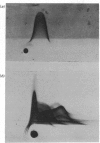
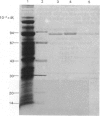
Abstract
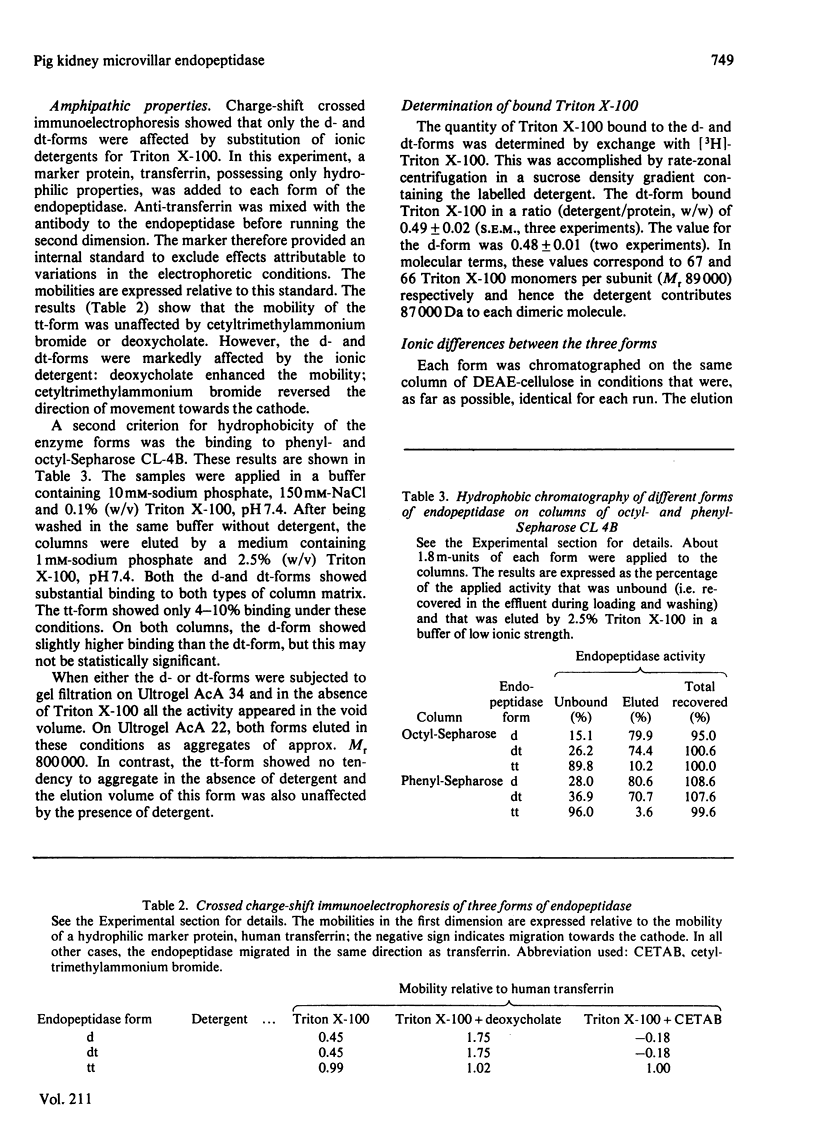
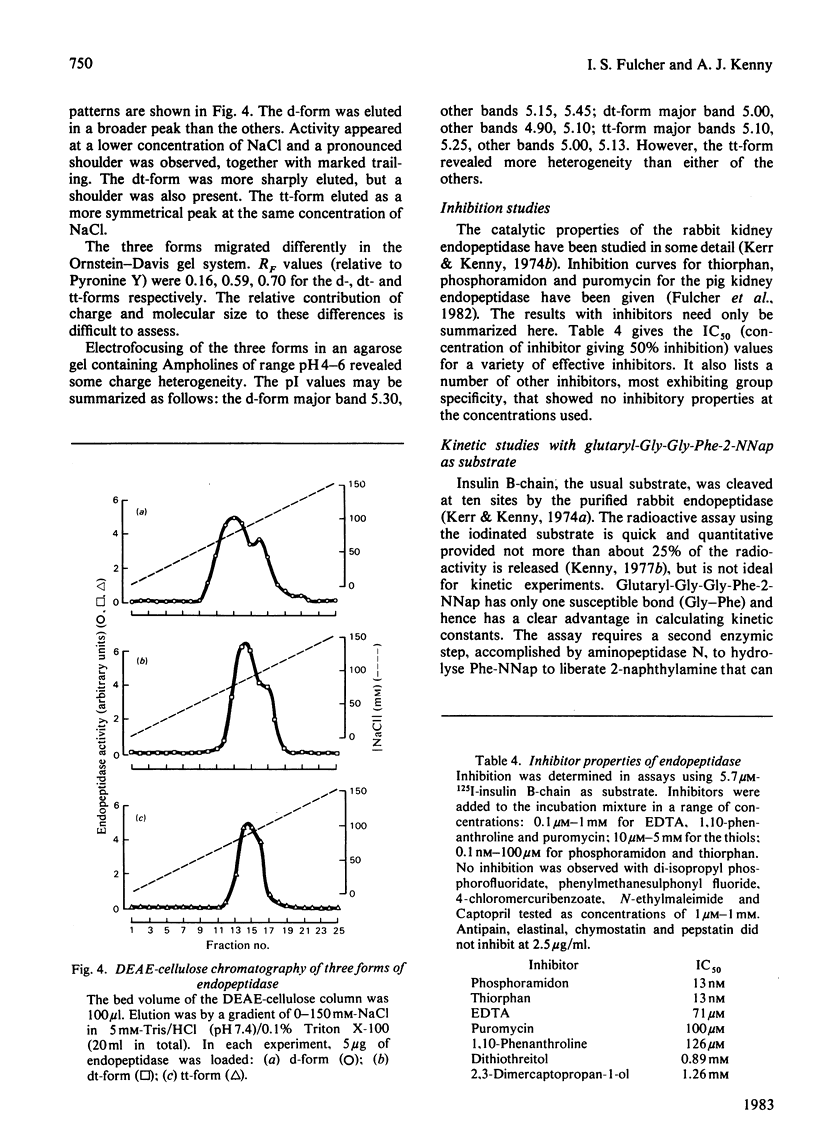
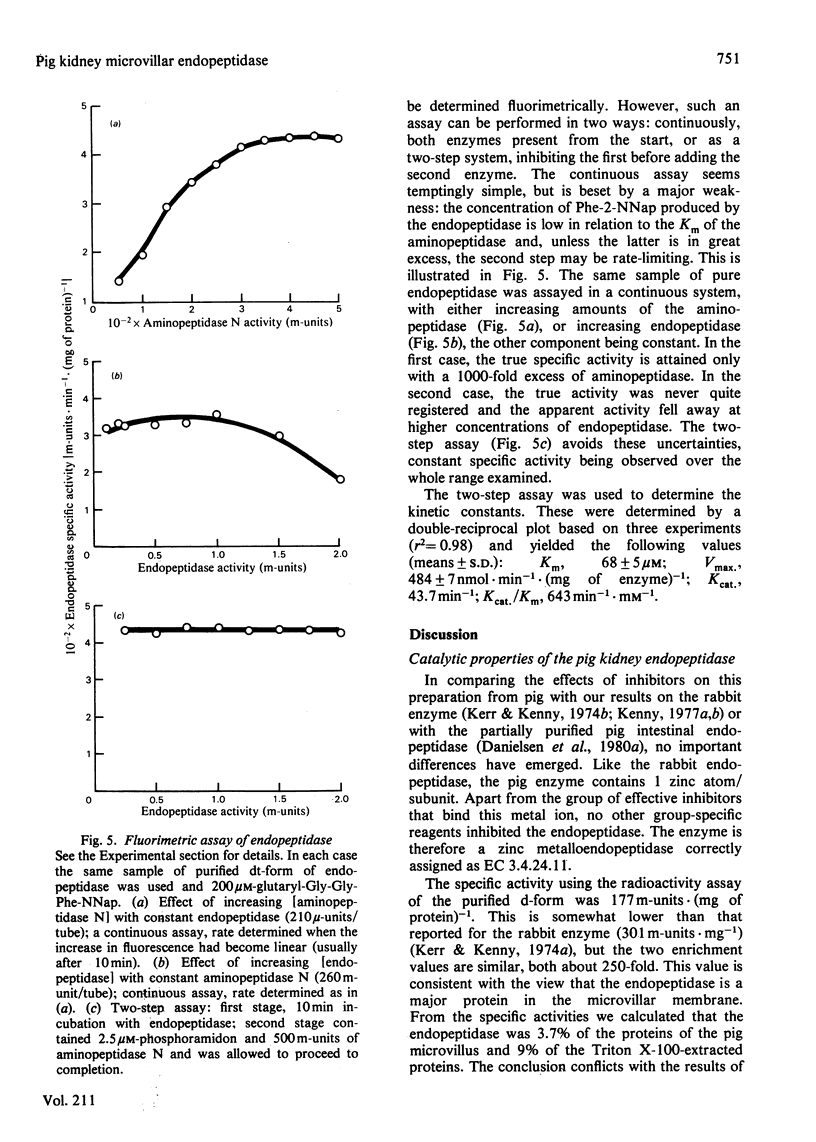
The purification of detergent-solubilized kidney microvillar endopeptidase (EC 3.4.24.11) by immuno-adsorbent chromatography is described. The product (the d-form) was 270-fold purified compared with the homogenate of kidney cortex and was obtained in a yield of 5%. It was free of other peptidase activities and homogeneous by electrophoretic analyses. It contained about 15% carbohydrate and one Zn atom/subunit. Two trypsin-treated forms were also characterized. One (dt-form) was obtained by treatment of the d-form. The other (tt-form) was the result of solubilizing the membrane by treatment with toluene and trypsin. All three forms had apparent subunit Mr values of approx. 89 000, but the d-form appeared to be slightly larger than the other two. Estimates of Mr by gel filtration showed that of the tt-form to be 216 000 whereas those of the other forms were 320 000. An estimate of the detergent (Triton X-100) bound to the d- and dt-forms accounted for this difference. By several criteria, including charge-shift crossed immunoelectrophoresis and hydrophobic chromatography, the d- and dt-forms were shown to be amphipathic molecules. In contrast, the tt-form was hydrophilic in its properties. Differences in ionic properties were also noted, consistent with