Abstract
Acute pancreatitis (AP) can result in acute kidney injury (AKI), which is linked to poor outcomes. We aimed to assess the relationship between the hematocrit-to-albumin ratio (HAR) and AKI in this population. This retrospective cohort study included consecutive patients diagnosed with AP and admitted to hospital. Data were systematically extracted from electronic medical records, covering baseline demographic and clinical characteristics. Total 1514 AP patients were enrolled, with 17% (257/1514) developing AKI. Multivariable-adjusted regression analysis, curve fitting, threshold effects analyses, and subgroup analyses were conducted to evaluate the relationship between HAR and AKI incidence in AP patients. Compared to the reference tertile of HAR, the adjusted OR values for the lower and higher tertiles of HAR were 1.25 (95% CI, 0.82–1.91, P = 0.297) and 1.50 (95% CI, 1.03–2.20, P = 0.037), respectively, after adjusting for covariates. The curve fitting results showed a J-shaped relationship between HAR and AKI (non-linear, p = 0.001), with an inflection point of 8.969. Furthermore, validation using the Medical Information Mart for Intensive Care (MIMIC-IV) database AP population revealed a similar relationship with an inflection point at 10.257. Our findings suggest a J-shaped relationship between HAR and AKI in AP patients, indicating higher risk of AKI when HAR exceeds 8.969.
Keywords: Acute pancreatitis, Acute kidney injury, Serum hematocrit/albumin ratio, Cohort study
Subject terms: Biomarkers, Diseases, Gastroenterology
Acute pancreatitis (AP) is an inflammatory condition of the pancreas characterized by its sudden onset and variable clinical course1–3.Over the past several decades, the incidence of AP has been increasing, leading to numerous hospital admissions and a significant economic burden4,5. The majority of patients experience with mild, self-limited acute pancreatitis and with a favorable prognosis6. Although the overall mortality rate of AP is below 5%, severe cases, often associated with the progression of systemic inflammatory response syndrome (SIRS) to multiorgan dysfunction syndrome, exhibit a mortality rate ranging from 30 to 50%4,7,8.
Acute kidney injury (AKI) occurs in 10 to 42% of patients with AP. The development of AKI is associated with high mortality rate, prolongs hospital stays and higher risk of chronic kidney disease development9,10. While some studies have investigated biomarkers and developed predictive models for AKI in AP patients, these studies often suffer from small sample sizes and lack robust predictive accuracy11,12. Therefore, the challenge of early AKI identification and timely therapeutic intervention in AP patients remains critical.
Hematocrit (HCT) represents the volume percentage of red blood cells in the blood. Previous studies have identified HCT as a biomarker for predicting mortality risk in sepsis and septic shock13,14. Albumin (ALB), a negative acute-phase reactant synthesized by the liver, constitutes 40% to 60% of total plasma protein and decreases during inflammation15. Prior studies has demonstrated a correlation between the difference in HCT and ALB levels (HCT-ALB) and various conditions, including eclampsia, severe infections16,17, and the prognosis of elderly sepsis ICU patients18. This study is the first to investigate the relationship between HCT-to-ALB ratio (HAR) and AKI in patients with acute pancreatitis.
Methods
Study design
Database and study population from the Chinese hospital
Studies involving human participants were reviewed and approved by the Hospital of Medicine Ethics Committee (ethical approval number: 2023-130-01), and all information about cases was obtained from a retrospective chart of patients with electronic medical records who underwent acute pancreatitis at the First College of Clinical Medical Science of China Three Gorges University from January 2019 to October 2023. Due to the retrospective nature of the study, the Ethics Committee of the First College of Clinical Medical Science of China Three Gorges University waived the need of obtaining informed consent.
Database and study population from the MIMIC-IV 2.0 Database
To better verify the applicability of the model in Chinese patients, we also reviewed participants from the MIMIC-IV 2.0 Clinical Database (MIMIC-IV 2.0), which holds information on more than 672 patients meeting the inclusion criteria admitted to Beth Israel Deaconess Medical Center’s ICUs in Boston, MA, from 2008 to 2019.The database is accessible to anybody who has passed the Collaborative Institutional Training Initiative exam (Certification number 46658537 for Wen Wu).
Inclusion and exclusion criteria
The inclusion criteria for this retrospective study were as follows: Patients who were admitted to the First College of Clinical Medical Science of China Three Gorges University; patients who were diagnosed with AP; The exclusion criteria were as follows: patients who were younger than 18 years, or older than 85 years; Pregnant or breastfeeding women; Length of hospital stay ≤ 24 h; patients with CKD; patients with malignant tumor; patients with chronic pancreatitis; patients who received renal transplantation or nephrectomy; incomplete medical data.
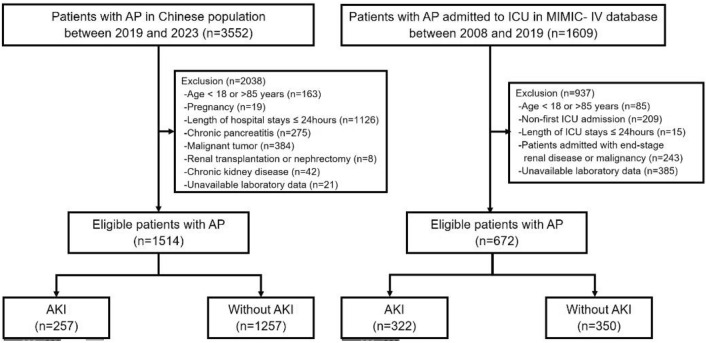
A total of 523,740 admissions were recorded in the MIMIC-IV database, of which 76,540 were admitted to the ICU. Hospital admission information for patients with AP was extracted according to International Classification of Diseases, with a total of 1609 had been admitted to the ICU (supplementary). After further screening, patients who meet the following criteria will be excluded (1): Patients younger than 18 years or older than 85 years of age at the time of the first admission; (2) Patients admitted repeatedly for acute pancreatitis, for whom only the first admission data were retained; (3) Patients who stayed in the ICU for less than 24 h; (4) Patients admitted with end-stage renal disease or malignancy; (5) Patients without recorded blood hematocrit and serum albumin data within 24 h of admission. Ultimately, 672 patients were enrolled in this study. The patient collecting and reviewing process was shown in Fig. 1.
Fig. 1.
Flowchart of the screening and enrollment of study participants. AKI acute kidney injury; AP Acute pancreatitis.
Clinical definitions and laboratory data
Diagnosis and Severity Classification of AP
According to the revised Atlanta classification (2012), the diagnosis of AP was established when at least two of the three diagnostic criteria, namely clinical presentation (typical epigastric abdominal pain), laboratory parameters (serum amylase or lipase levels at least three times higher than the upper limit of normal), and abdominal cross-sectional radiographic evidences (including abdominal ultrasound, computed tomography, or magnetic resonance imaging), were met19,20. A limited number of diagnosed AP patients were eventually confirmed by undergoing exploratory laparotomy.
