Abstract
Background
Heart donation (HD) by those with death determination by circulatory criteria (DDCC) has been proposed as a method to increase the heart donor pool in response to the growing need for heart transplantation (HT). However, the potential level of HD after DDCC in the province of Québec has not yet been reported. This study aims to assess the suitability for HD among donors with DDCC, and to estimate its impact on HT activity.
Methods
Donation records by those with DDCC in the province of Québec, from January 2016 to December 2020, were retrospectively reviewed for donor and predonation characteristics. Predetermined exclusion criteria were used to evaluate eligibility for HD.
Results
Of the 122 patients with DDCC who were included, 42 (34%) were identified as potentially-eligible heart donors. The median age of potentially-eligible donors was 52 years; 60% were female; and the most prevalent causes leading to organ donation in this group were medical aid in dying (26%), traumatic brain injury (26%), and anoxia (24%). A 19% increase (42 of 225) in potential HT activity was estimated using strict criteria. In only one case did functional warm ischemia time exceed the 30-minute limit.
Conclusions
Using those with DDCC as a new source of heart donors can significantly increase the volume of heart donation in the province of Québec. Implementing an HD program for those with DDCC in Québec may reduce waiting time and increase the number of heart recipients.
RÉsumÉ
Contexte
Le don de cœur d’une personne déclarée décédée selon des critères circulatoires est proposé comme moyen d’accroître le bassin de donneurs pour répondre aux besoins croissants en transplantation cardiaque. Aucun rapport ne fait néanmoins état à ce jour du nombre potentiel de dons de cœur après détermination du décès selon des critères circulatoires dans la province du Québec. Cette étude vise à évaluer l’adéquation du don de cœur par des personnes déclarées décédées selon des critères circulatoires et à estimer son impact sur l’activité dans le domaine de la transplantation cardiaque.
Méthodologie
Les dossiers de donneurs déclarés décédés selon des critères circulatoires dans la province du Québec entre janvier 2016 et décembre 2020 ont été examinés de manière rétrospective afin de connaître les caractéristiques des donneurs et les caractéristiques avant le don. Des critères d’exclusion préétablis ont été utilisés pour évaluer l’admissibilité au don de cœur.
Résultats
Parmi les 122 personnes incluses dont le décès a été déclaré selon des critères circulatoires, 42 (34 %) ont été jugées potentiellement admissibles au don de cœur. L’âge médian des donneurs potentiels était de 52 ans; 60 % étaient des femmes, et les raisons ayant mené le plus souvent au don d’organes dans ce groupe ont été l’aide médicale à mourir (26 %), le traumatisme cérébral (26 %) et l’anoxie (24 %). La hausse de l’activité dans le domaine de la transplantation cardiaque (42 sur 225) a été estimée à 19 % en se fondant sur des critères rigoureux. Dans un seul cas, le temps d’ischémie chaude fonctionnelle a dépassé la limite des 30 minutes.
Conclusions
Utiliser des personnes déclarées décédées selon des critères circulatoires comme nouvelle source de donneurs cardiaques permettrait d’augmenter considérablement le volume de dons de cœur au Québec. Mettre sur pied un programme québécois de don de cœur ciblant ces personnes permettrait de réduire le temps d’attente et d’augmenter le nombre de receveurs d’une greffe.
Receiving a heart transplant (HT) can greatly improve the life expectancy and quality of life in selected patients with end-stage heart failure.1 However, access to HT is limited by donor-heart availability, which in Canada is restricted to donations that occur after neurologic determination of death (NDD). An organ shortage has led to increased waiting times. In the past decade, in the province of Québec, the average waiting time for an HT has varied between 231 days (2018 and 2019) and 342 days (2022).2 As a result of these long waiting times, as many as 1 in 4 Canadian adults on the HT waiting list either die, or become ineligible for an HT, due to worsening of their condition.3 The volume of heart donation (HD) is expected to remain stable, whereas the need for HT likely will increase as terminal heart failure becomes more prevalent.4 This situation has led to renewed international interest in HD after death determination by circulatory criteria (DDCC), to expand the donor pool. In the US, 5% of HTs are performed following HD by those with DDCC,5 and this percentage is expected to increase as programs develop more experience with donation by those with DDCC.
