulfide forms of arsenic are difficult to dissolve in the chalcopyrite phase. Therefore, the concentration of arsenic enriched in the chalcopyrite phase is extremely low, making it not the primary area of arsenic enrichment in copper matte.
Magnetite phase
The majority of the phases in copper matte are metallic sulfides, but industrial copper matte typically dissolves 2–4% oxygen. This is due to the presence of iron sulfide in the copper matte; iron being an oxygen-affine metal, makes iron sulfide prone to oxidation, thereby dissolving oxygen into the copper matte phase. The oxygen content in copper matte depends on the activity of iron sulfide; the higher the activity of iron sulfide, the more oxygen theoretically dissolves. Iron, serving as the carrier of oxygen in copper matte, is commonly present in the form of the magnetite phase. Magnetite has several negative impacts on copper smelting: its presence increases the viscosity of the slag, leading to difficulties in slag disposal; it also erodes the refractory bricks inside the smelting furnace, hence the production process aims to minimize the formation of magnetite phase. It is observed that, apart from Fe and O, this region also contains Cu. This is because under the oxygen pressure conditions of copper matte smelting, the solubility of copper in the magnetite phase increases, forming a spinel solid solution, CuFe2O4, which is very stable at copper smelting temperatures. In this region, the arsenic content is approximately zero, indicating that the magnetite phase not only fails to capture and dissolve arsenic but may also contribute to its volatilization. The oxygen in the magnetite phase combines with arsenic to form gaseous arsenic oxides, which then volatilize into the smelting flue gases.
Copper-based alloy phase
From the backscattered electron images of the copper matte in the scanning electron microscope, it’s distinctly observable that the copper matte samples contain some “bright” areas. Based on the characteristics of the backscatter mode, elements with higher relative atomic masses appear brighter in this mode. Combining this with energy dispersive spectroscopy (EDS) analysis, it is evident that these regions have extremely low contents of O and S elements, with the majority being metallic elements such as Cu, Fe, Pb, Zn, etc. Considering the results of the previous Mineral Liberation Analysis (MLA), it can be inferred that these regions likely represent copper-based alloy phases.
Although copper matte smelting occurs under oxygen-rich conditions, the presence of abundant metal sulfides in the furnace leads to the separation of sulfur from metals under high-temperature and oxygen-rich conditions, potentially creating localized reducing atmospheres. Within these atmospheres, some metal sulfides might not be fully oxidized, thus forming metallic phases. These metallic phases are bonded together through metallic bonds, forming complex alloy compounds. The arsenic content in this region is significantly higher than in the previous two regions, with concentrations ranging between 1 wt% and 20 wt%. This suggests that arsenic readily enriches in these complex alloy compounds. According to studies by Itagaki and others, arsenic shows a marked preference for copper-rich and iron-rich alloys, with both metallic copper and iron readily forming arsenic-containing alloy compounds. The possible reactions that may occur in this process are as follows:
 |
1 |
 |
2 |
 |
3 |
 |
4 |
 |
5 |
 |
6 |
 |
7 |
 |
8 |
Although arsenic readily forms arsenic-containing alloy compounds with many metals in this process, it’s important to note that in these compounds, other arsenic-containing alloy compounds tend to undergo vaporization in the presence of oxygen, causing the arsenic bonded with them to volatilize and separate. However, copper is a metal that is not oxygen-friendly, making it challenging to separate arsenic from copper arsenic alloy compounds through oxidation and vaporization mechanisms.
Arsenic-antimony alloy phase
It can be observed that there are some bright regions dispersed in the copper matte. Based on the spectroscopic information, their arsenic content can be as high as 50 wt%, making it the phase with the highest arsenic content among all phases in the copper matte. Combining the elemental composition revealed by spectroscopy, it can be deduced that this phase is the arsenic-antimony alloy phase. Arsenic and antimony both belong to the nitrogen group elements, share similar chemical properties, and can readily form alloy phases in the metallic state. Although this phase has the highest proportion of arsenic, its overall content is minimal and does not significantly impact copper smelting.
Conclusions
The higher the copper grade in copper matte, the higher the arsenic content. The main phases in copper matte are chalcopyrite phases composed of copper sulfide (Cu2S) and iron sulfide (FeS), along with small amounts of lead sulfide (PbS) and zinc sulfide (ZnS) captured during smelting. In addition, due to the small amount of oxygen dissolved during smelting, there is a minor presence of magnetite (Fe3O4) phase in copper matte. Thermal analysis of copper matte demonstrates its remarkable structural stability, with no significant decomposition observed even at temperatures as high as 1200 °C.