Adult patients with acute pancreatitis (AP) were categorized into three groups: mild AP (MAP), moderately severe AP (MSAP), and severe AP (SAP) depending on the presence of organ dysfunction and the duration of organ dysfunction being less than 48 h and more than 48 hours3. For adult AP patients with evolving disease severity, the most severe classification of AP during hospitalization was considered the ultimate one.
Diagnosis and classification of AKI
The diagnostic criteria of AKI were based on the guidelines of the Kidney Disease Improving Global Outcomes (KIDGO) (2012)21criteria, as follows:
(i) Serum creatinine increased by more than 26.5 µmol/L (0.3 mg/dL) or a percentage increase in serum creatinine level of ≥ 50% within 48 h; (ii) The urine volume lasted for more than 6 h and was less than 0.5 mg/kg/h; (iii) Serum creatinine increased 1.5 times higher than the baseline level. Baseline serum creatinine level was defined as the lowest serum creatinine level measured within 2 days prior to admission to hospital. If no serum creatinine level was measured, the serum creatinine level recorded in the first measurement within 2 days after admission to hospital was considered as baseline serum creatinine level.
The BISAP score
The Bedside Index for Severity in Acute Pancreatitis (BISAP) score22 was developed in 2008 and designed as a predictor of mortality based on 5 variables: blood urea nitrogen (BUN) level greater than 25 mg/dL, impaired mental status, meeting at least two of the systemic inflammatory response syndrome (SIRS) criteria, age older than 60 years, or radiographic evidence of pleural effusion within the first 24 h of admission. Those with a score of 3 or above while receiving 1 point for each positive criterion were defined as having severe acute pancreatitis, and those with a score of 2 or less were defined as having mild acute pancreatitis according to the BISAP scoring system23.
Data collection
General information was collected, including sex, age, the length of hospital stays, systolic blood pressure (SBP), demand for continuous renal replacement treatment (CRRT), mechanical ventilation (MV) and blood transfusion, sequential organ failure assessment (SOFA) score and Bedside Index of Severity in Acute Pancreatitis (BISAP) score within 24 h of admission. Blood routine parameters including white blood cell count, hemoglobin (HGB) content, and hematocrit (HCT) were also collected. Biochemical parameters including albumin (ALB), blood urea nitrogen (BUN), serum creatinine (CREA), fasting plasma glucose (FPG), sodium ion concentration (Na+), calcium ion concentration (Ca2+), alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), C-reactive protein (CRP), procalcitonin (PCT), The HCT/ALB ratio (HAR) was calculated by dividing the hematocrit (HCT, %) by the serum albumin level (ALB, g/dl). The endpoint event defined in this study was acute kidney injury (AKI) in hospital stay caused by acute pancreatitis (AP).
Statistical analysis
Continuous variables are shown as means with standard deviations, while categorical variables are shown as frequencies or percentages. For continuous variables, statistical differences were determined using one-way ANOVA (for normally distributed data) or Kruskal–Wallis H test (for skewed data), while the Chi-square or Fisher’s exact test was used for categorical variables.
Subjects were grouped into tertiles by HAR levels (HAR < 9.39, 9.39 ≤ HAR < 10.83, HAR ≥ 10.83), multiple logistic regression analysis and smooth curve fitting were performed to explore the association between HAR and AKI. According to the guidelines in the Strengthening the Reporting of Observational Studies statement24, we simultaneously analyzed both non-adjusted and multivariable-adjusted models. Covariates were adjusted if variables were within P < 0.1 as determined by univariate analysis, and if adding or removing the covariate from the model altered the corresponding odds ratio by at least 10%. We used 3 models: in the adjusted model 1, we adjusted for age and sex; in the adjusted model 2, we adjusted for age, sex, hypertension, diabetes mellitus (DM), coronary heart disease (CHD), heart rate (HR), respiratory rate (RR) and pulse oxygen saturation (SpO2); in the adjusted model 3, we adjusted for age, sex, hypertension, DM, CHD, HR, RR, SpO2, etiology, blood transfusion, SOFA score, BISAP score, white blood cell count (WBC), and fasting plasma glucose (FPG). Baseline variables that were considered clinically relevant or had a change in effect estimate of > 10% were chosen as confounders. The smooth curve fitting was established and adjusted according to the covariables contained in model 3. The nonlinear relationship between HAR and AKI was observed after logical regression. For the missing data, we used the multiple imputation method to fill in the missing values25. The details of the missing value are shown in supplementary Table S4. Descriptive analyses report observed data only, while regression models include all patients with multiple imputed data. Meanwhile, we utilized the same statistical analysis method on the MIMIC-IV database.
As additional exploratory analyses, possible modifications on the association of HAR and AKI were evaluated by stratified analyses and interaction testing.
A two-tailed P < 0.05 was considered to be statistically significant in all analyses. All the analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and Free Statistics software versions 1.9.
Results
Patients’ baseline and clinical data
The enrolled AP patients of Chinese population were divided into AKI group (n = 257) and non-AKI group (n = 1257) according to occurrence of AKI. There was no significant difference in patients’ BMI, amylase and prealbumin between the two groups (p > 0.05), but there were significant differences in sex, age, length of hospital stays, demand for MV, blood transfusion and CRRT, ALB, PCT, CRP, TG, CREA, and HAR (p < 0.001). All results of two databases are shown in Table 1. A total of 1,514 patients were recruited and 257 (17%) of them developed AKI. The baseline demographic characteristics of the study population, stratified by HAR tertiles, are summarized in Table 2. The mean age of the participants was 53.0 (41.0, 65.0) years old, of whom 53.6% were male, and 46.4% were female. Serum HAR levels were positively associated with white blood cell count, hemoglobin, hematocrit, C-reactive protein and demand for mechanical ventilation and CRRT. Furthermore, we also recruited 672 cases from the MIMIC-IV database as the validation cohort, among which 57.6% were male, and 42.4% were female. All results are shown in Table 2 and Supplementary Table S1. Supplementary Table S2 presents the baseline data of non-ICU and ICU patients in the Chinese population.
Table 1.