the loss, in the case of the dt-form, of a positively charged peptide. The results indicate that the native endopeptidase is a dimeric molecule, each subunit being anchored in the membrane by a relatively small region of the polypeptide close to one or other terminus. The d- and dt-forms had similar enzyme activity when assayed by the hydrolysis of 125I-insulin B-chain. Chelating agents and phosphoramidon inhibited the endopeptidase. The kinetic constants were determined by a new two-stage fluorimetric assay using glutarylglycylglycylphenylalanine 2-naphthylamide as substrate and aminopeptidase N (EC 3.4.11.2) to hydrolyse phenylalanine 2-naphthylamide. The Km was 68 microM and Vmax. 484nmol X min-1 X (mg of protein)-1.
Full text
PDF



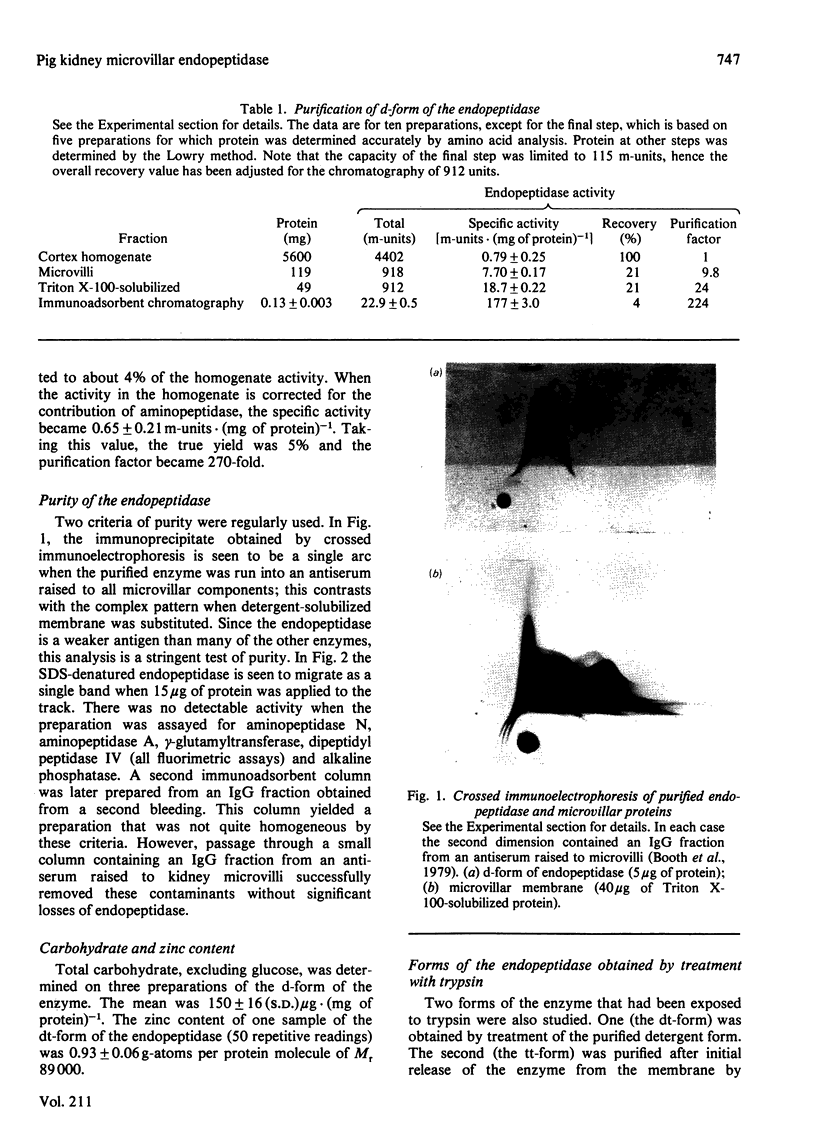
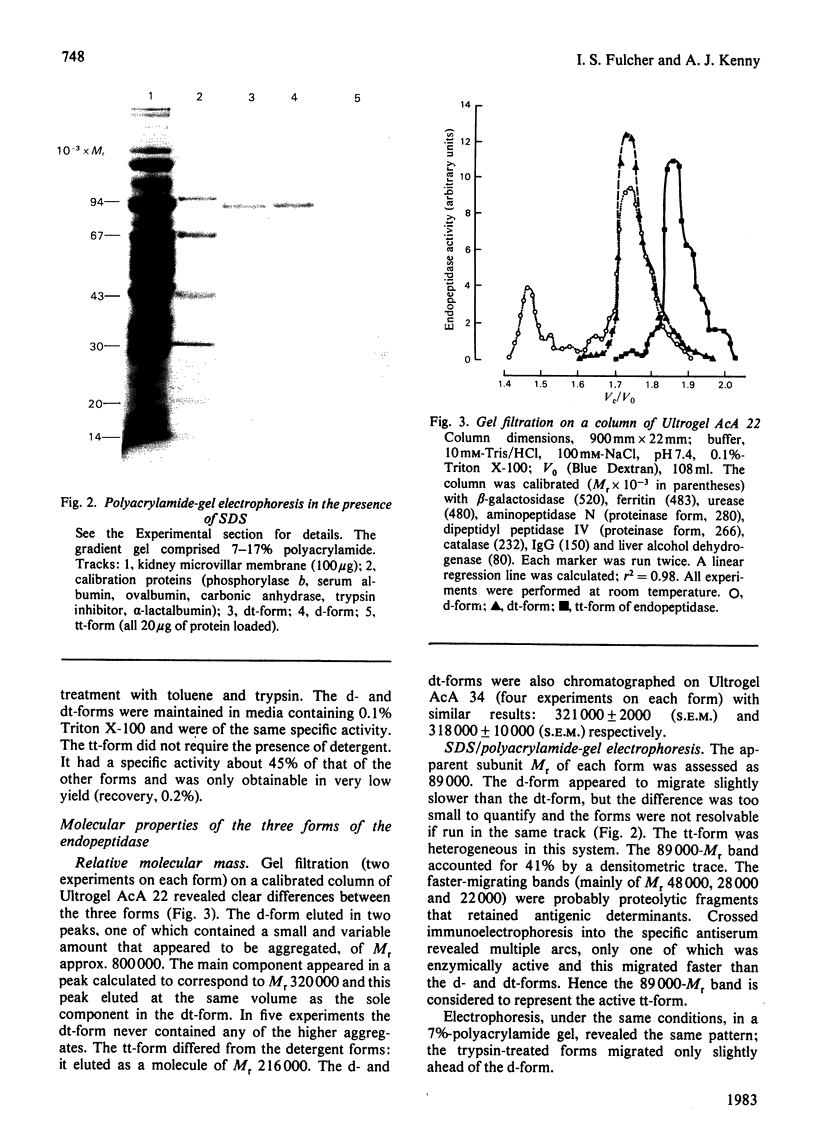


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almenoff J., Wilk S., Orlowski M. Membrane bound pituitary metalloendopeptidase: apparent identity to enkephalinase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):206–214. doi: 10.1016/0006-291x(81)91508-4. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Shannon J. D., Bond J. S. Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem J. 1981 Dec 1;199(3):591–598. doi: 10.1042/bj1990591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Hubbard L. M., Kenny A. J. Proteins of the kidney microvillar membrane. Immunoelectrophoretic analysis of the membrane hydrolases: identification