The arsenic in copper matte exists in four main forms: arsenic sulfides, arsenic oxides, arsenates, and residual arsenic. The majority of arsenic is in the form of residual arsenic. Arsenic in copper matte exhibits extremely high stability and is resistant to exchange with the external environment. Mass loss analysis of copper matte at 1200 °C does not exceed 10%. Furthermore, within the temperature range of 800 °C to 1200 °C, the arsenic content in copper matte first decreases and then increases, indicating that high temperatures promote the retention of arsenic in copper matte and inhibit its gas-phase volatilization.
In high-grade copper matte, arsenic primarily exists in complex copper-based alloy compounds, where arsenic combines with other metals through metal bonding. In low-grade copper matte, aside from the complex alloy phase with the highest arsenic content, there are also some glassy phases. These glassy phases have a fixed network structure that retains arsenic, making it easy for arsenic to be present in the glassy phase. Copper matte mainly contains four distinct phase regions: chalcopyrite phase, magnetite phase, copper-based alloy compound phase, and arsenic-antimony alloy phase. The magnetite phase region has the lowest arsenic content, approximately equal to 0. The chalcopyrite phase region has the widest range of arsenic content because it is the most abundant phase in copper matte. However, the dissolved arsenic proportion in the chalcopyrite phase is extremely low, not exceeding 0.1%. The arsenic-antimony alloy phase has the highest proportion of arsenic, but its content is very low, having minimal impact on copper matte. The copper-based alloy compound phase contains the highest arsenic content in copper matte, approximately ranging from 1 to 10%. Due to the highly stable metal bonds between Cu and As, it is challenging to separate arsenic from this phase, which is a key factor in the difficulty of completely removing arsenic from copper matte.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Hunan Provincial Natural Science Foundation of China, 2022JJ50259. The Research Foundation of Education Bureau of Hunan, China, 22B0279.
Author contributions
Wang Dawei performed the data analysis and wrote the manuscript; Tang Jinyao performed the formal analysis; Song Yu xia performed the validation.
Data availability
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang, Y., Jin, B., Huang, Y., Song, Q. & Wang, C. Two-stage leaching of zinc and copper from arsenic-rich copper smelting hazardous dusts after alkali leaching of arsenic. Sep. Purif. Technol.220, 250–258 (2019). [Google Scholar]
- 2.Chen, Y. et al. Characterization of copper smelting flue dusts from a bottom-blowing bath smelting furnace and a flash smelting furnace. Metall. Mater. Trans. B. 51, 2596–2608 (2020). [Google Scholar]
- 3.Zhao, B. & Liao, J. Development of bottom-blowing copper smelting technology: A review. Metals. 12, 190 (2022). [Google Scholar]
- 4.Yang, W. et al. Leaching behaviors of copper and arsenic from high-arsenic copper sulfide concentrates by oxygen-rich sulfuric acid leaching at atmospheric pressure. J. Environ. Chem. Eng.10, 107358 (2022). [Google Scholar]
- 5.Padilla, R., Aracena, A. & Ruiz, M. C. Reaction mechanism and kinetics of enargite oxidation at roasting temperatures. Metall. Mater. Trans. B. 43, 1119–1126 (2012). [Google Scholar]
- 6.Li, X. et al. Pyrolysis of arsenic-bearing gypsum sludge being substituted for calcium flux in smelting process. J. Anal. Appl. Pyrol.130, 19–28 (2018). [Google Scholar]
- 7.Montenegro, V., Sano, H. & Fujisawa, T. Recirculation of high arsenic content copper smelting dust to smelting and converting processes. Miner. Eng.49, 184–189 (2013). [Google Scholar]
- 8.de las Torres, A. G., Ríos, G., Almansa, A. R., Sánchez-Rodas, D. & Moats, M. S. Solubility of bismuth, antimony and arsenic in synthetic and industrial copper electrorefining electrolyte. Hydrometallurgy. 208, 105807 (2022). [Google Scholar]
- 9.Chen, C., Zhang, L. & Jahanshahi, S. Thermodynamic modeling of arsenic in copper smelting processes. Metall. Mater. Trans. B. 41, 1175–1185 (2010). [Google Scholar]
- 10.Xu, B. et al. A review of the comprehensive recovery of valuable elements from copper smelting open-circuit dust and arsenic treatment. Jom. 72, 3860–3875 (2020). [Google Scholar]