Baseline characteristics of included patients grouped by the occurrence of AKI in two databases of Chinese population and MIMIC-IV.
| Chinese population | MIMIC-IV population | |||||
|---|---|---|---|---|---|---|
| Characteristics | No-AKI (n = 1257) | AKI (n = 257) | p-value | No-AKI (n = 350) | AKI (n = 322) | p-value |
| Sex (%) | < 0.001 | 0.135 | ||||
| Male | 642 (51.1) | 170 (66.1) | 192 (54.9) | 195 (60.6) | ||
| Female | 615 (48.9) | 87 (33.9) | 158 (45.1) | 127 (39.4) | ||
| Age (years) | 53.0 (41.0, 64.0) | 56.0(44.0, 69.0) | 0.002 | 54.0 (43.0, 67.0) | 59.5 (47.0, 72.8) | 0.001 |
| Death (%) | 5 (0.4) | 18 (7) | < 0.001 | 18 (5.1) | 78 (24.2) | < 0.001 |
| LOS(days) | 13.0 (10.0, 18.0) | 16.0 (10.0, 26.0) | < 0.001 | 9.0 (5.0, 16.0) | 16.0 (8.0, 26.0) | < 0.001 |
| ICU (%) | 115 (9.1) | 132 (51.4) | < 0.001 | 350(100) | 322(100) | |
| Hypertension (%) | 304 (24.2) | 88 (34.2) | < 0.001 | 204 (58.3) | 171 (53.1) | 0.177 |
| DM (%) | 177 (14.1) | 48 (18.7) | 0.059 | 121 (34.6) | 134 (41.6) | 0.06 |
| CHD (%) | 77 (6.1) | 26 (10.1) | 0.021 | 58 (16.6) | 65 (20.2) | 0.226 |
| COPD (%) | 48 (3.8) | 16 (6.2) | 0.081 | N/A | N/A | |
| Weight (kg) | 64.0 (56.0, 75.0) | 65.0 (55.0, 75.0) | 0.704 | 79.1 (68.8, 96.5) | 83.2 (71.1, 99.4) | 0.016 |
| Height (cm) | 163.0(158.0, 170.0) | 167.0(158.0, 172.0) | 0.001 | 170.0(163.0, 178.0) | 170.0(163.0, 178.0) | 0.877 |
| BMI | 24.1 (21.8, 26.7) | 24.2 (20.8, 26.9) | 0.437 | N/A | N/A | |
| SBP (mmHg) | 130.0(119.0, 143.0) | 123.0(110.0, 140.0) | < 0.001 | 130.0(113.0, 146.0) | 122.0(105.2, 143.0) | 0.005 |
| DBP (mmHg) | 80.0 (74.0, 90.0) | 80.0 (66.0, 89.0) | < 0.001 | 76.0 (64.0, 88.8) | 68.0 (58.0, 81.8) | < 0.001 |
| HR (bpm) | 78.0 (72.0, 90.0) | 90.0 (76.0, 110.0) | < 0.001 | 99.0 (83.0, 114.0) | 98.0 (83.0, 115.0) | 0.599 |
| Temperature | 36.5 (36.4, 36.6) | 36.5 (36.4, 36.8) | 0.013 | N/A | N/A | |
| RR (bpm) | 20.0 (19.0, 20.0) | 20.0 (19.0, 21.0) | 0.009 | 21.0 (17.0, 24.8) | 22.0 (19.0, 27.0) | < 0.001 |
| SpO2(%) | 99.0 (98.0, 100.0) | 98.0 (96.0, 100.0) | < 0.001 | N/A | N/A | |
| P/F | N/A | N/A | 231.7 (172.9, 374.0) | 207.9 (138.2, 296.3) | < 0.001 | |
| Ventilation (%) | 73 (5.8) | 85 (33.1) | < 0.001 | 1.6 (0.7, 3.2) | 3.4 (1.2, 11.0) | < 0.001 |
| Transfusion (%) | 70 (5.6) | 98 (38.1) | < 0.001 | 26 (7.4) | 35 (10.9) | 0.121 |
| CRRT (%) | 21 (1.7) | 71 (27.6) | < 0.001 | 11 (3.1) | 44 (13.7) | < 0.001 |
| PLT (× 109L) | 181.0(139.0, 234.0) | 172.0(110.0, 227.0) | 0.004 | 198.0(138.2, 285.5) | 177.0(122.2, 242.0) | < 0.001 |
| PCT (ng/ml) | 0.1 (0.0, 0.6) | 1.1 (0.2, 6.5) | < 0.001 | N/A | N/A | |
| CRP (mg/L) | 31.4 (5.9, 119.0) | 88.7 (16.9, 200.2) | < 0.001 | N/A | N/A | |
| ALB(g/dl) | 3.9 (3.5, 4.3) | 3.5 (2.9, 4.0) | < 0.001 | 3.1 (2.7, 3.6) | 3.0 (2.4, 3.3) | < 0.001 |
| FPG (mmol/L) | 6.6 (5.3, 8.9) | 7.5 (5.7, 11.2) | < 0.001 | 6.7 (5.3, 9.3) | 7.9 (5.9, 12.3) | < 0.001 |
| TG (mmol/L) | 1.5 (1.0, 3.4) | 1.8 (1.2, 6.4) | < 0.001 | 1.6 (1.1, 3.3) | 2.0 (1.2, 5.2) | 0.008 |
| Ca (mmol/L) | 2.2 (2.1, 2.4) | 2.1 (1.9, 2.3) | < 0.001 | 2.0 (1.9, 2.2) | 2.0 (1.8, 2.1) | < 0.001 |
| CREA (µmol/L) | 65.8 (53.8, 77.0) | 143.0 (107.0, 226.0) | < 0.001 | 70.7 (53.0, 88.4) | 145.9 (97.2, 265.2) | < 0.001 |
| AMY(U/L) | 163.0 (64.5, 716.0) | 202.0(92.0, 607.0) | 0.065 | 168.0(64.5, 425.0) | 280.0(78.2, 746.0) | < 0.001 |
| PA (mg/L) | 162.3(111.0, 210.0) | 144.8(92.0, 230.0) | 0.625 | N/A | N/A | |
|
Severity of AP (%) Mild and moderate |
1181 (94) | 162 (63) | < 0.001 | N/A | N/A | |
| Severe | 76 (6) | 95 (37) | ||||
| Etiology of AP (%) | < 0.001 | N/A | N/A | |||
| Biliary | 887 (70.6) | 132 (51.4) | ||||
| Hyperlipidemic | 289 (23) | 65 (25.3) | ||||
| Alcoholic | 42 (3.3) | 9 (3.5) | ||||
| Others | 39 (3.1) | 51 (19.8) | ||||
| Lac (mmol/L) | N/A | N/A | 1.5 (1.1, 2.0) | 2.0 (1.4, 3.5) | < 0.001 | |
| HAR | 10.0 (9.0, 11.0) | 10.7 (9.2, 12.8) | < 0.001 | 11.4 ± 2.6 | 12.2 ± 3.6 | 0.002 |
LOS Length of hospital stay, DM Diabetes mellitus, CHD Coronary heart disease, SBP Systolic blood pressure, DBP Diastolic blood pressure, HR Heart rate, RR Respiratory rate, SpO2 Pulse oxygen saturation, P/F PaO2/FiO2, Ventilation noninvasive or invasive mechanical ventilation, Transfusion blood transfusion, CRRT Continuous renal replacement treatment, WBC White blood cell count, HGB Hemoglobin, HCT Hematocrit, PCT Procalcitonin, CRP C-reactive protein, AST Aspartate aminotransferase, ALB Albumin, FPG Fasting plasma glucose, TG Triglyceride, Na Sodium, Ca Calcium, CREA Creatinine, Lac Lactic acid, AKI Acute kidney injury, SOFA Sequential organ failure assessment score, BISAP Bedside Index of Severity in Acute Pancreatitis score, HAR Dividing the hematocrit level (%) by the ALB level (g/dl).