and resolution of the detergent- and proteinase-solubilized forms. Biochem J. 1979 May 1;179(2):397–405. doi: 10.1042/bj1790397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaplin M. F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- Clarke S. The size and detergent binding of membrane proteins. J Biol Chem. 1975 Jul 25;250(14):5459–5469. [PubMed] [Google Scholar]
- Danielsen E. M., Norén O., Sjöström H., Ingram J., Kenny A. J. Proteins of the kidney microvillar membrane. Aspartate aminopeptidase: purification by immunoadsorbent chromatography and properties of the detergent- and proteinase-solubilized forms. Biochem J. 1980 Sep 1;189(3):591–603. doi: 10.1042/bj1890591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Vyas J. P., Kenny A. J. A neutral endopeptidase in the microvillar membrane of pig intestine. Partial purification and properties. Biochem J. 1980 Nov 1;191(2):645–648. doi: 10.1042/bj1910645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgenhauer K., Glenner G. G. The enzymatic hydrolysis of amino acid beta-naphthylamides. II. Partial purification and properties of a particle-bound cobalt-activated rat kidney aminopeptidase. J Histochem Cytochem. 1966 May;14(5):401–413. doi: 10.1177/14.5.401. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Matsas R., Turner A. J., Kenny A. J. Kidney neutral endopeptidase and the hydrolysis of enkephalin by synaptic membranes show similar sensitivity to inhibitors. Biochem J. 1982 May 