- 11.Zhang, Y., Feng, X., Qian, L., Luan, J. & Jin, B. Separation of arsenic and extraction of zinc and copper from high-arsenic copper smelting dusts by alkali leaching followed by sulfuric acid leaching. J. Environ. Chem. Eng.9, 105997 (2021). [Google Scholar]
- 12.Che, J., Zhang, W., Deen, K. M. & Wang, C. Eco-friendly treatment of copper smelting flue dust for recovering multiple heavy metals with economic and environmental benefits. J. Hazard. Mater.465, 133039 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Guo, X., Chen, Y., Wang, Q. & Wang, S. Tian Q-h. copper and arsenic substance flow analysis of pyrometallurgical process for copper production. Trans. Nonferrous Met. Soc. China. 32, 364–376 (2022). [Google Scholar]
- 14.Zhou, H., Liu, G., Zhang, L. & Zhou, C. Mineralogical and morphological factors affecting the separation of copper and arsenic in flash copper smelting slag flotation beneficiation process. J. Hazard. Mater.401, 123293 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Hassan, K. M., Fukushi, K., Turikuzzaman, K. & Moniruzzaman, S. M. Effects of using arsenic–iron sludge wastes in brick making. Waste Manage.34, 1072–1078 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Alka, S. et al. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod.278, 123805 (2021). [Google Scholar]
- 17.Torres AIGdl, Ríos, G., Almansa, A. R., Sánchez-Rodas, D. & Moats, M. S. Solubility of bismuth, antimony and arsenic in synthetic and industrial copper electrorefining electrolyte. Hydrometallurgy. 208, 105807 (2022). [Google Scholar]
- 18.Yu, T. et al. Efficient removal of bismuth with supersoluble amorphous antimony acids: An insight into synthesis mechanism and sb (V)-Bi (III) interaction behaviors. Chem. Eng. J.420, 127617 (2021). [Google Scholar]
- 19.Wang, X. et al. Promotion of copper electrolyte self-purification with antimonic oxides. Hydrometallurgy. 175, 28–34 (2018). [Google Scholar]
- 20.Wang, X., Wang, X. & Wang, M. Characteristics of BiAsO4 precipitate formation in copper electrolyte. Hydrometallurgy. 171, 95–98 (2017). [Google Scholar]
- 21.Jafari, S. et al. Effect of typical impurities for the formation of floating slimes in copper electrorefining. Int. J. Miner. Process.168, 109–115 (2017). [Google Scholar]
- 22.Benabdallah, N. et al. Increasing the circularity of the copper metallurgical industry: Recovery of sb (III) and Bi (III) from hydrochloric solutions by integration of solvating organophosphorous extractants and selective precipitation. Chem. Eng. J.453, 139811 (2023). [Google Scholar]
- 23.González de las Torres, A. I., Moats, M. S., Ríos, G., Rodríguez Almansa, A. & Sánchez-Rodas, D. Removal of sb impurities in copper electrolyte and evaluation of as and Fe species in an electrorefining plant. Metals. 11, 902 (2021). [Google Scholar]
- 24.Xiao, F., Cao, D. & Mao, J. Shen X-n, Ren F-z. Role of trivalent antimony in the removal of as, Sb, and Bi impurities from copper electrolytes. Int. J. Minerals Metall. Mater.20, 9–16 (2013). [Google Scholar]
- 25.Zhao, Z. et al. Arsenic removal from copper slag matrix by high temperature sulfide-reduction-volatilization. J. Hazard. Mater.415, 125642 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Xu, F. et al. Distribution and Control of Arsenic during copper converting and refining. Metals. 13, 85 (2022). [Google Scholar]
- 27.Zheng, Y., Peng, Y., Lang, K. & Chen, W. Separation and recovery of Cu and as from copper electrolyte through electrowinning and SO2 reduction. Trans. Nonferrous Met. Soc. China. 23, 2166–2173 (2013). [Google Scholar]
- 28.Nie, H., Cao, C., Xu, Z. & Tian, L. Novel method to remove arsenic and prepare metal arsenic from copper electrolyte using titanium (IV) oxysulfate coprecipitation and carbothermal reduction. Sep. Purif. Technol.231, 115919 (2020). [Google Scholar]
- 29.Sohn, H., Fukunaka, Y., Oishi, T., Sohn, H. & Asaki, Z. Kinetics of as, Sb, Bi and Pb volatilization from industrial copper matte during ar + O 2 bubbling. Metall. Mater. Trans. B. 35, 651–661 (2004). [Google Scholar]
- 30.Gitsu, D., Golban, I., Makeichik, A., Muntyanu, F. & Onu, M. The Thermopower and the Thermomagnetic Power in Arsenic—Antimony alloys at low temperature. Phys. Status Solidi (b). 100, 401–406 (1980). [Google Scholar]
- 31.Mendoza, D. G., Hino, M. & Itagaki, K. Phase relations and activity of arsenic in Cu-Fe-S-As system at 1473 K. Mater. Trans.42, 2427–2433 (2001). [Google Scholar]
- 32.Zhao, Z. et al. XPS and FTIR studies of sodium arsenate vitrification by cullet. J. Non-cryst. Solids. 452, 238–244 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.