Table2.
Baseline characteristics of included patients grouped by tertile of HAR in two databases of Chinese population and MIMIC-IV.
| Characteristics | Chinese population | MIMIC-IV population | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 (n = 505) < 9.39 |
T2 (n = 504) 9.39–10.83 |
T3 (n = 505) ≥ 10.83 |
p | T1 (n = 224) < 10.133 |
T2 (n = 224) 10.133–12.793 |
T3 (n = 224) ≥ 12.793 |
p | |
| Sex (%) | < 0.001 | < 0.001 | ||||||
| Male | 169 (33.5) | 289 (57.3) | 354 (70.1) | 124 (55.4) | 121 (54) | 142 (63.4) | ||
| female | 336 (66.5) | 215 (42.7) | 151 (29.9) | 100 (44.6) | 103 (46) | 82 (36.6) | ||
| Age (years) | 53. (41.0, 65.0) | 53.0 (41.0, 66.0) | 54.0 (41.0, 65.0) | 0.957 | 54.0 (44.0, 69.0) | 58.0 (46.0, 72.0) | 56.0 (45.0, 70.0) | 0.598 |
| Death (%) | 5 (1) | 7 (1.4) | 11 (2.2) | 0.291 | 28 (12.5) | 24 (10.7) | 44 (19.6) | 0.017 |
| ICU (%) | 56 (11.1) | 64 (12.7) | 127 (25.1) | < 0.001 | N/A | N/A | N/A | N/A |
| LOS (days) | 16.3 ± 11.2 | 15.1 ± 12.4 | 18.5 ± 14.2 | < 0.001 | 10.0 (5.0, 19.0) | 10.5 (5.0, 19.0) | 14.0 (7.0, 26.0) | < 0.001 |
| Hypertension (%) | 130 (25.7) | 123 (24.4) | 139 (27.5) | 0.525 | 115 (51.3) | 125 (55.8) | 135 (60.3) | 0.164 |
| DM (%) | 81 (16) | 66 (13.1) | 78 (15.4) | 0.38 | 84 (37.5) | 88 (39.3) | 83 (37.1) | 0.876 |
| CHD (%) | 35 (6.9) | 34 (6.7) | 34 (6.7) | 0.99 | 50 (22.3) | 45 (20.1) | 28 (12.5) | 0.019 |
| SBP (mmHg) | 126.0 (116.0, 143.0) | 130.0 (119.0, 143.0) | 130.0 (116.0, 143.0) | 0.206 | 131.5 (112.0, 148.2) | 124.0 (110.0, 139.2) | 125.5 (108.0, 148.0) | 0.074 |
| DBP (mmHg) | 80.0 (70.0, 89.0) | 80.0 (72.0, 90.0) | 81.0 (74.0, 91.0) | 0.004 | 72.0 (60.0, 82.2) | 72.0 (60.0, 86.2) | 74.0 (62.0, 89.0) | 0.324 |
| HR (bpm) | 78.0 (70.0, 88.0) | 79.5 (72.0, 90.2) | 85.0 (76.0, 102.0) | < 0.001 | 93.0 (80.0, 108.0) | 95.0 (83.0, 113.0) | 105.5 (90.0, 122.0) | < 0.001 |
| RR (bpm) | 20.0 (18.0, 20.0) | 20.0 (19.0, 20.0) | 20.0 (19.0, 21.0) | < 0.001 | 21.0 (16.0, 25.0) | 21.0 (18.0, 24.0) | 23.5 (20.0, 28.0) | < 0.001 |
| SpO2 (%) | 99.0 (98.0, 100.0) | 99.0 (97.0, 100.0) | 98.0 (97.0, 100.0) | < 0.001 | N/A | N/A | N/A | N/A |
| P/F | N/A | N/A | N/A | N/A | 266.6 (188.9, 382.0) | 217.9 (156.2, 328.8) | 199.8 (133.8, 295.7) | < 0.001 |
| Ventilation (%) | 35 (6.9) | 39 (7.7) | 84 (16.6) | < 0.001 | 165 (73.7) | 193 (86.2) | 198 (88.4) | < 0.001 |
| Transfusion (%) | 52 (10.3) | 42 (8.3) | 74 (14.7) | 0.005 | 20 (8.9) | 18 (8) | 23 (10.3) | 0.71 |
| CRRT (%) | 16 (3.2) | 23 (4.6) | 53 (10.5) | < 0.001 | 15 (6.7) | 15 (6.7) | 25 (11.2) | 0.138 |
| WBC (× 109/L) | 8.4 (5.7, 12.1) | 9.9 (6.8, 13.6) | 11.1 (7.8, 15.6) | < 0.001 | 10.0 (5.9, 15.2) | 11.4 (7.7, 17.1) | 13.6 (10.4, 19.3) | < 0.001 |
| HGB (g/L) | 117.0 (104.0, 130.0) | 131.0 (118.0, 145.2) | 142.0 (126.0, 155.0) | < 0.001 | 101.0 (86.0, 114.0) | 114.0 (104.0, 128.0) | 129.0 (111.0, 143.0) | < 0.001 |
| HCT (%) | 35.3 (31.7, 38.4) | 39.4 (35.9, 42.5) | 42.1 (37.3, 46.0) | < 0.001 | 30.0 (26.0, 33.6) | 34.5 (31.2, 38.4) | 39.0 (34.1, 43.3) | < 0.001 |
| PCT (ng/ml) | 0.1 (0.0, 0.4) | 0.1 (0.0, 0.6) | 0.5 (0.1, 2.0) | < 0.001 | N/A | N/A | N/A | N/A |
| CRP (mg/L) | 14.3 (3.3, 79.6) | 26.0 (6.2, 103.2) | 106.8 (24.8, 202.9) | < 0.001 | N/A | N/A | N/A | N/A |
| AST (U/L) | 47.0 (23.0, 187.0) | 39.5 (23.0, 135.5) | 39.0 (23.0, 111.0) | 0.108 | 69.5 (36.0, 173.5) | 72.0 (34.0, 230.0) | 75.5 (35.0, 141.0) | 0.922 |
| ALB (g/dl) | 4.1 (3.8, 4.5) | 3.9 (3.6, 4.3) | 3.4 (2.9, 3.8) | < 0.001 | 3.5 (3.1, 3.8) | 3.1 (2.8, 3.4) | 2.5 (2.2, 3.0) | < 0.001 |
| FPG (mmol/L) | 6.5 (5.3, 8.7) | 6.5 (5.1, 9.0) | 7.1 (5.6, 9.9) | 0.001 | 7.2 (5.6, 9.3) | 7.4 (5.4, 10.2) | 7.6(5.5, 10.0) | 0.733 |
| TG (mmol/L) | 1.5 (0.9, 3.2) | 1.5 (1.0, 3.8) | 1.6 (1.0, 3.8) | 0.233 | 1.8(1.1, 3.3) | 1.5 (1.0, 2.7) | 2.2 (1.2, 5.8) | < 0.001 |
| Na (mmol/L) | 139.8 (137.3, 141.9) | 140.0 (137.1, 141.9) | 138.8 (136.0, 141.2) | < 0.001 | 138.0 (135.0, 141.0) | 138.0 (135.0, 141.2) | 138.0 (135.0, 141.0) | 0.984 |
| Ca (mmol/L) | 2.2 (2.1, 2.4) | 2.2 (2.1, 2.4) | 2.1 (1.9, 2.3) | < 0.001 | 2.1 (2.0, 2.2) | 2.1 (1.9, 2.2) | 1.9 (1.7, 2.0) | < 0.001 |
| CREA (µmol/L) | 62.0 (51.0, 76.0) | 68.0 (57.0, 82.8) | 73.0 (60.0, 99.0) | < 0.001 | 97.2 (61.9, 170.2) | 88.4 (61.9, 141.4) | 97.2 (61.9, 176.8) | 0.209 |