1;203(2):519–522. doi: 10.1042/bj2030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. G., Kenny J. Studies on the enzymology of purified preparations of brush border from rabbit kidney. Biochem J. 1973 May;134(1):43–57. doi: 10.1042/bj1340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Sarvas M., Simons K. Asymmetric and symmetric membrane reconstitution by detergent elimination. Studies with Semliki-Forest-virus spike glycoprotein and penicillinase from the membrane of Bacillus licheniformis. Eur J Biochem. 1981 May;116(1):27–35. doi: 10.1111/j.1432-1033.1981.tb05296.x. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Charge shift electrophoresis: simple method for distinguishing between amphiphilic and hydrophilic proteins in detergent solution. Proc Natl Acad Sci U S A. 1977 Feb;74(2):529–532. doi: 10.1073/pnas.74.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Kenny A. J. Endopeptidases in the brush border of the kidney proximal tubule. Ciba Found Symp. 1977;(50):209–215. doi: 10.1002/9780470720318.ch12. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Fulcher I. S., McGill K. A., Kershaw D. Proteins of the kidney microvillar membrane. Reconstitution of endopeptidase in liposomes shows that it is a short-stalked protein. Biochem J. 1983 Jun 1;211(3):755–762. doi: 10.1042/bj2110755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Fulcher I. S., Ridgwell K., Ingram J. Microvillar membrane neutral endopeptidases. Acta Biol Med Ger. 1981;40(10-11):1465–1471. [PubMed] [Google Scholar]