| Lac (mmol/L) | N/A | N/A | N/A | N/A | 1.6 (1.1, 2.2) | 1.6 (1.2, 2.6) | 1.9 (1.3, 3.7) | < 0.001 |
| AKI (%) | 69 (13.7) | 67 (13.3) | 121 (24) | < 0.001 | 104 (46.4) | 86 (38.4) | 132 (58.9) | < 0.001 |
| SOFA | 2.4 ± 2.1 | 2.5 ± 2.4 | 3.3 ± 2.9 | < 0.001 | 5.0 (3.0, 8.0) | 4.0 (3.0, 8.0) | 6.5 (3.0, 10.0) | 0.011 |
| Etiology (%) | 0.198 | N/A | N/A | N/A | N/A | |||
| Biliary | 351 (69.5) | 343 (68.1) | 325 (64.4) | |||||
| Hyperlipidemic | 111 (22) | 121 (24) | 122 (24.2) | |||||
| Alcoholic | 11 (2.2) | 16 (3.2) | 24 (4.8) | |||||
| Others | 32 (6.3) | 24 (4.8) | 34 (6.7) | |||||
| Severity (%) | < 0.001 | N/A | N/A | N/A | N/A | |||
| Mild and moderate | 476 (94.3) | 458 (90.9) | 409 (81) | |||||
| Severe | 29 (5.7) | 46 (9.1) | 96 (19) | |||||
| BISAP | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 2.0 (0.0, 2.0) | < 0.001 | N/A | N/A | N/A | N/A |
LOS Length of hospital stay, DM Diabetes mellitus, CHD Coronary heart disease, SBP Systolic blood pressure, DBP Diastolic blood pressure, HR Heart rate, RR Respiratory rate, SpO2 Pulse oxygen saturation, P/F PaO2/FiO2, Ventilation noninvasive or invasive mechanical ventilation, Transfusion blood transfusion, CRRT Continuous renal replacement treatment, WBC white blood cell count, HGB Hemoglobin, HCT Hematocrit, PCT Procalcitonin, CRP C-reactive protein, AST Aspartate aminotransferase, ALB Albumin, FPG Fasting plasma glucose, TG Triglyceride, Na Sodium, Ca Calcium, CREA creatinine, Lac Lactic acid, AKI Acute kidney injury, SOFA Sequential organ failure assessment score, BISAP Bedside Index of Severity in Acute Pancreatitis score, HAR Dividing the hematocrit level (%) by the ALB level (g/dl).
Mean and interquartile range for continuous variables: P value was calculated by weighted linear regression model. % for categorical variables: P value was calculated by weighted chi-square test, P < 0.05 was considered statistically significant.
Univariate and multivariate analyses of HAR and AKI
Univariate analysis showed that age, sex, hypertension, coronary heart disease, systolic blood pressure, diastolic blood pressure, heart rate, respiratory rate, SpO2, severity, etiology, blood transfusion, Ventilation, CRRT, SOFA score, BISAP score, WBC, ALB, TG and Ca2+ were significant confounding factors influencing AKI incidence (p < 0.001) (Supplementary Table S3).
In the multiple logistic regression analysis (Table 3), as compared with the reference tertile of HAR (T2 9.39–10.83), the adjusted OR values for lower tertile of HAR (T1 < 9.39), and higher tertile of HAR (T3 ≥ 10.83) were 1.25(95% CI, 0.82–1.91, p = 0.297) and 1.50 (95% CI, 1.03–2.20, p = 0.037), respectively, were associated with a higher risk of AKI incidence, after adjusting for covariates. All results of two databases are presented in Table 3.
Table 3.
Multiple logistic regression analysis for HAR and AKI in Chinese population and MIMIC-IV database.
| Non-adjusted model | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| OR (95%Cl), P value | OR (95% Cl), P value | OR (95% Cl), P value | OR (95% Cl), P value | |
| Chinese population | ||||
| HAR, Continuous | 1.24 (1.17–1.32), < 0.001 | 1.21(1.13–1.29), < 0.001 | 1.09 (1.02–1.17), 0.013 | 1.1 (1.02–1.18), 0.016 |
| HAR, Categories | ||||
| Tertile 1 | 1.03 (0.72–1.48), 0.863 | 1.20 (0.83–1.74), 0.337 | 1.31 (0.89–1.93), 0.17 | 1.25 (0.82–1.91),0.297 |
| Tertile 2 | Reference | Reference | Reference | Reference |
| Tertile 3 | 2.06 (1.48–2.85), < 0.001 | 1.93 (1.38–2.69), < 0.001 | 1.45 (1.02–2.05), 0.04 | 1.50 (1.03–2.20), 0.037 |
| P for trend | < 0.001 | 0.003 | 0.464 | 0.275 |
| MIMIC-IV population | ||||
| HAR, Continuous | 1.08 (1.03–1.14), 0.002 | 1.08 (1.02–1.13),0.004 | 1.05 (1.00–1.11), 0.06 | 0.98 (0.92–1.05), 0.649 |
| HAR, Categories | ||||
| Tertile 1 | 1.39 (0.95–2.03), 0.086 | 1.43 (0.98–2.09), 0.065 | 1.57 (1.06–2.32), 0.025 | 1.69 (1.07–2.67), 0.024 |
| Tertile 2 | Reference | Reference | Reference | Reference |
| Tertile 3 | 2.30 (1.58–3.36), < 0.001 | 2.30 (1.57–3.38), < 0.001 | 2.08 (1.39–3.12), < 0.001 | 1.66 (1.03–2.68), 0.038 |
| P for trend | 0.008 | 0.014 | 0.201 | 0.826 |
AKI Acute kidney injury, HAR Hematocrit to ALB ratio, T Tertile, OR Odds ratio, 95%CI 95% Confidence interval.
Results for each model are presented as OR (95% CI), P value.
HAR of Chinese population Tertile 1: < 9.39; Tertile 2: 9.39–10.83; Tertile 3: ≥ 10.83.
Model 1 of Chinese population: adjusted for age and sex.
Model 2 of Chinese population: adjusted for age, sex, hypertension, DM, CHD, HR, RR and SpO2.
Model 3 of Chinese population: adjusted for age, sex, hypertension, DM, CHD, HR, RR, SpO2, etiology, blood transfusion, SOFA score, BISAP score, WBC, and FPG.
HAR of MIMIC-IV database Tertile 1: < 10.13; Tertile 2: 10.13–12.79; Tertile 3: ≥ 12.79.
Model 1 of MIMIC-IV database: adjusted for age and sex.
Model 2 of MIMIC-IV database: adjusted for age, sex, hypertension, DM, CHD, HR, RR and P/F.
Model 3 of MIMIC-IV database: adjusted for age, sex, hypertension, DM, CHD, HR, RR, P/F, blood transfusion, SOFA score, Lac, WBC, and FPG.
Smooth curve fitting, threshold effect analyses between HAR and AKI
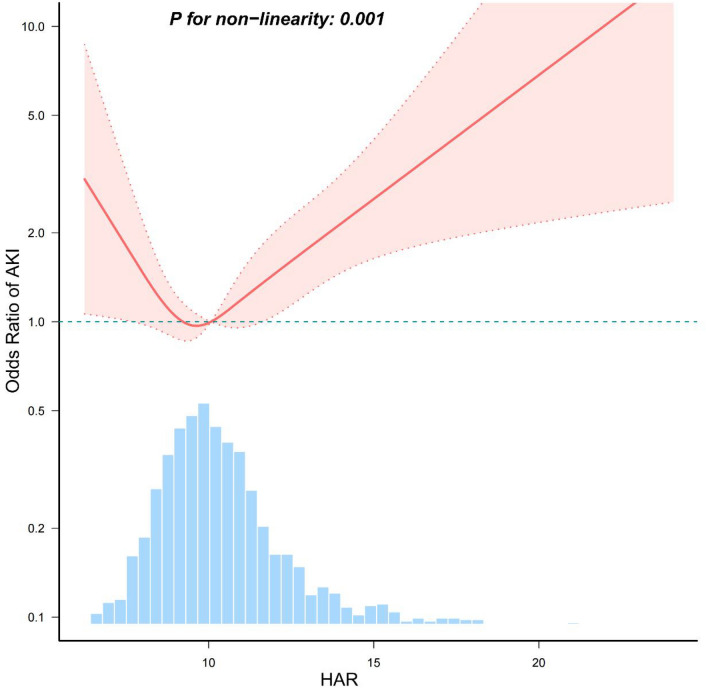
After adjusting for covariates in model 3, we found a J-shaped relationship between HAR and AKI in curve fitting (P for non-linearity < 0.001) (Fig. 2). The data were fitted to a piecewise multiple logistic regression model with two different slopes. In our study, the P value for the likelihood ratio test was 0.029 and 0.001 (Table 4); therefore, we utilized a two-part model to fit the association between serum HAR levels and AKI incidence in patients with acute pancreatitis. We discovered an inflection point at 8.969 in Chinese population. Similarly, in the MIMIC-IV population, a J-shaped relationship between HAR and AKI was identified in curve fitting (P for non-linearity < 0.001) (Supplementary Fig. S1), with an inflection point at 10.257.
Fig. 2.
Restricted cubic spline analysis of nonlinear association between HAR and AKI in patients with acute pancreatitis in the Chinese population. Adjusted for all covariates as model 3. Solid lines represent the odds ratio of AKI and dotted lines represent the corresponding 95% CI. OR = 1 was set as the reference line. (takes the upper limit of 100%).
Table 4.
Threshold effect analysis of HAR and AKI using two-piecewise regression models in two databases.
| Chinese population | MIMIC-IV population | |||
|---|---|---|---|---|
| Two-piecewise linear regression model | OR 95%CI | P value | OR 95%CI | P value |
| Inflection point (K) | 8.969 | 10.257 | ||
| HAR < K | 0.615 (0.38, 0.996) | 0.048 | 0.684 (0.499, 0.937) | 0.018 |
| HAR ≥ K | 1.162 (1.035, 1.304) | 0.011 | 1.256 (1.097, 1.44) | 0.001 |
| Likelihood Ratio test | 0.029 | 0.001 | ||
AKI Acute kidney injury, HAR Hematocrit to albumin ratio, OR Odds ratio, 95%CI 95% Confidence interval.
Subgroup analyses
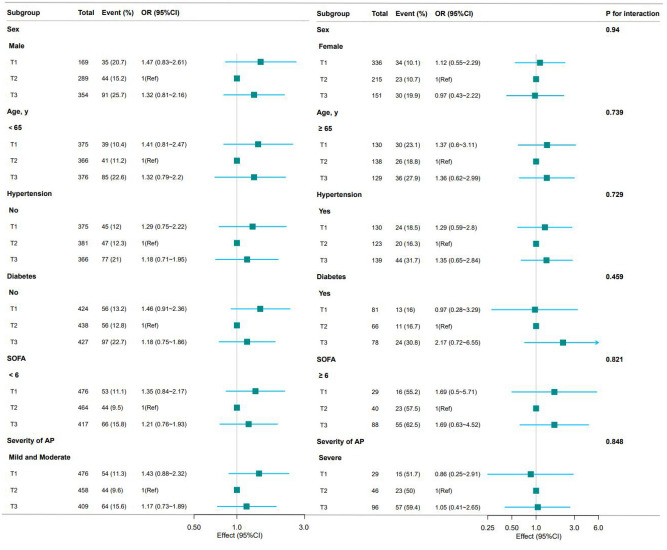
To verify the stability of the J-shaped relationship between HAR and AKI, we performed curve fitting stratified by sex, age, hypertension, DM, SOFA score, and severity of AP, respectively. No significant interactions were found in any subgroups after stratifying by sex, age, hypertension, DM, SOFA score, and severity of AP (all P for interaction > 0.05) (Fig. 3 and Supplementary Fig. S2).
Fig. 3.
Subgroup analyses for the association between HAR and AKI. Each stratified group was adjusted for model 3 (age, sex, hypertension, DM, CHD, HR, RR, SpO2, etiology, blood transfusion, BISAP score, WBC, and FPG).