- Kenny A. J., Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol Rev. 1982 Jan;62(1):91–128. doi: 10.1152/physrev.1982.62.1.91. [DOI] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The molecular weight and properties of a neutral metallo-endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):489–495. doi: 10.1042/bj1370489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Schwartz J. C. Properties of "enkephalinase" from rat kidney: comparison of dipeptidyl-carboxypeptidase and endopeptidase activities. Biochem Biophys Res Commun. 1982 May 31;106(2):276–285. doi: 10.1016/0006-291x(82)91106-8. [DOI] [PubMed] [Google Scholar]
- Mumford R. A., Pierzchala P. A., Strauss A. W., Zimmerman M. Purification of a membrane-bound metalloendopeptidase from porcine kidney that degrades peptide hormones. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6623–6627. doi: 10.1073/pnas.78.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino N., Powers J. C. Design of potent reversible inhibitors for thermolysin. Peptides containing zinc coordinating ligands and their use in affinity chromatography. Biochemistry. 1979 Oct 2;18(20):4340–4347. doi: 10.1021/bi00587a012. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Wilk S. Purification and specificity of a membrane-bound metalloendopeptidase from bovine pituitaries. Biochemistry. 1981 Aug 18;20(17):4942–4950. doi: 10.1021/bi00520a021. [DOI] [PubMed] [Google Scholar]