Discussion
This study investigated the relationship between serum hematocrit-to-albumin ratio (HAR) levels and the incidence of AKI in patients with AP. We found that HAR independently correlates with the occurrence of AKI, demonstrating a J-shaped association curve. Specifically, the incidence of AKI initially decreased and subsequently increased with rising HAR levels. Subgroup analyses and interaction tests revealed that this relationship remained stable across different population groups. To further validate these findings, we conducted an analysis of MIMIC-IV database, which also revealed a J-shaped association between serum HAR levels and AKI incidence.
In healthy individuals, hematocrit (HCT) and plasma albumin (ALB) levels are stable, with HCT ranging from 40 to 45% and ALB from 35 to 45 g/L under normal conditions, respectively26. Elevated HCT levels are associated with a poor prognosis of sepsis, cardiopulmonary bypass surgery and tumor27,28. In AP patients, HCT may increase due to substantial fluid loss, resulting in hemoconcentration and chronic hypoxia. Conversely, reductions in HCT are observed in various pathological states including anemia, bleeding, malnutrition, surgery, trauma, tumors and chronic infections. Additionally, the sole measurement of HCT presents limitations, as various conditions are associated with elevated HCT levels, including dehydration, heat stroke, excessive muscle activity, liver dysfunction, diabetic ketoacidosis, myeloproliferative disorders, and chronic pulmonary diseases.
ALB is critical for maintaining blood volume and plasma colloidal osmotic pressure. Under hypermetabolic conditions, albumin levels may decrease due to reduced synthesis, accelerated catabolism, and increased vascular permeability, which leading to protein leakage29. Various studies have established a significant association between albumin concentrations and inflammation severity, as well as with disease prognosis and mortality rates 15,30,31. However, it is essential to recognize that albumin levels are influenced by factors such as nutritional status, dehydration, and chronic inflammation. These variables can affect the accuracy of prognostic assessments based solely on albumin concentrations.
The capacity index of HCT and ALB has been emphasized in numerous studies32,33. Taking into account these factors, the hematocrit-to-albumin ratio (HCT/ALB) may serve as a more dependable prognostic indicator compared to utilizing each measurement independently. Current studies mainly focus on the differences between HCT and ALB levels (HCT-ALB). Dai et al. proposed that an HCT-ALB level exceeding 12.65 could serve as a potential biomarker for diagnosing hypertensive pregnancy disorders, such as preeclampsia and eclampsia17. Furthermore, Dai et al. found that HCT-ALB levels are elevated in patients with septic shock compared to those with hemorrhagic shock. The HCT-ALB level above 6.8 proves useful in distinguishing these conditions16. Additionally, they also observed that HCT-ALB level above 10.25 might facilitate the rapid diagnosis of severe infections34. Wang et al. confirmed the importance of HCT-ALB levels in predicting the outcomes of elderly sepsis patients, using data from the MIMIC-IV and eICU-CRD databases18. Our research is the first to explore the association between the HAR and AKI in acute pancreatitis, where hematocrit and albumin levels reflect volume status, inflammation, and nutritional condition, which were key elements in AKI pathogenesis.
In individuals diagnosed with acute pancreatitis, the incidence of AKI increases during the acute phase characterized by capillary leakage syndrome (CLS), systemic inflammatory response syndrome (SIRS), hypovolemia. During this phase, individuals with acute pancreatitis encounter pathogenic micro-organisms that trigger the immune system and prompt the release of numerous inflammatory mediators such as tumor necrosis factor and interleukins35. These mediators can compromise the integrity of the vascular endothelial barrier, enhance capillary permeability, and lead to substantial plasma extravasation36. The gap between capillary endothelial cells measures approximately 6–7 nm, while the diameter of albumin is slightly larger at 7.2 nm. This size discrepancy results in substantial extravasation of albumin and water from the vascular to the interstitial spaces, causing to tissue edema and intravascular hypovolemia. Moreover, patients with acute pancreatitis often experience a hypermetabolic state, increasing albumin consumption, while intestinal dysfunction can lead to significant albumin loss, ultimately reducing serum albumin levels.
Conversely, the diameter of red blood cells is much larger, measuring 7–8 µm (about 1000 times larger than the gap between endothelial cells), preventing them from passing through the endothelial gaps and leading to an elevated HCT in the context of hypovolemia. These pathophysiological collectively contribute to the development of pathologic capillary leakage syndrome, which further exacerbates the risk of AKI and results in an elevated HAR37,38. Our findings suggest a J-shaped relationship between HAR and AKI in AP patients, there is higher risk of AKI when HAR exceeds 8.969. However, the inflection point in MIMIC-IV cohort was observed at a HAR value of 10.257. This discrepancy may be attributable to racial differences between the study populations and variations in laboratory testing methodologies.
Overall, the HAR connects the pathophysiologic trajectory of AP by reflecting both the volume status and the SIRI-induced capillary leak syndrome. The severity of AP with CLS is linked to an increased HAR due to hemoconcentration and albumin extravasation. The dynamic shifts in HAR may provide valuable insights for managing AP. An increase in HAR should alert clinicians to consider the possibilities of volume depletion, which may contribute to AKI development. Accordingly, therapeutic measures, including albumin infusion and the restoration of effective circulating blood volume, should be tailored based on HAR levels to improve the prognosis of AKI39,40.
Our study boasts several strengths. First, it is the largest to date that investigates acute pancreatitis-related AKI within the Chinese population, with external validation provided by the MIMIC-IV database. Second, we employed statistical methods to elucidate the curvilinear relationship between HAR levels and AKI risk, moving beyond a simple linear model. We conducted sensitivity analyses based on varying HAR levels, and identified the inflection point via smooth curve fitting instead of arbitrary thresholds categorization.
Our research has certain limitations. Initially, the retrospective data collection at a single academic center could have resulted in selection bias. Secondly, in our current dataset, a significant portion of patients were non-ICU cases, where detailed fluid administration records are often less meticulously maintained, posing challenges in accurately recording fluid balance during resuscitation. Finally, despite the strong association between HAR and AKI, our findings cannot establish causality due to the observational nature of the study. More prospective studies are necessary to confirm these relationships.
Conclusions
This study discovered a significant correlation between the serum HAR and the incidence of AKI. The relationship exhibited a J-shaped curve, with an inflection point at 8.969. Further research is essential to validate these results and elucidate the underlying mechanisms of the HAR-AKI association.
Supplementary Information
Acknowledgements
We extend our gratitude to Dr. Jie Liu from the Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital, for his insightful review and comments on the manuscript. We also thank Mingzi Tao and Tao Wu for their contributions to structured data extraction.
Author contributions
Wen Wu and Yupei Zhang conceived, designed the study and obtained the data, which was analyzed by Wen Wu and Chunzhen Zhang. Yupei Zhang and Wen Wu interpreted the data and results and drafted the manuscript. Zhaohui Zhang and Xingguang Qu critically revised the manuscript for intellectual content. All authors contributed to revising the article and approved the final version.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data is provided within the manuscript or supplementary information files.
Competing interests
The authors declare no competing interests.
Ethics approval
This retrospective study was approved by the Ethics Committee of the First College of Clinical Medical Science of China Three Gorges University (ethical approval number: 2023-130-01), and informed consent was waived. This study was conducted in accordance with the Declaration of Helsinki.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wen Wu and Yupei Zhang have contributed equally to this paper as co-first author.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77842-4.
References
- 1.Lee, P. J. & Papachristou, G. I. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol.16, 479–496 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Whitcomb, D. C. Acute pancreatitis. N Engl. J. Med.354(20), 2142–2150 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Banks, P. A. et al. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut62, 102–111 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Silva-Vaz, P. et al. Murine models of acute pancreatitis: A critical appraisal of clinical relevance. IJMS20, 2794 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Dijk, S. M. et al. Acute pancreatitis: Recent advances through randomised trials. Gut66, 2024–2032 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Singh, V. K. et al. An assessment of the severity of interstitial pancreatitis. Clin. Gastroenterol. Hepatol.9, 1098–1103 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Wu, B. U. et al. Dynamic measurement of disease activity in acute pancreatitis: The pancreatitis activity scoring system. Am. J. Gastroenterol.112, 1144–1152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan, M. et al. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobiliary Pancreat Dis. Int.16, 424–430 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Lin, H.-Y. et al. Acute renal failure in severe pancreatitis: A population-based study. Upsala J. Med. Sci.116, 155–159 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamada, S., Masamune, A. & Shimosegawa, T. Management of acute pancreatitis in Japan: Analysis of nationwide epidemiological survey. WJG22, 6335 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu, L. et al. The systemic immune-inflammation index may be a novel and strong marker for the accurate early prediction of acute kidney injury in severe acute pancreatitis patients. J. Invest. Surg.35, 962–966 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Yang, D. et al. Development and validation of a predictive model for acute kidney injury in patients with moderately severe and severe acute pancreatitis. Clin. Exp. Nephrol.26, 770–787 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Block, S., Büchler, M., Bittner, R. & Beger, H. G. Sepsis indicators in acute pancreatitis. Pancreas2, 499–505 (1987). [DOI] [PubMed] [Google Scholar]
- 14.Juncal, V. R., Britto Neto, L. A. D., Camelier, A. A., Messeder, O. H. C. & Farias, A. M. D. C. Impacto clínico do diagnóstico de sepse à admissão em UTI de um hospital privado em Salvador. Bahia. J. Bras. pneumol.37, 85–92 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Ni, T. et al. Association between albumin or prealbumin levels at different stages and prognosis in severe acute pancreatitis: a 5-year retrospective study. Sci. Rep.12, 16792 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai, D. et al. Feasibility study of the difference between hematocrit and albumin for identifying hemorrhagic shock and septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue30, 1137–1140 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Dai, D.-M. et al. Hematocrit and plasma albumin levels difference may be a potential biomarker to discriminate preeclampsia and eclampsia in patients with hypertensive disorders of pregnancy. Clin. Chim. Acta464, 218–222 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Wang, Z. et al. The relationship between hematocrit and serum albumin levels difference and mortality in elderly sepsis patients in intensive care units—a retrospective study based on two large database. BMC Infect Dis.22, 629 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyer, G. et al. Clinical practice guideline: Acute and chronic pancreatitis. Deutsches Ärzteblatt Int.119, 495–501 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou, W., Dong, S., Chen, Z., Li, X. & Jiang, W. New challenges for microRNAs in acute pancreatitis: Progress and treatment. J. Transl. Med.20, 192 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract.120, c179–c184 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Annane, D. et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl. J. Med.378, 809–818 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Hagjer, S. & Kumar, N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis—a prospective observational study. Int. J. Surg.54, 76–81 (2018). [DOI] [PubMed] [Google Scholar]
- 24.von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet370, 1453–1457 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Zhang, Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann. Transl. Med.4, 30 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vercueil, A., Grocott, M. P. W. & Mythen, M. G. Physiology, pharmacology, and rationale for colloid administration for the maintenance of effective hemodynamic stability in critically Ill patients. Transfus. Med. Rev.19, 93–109 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Hannan, E. L. et al. Predictors of postoperative hematocrit and association of hematocrit with adverse outcomes for coronary artery bypass graft surgery patients with cardiopulmonary bypass. J. Cardiac Surg.25, 638–646 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Peng, D., Li, Z.-W., Liu, F., Liu, X.-R. & Wang, C.-Y. Predictive value of red blood cell distribution width and hematocrit for short-term outcomes and prognosis in colorectal cancer patients undergoing radical surgery. World J. Gastroenterol.30, 1714–1726 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braamskamp, M. J. A. M., Dolman, K. M. & Tabbers, M. M. Clinical practice: Protein-losing enteropathy in children. Eur. J. Pediatr.169, 1179–1185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, H. J. & Lee, H. W. Important predictor of mortality in patients with end-stage liver disease. Clin. Mol. Hepatol.19, 105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu, H., Zheng, X., Ai, J. & Yang, L. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: A systematic review and meta-analysis of 13,110 patients. Int. Immunopharmacol.114, 109496 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Ling, H. Z. et al. Calculated plasma volume status and prognosis in chronic heart failure. Eu. J. Heart Fail17, 35–43 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Crosignani, A. et al. Intravenous fluid therapy in patients with severe acute pancreatitis admitted to the intensive care unit: A narrative review. Ann. Intensive Care12, 98 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai, D.-M. et al. Difference in hematocrit and plasma albumin levels as an additional biomarker in the diagnosis of infectious disease. Aoms16, 522–530 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minasyan, H. Sepsis and septic shock: Pathogenesis and treatment perspectives. J. Crit. Care40, 229–242 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Berg, D. & Gerlach, H. Recent advances in understanding and managing sepsis. F1000Res7, 1570 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orfanos, S. E. et al. Angiopoietin-2 is increased in severe sepsis: Correlation with inflammatory mediators. Crit. Care Med.35(1), 199–206 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Ghim, M. et al. Visualization of three pathways for macromolecule transport across cultured endothelium and their modification by flow. Am. J. Physiol.-Heart Circul. Physiol.313, H959–H973 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao, L., Huang, Y., He, T. & Hu, X. Clinical study on hematocrit used as a predictor for evaluation of resuscitation effect in the early shock stage after burn. Zhonghua Shao Shang Za Zhi29, 235–238 (2013). [PubMed] [Google Scholar]
- 40.Li, L. et al. Early rapid fluid therapy is associated with increased rate of noninvasive positive-pressure ventilation in hemoconcentrated patients with severe acute pancreatitis. Dig. Dis. Sci.65, 2700–2711 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.