In Canada, donors with DDCC for lungs, livers, and kidneys account for 20% of deceased donors and have allowed for a significant increase in organ transplantation.3 Donation by those with DDCC increasingly is becoming the standard-of-care. From 2009 to 2018, donation by those with DDCC grew by over 400%.6 However, HD after DDCC has not been implemented yet, due to concerns over ischemic injury and ethical issues. Several countries have since performed HTs with hearts from donors with DDCC, with outcomes similar to those for HTs performed with hearts from donors with NDD .7 In light of these encouraging results and the nationwide support8,9 for HD by those with DDCC, implementing a program of HD by those with DDCC is worth considering. However, more knowledge is needed about the potential for HD by those with DDCC in Canada and whether it would significantly increase HT activity. Internationally, studies report many unused hearts after DDCC,10 and an estimated 11%-22% of donors with DDCC could be considered for HD.11, 12, 13 Such consideration potentially could lead to a 56% increase in HT activity,14, 15, 16 which could decrease the incidence of waiting-list mortality by 40%.13 As features vary among regions, a clearer picture of the potential for HD by those with DDCC, specifically in Canada, is needed to assess the relevance of implementing such a program and to set up a system capable of managing these donations.
This study aims to determine the number of those with DDCC who are potentially suitable for HD, and to evaluate the potential for an increase in cardiac graft offers and HT. Given that organ donation in Canada is overseen by provincial entities, we focus here on the province of Québec.
Material and Methods
This study was approved by the Montreal Heart Institute and the Transplant Québec Institutional Review Boards (#2022-3081).
Study Design
This cross-sectional study included all donors with DDCC aged ≤ 60 years who were referred to Transplant Québec for organ donation from a Québec healthcare facility from January 1, 2016 to December 31, 2020. Donors with a history of malignancy that had not prevented organ donation were included. The Transplant Québec database was used to collect anonymized demographic, clinical, and perioperative data.
Data and Definitions
Suitability for HD was assessed based on the exclusion criteria described in the literature and the consensus statement.17, 18, 19 The exclusion criteria included the following: history of heart disease; inotropic support before procurement; elevated cardiac enzyme levels; insulin-dependent diabetes; history of malignancy; viral hepatitis; active smoking; uncontrolled high blood pressure; and a predicted functional warm ischemia time (FWIT) of > 30 minutes.
As previously defined, the FWIT is the time from when systolic blood pressure (SBP) drops to < 50 mm Hg until heart cardioplegia occurs or reperfusion starts.20,21 Given that no heart procurement was performed, the heart perfusion time was extrapolated using the literature. Experience with DCD has described the time required to establish reperfusion after the skin incision as varying from 1 to 6 minutes.22,23 Based on these reports, we calculated the FWIT by adding 5 minutes to the time between SBP being < 50 mm Hg and the skin incision, to simulate the time required to reestablish heart perfusion. The limit for an acceptable FWIT was set at 30 minutes, based on clinical experience with HD by those with DDCC.24
Statistics
Continuous data with a normal distribution are expressed with means and standard deviations, whereas continuous data with a non-normal distribution are presented with medians and interquartile ranges. Continuous variables were compared between groups using the Mann-Whitney U test. Categorical variables are expressed as count and percentages, and were compared using the χ2 test. Donor characteristics are presented for all included donors with DDCC, to allow for an overview of current donation practice with such donors in Québec. A separate analysis of donors potentially eligible for HD also is provided. The expected impact of HD by donors with DDCC on HT activity for different combinations of eligibility criteria is discussed.
Results
HD activity by those with DDCC and potential for HD in Québec
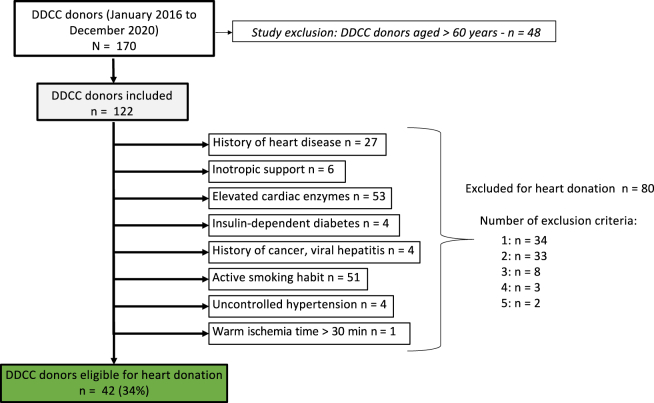
During the study period, 268 patients were referred for donation after DDCC in the province of Québec. Among them, 76 were declared ineligible for organ donation upon further investigation, and 22 did not die following the withdrawal of life-sustaining therapies (WLST). Organs were procured from 170 donors, of whom 122 were aged ≤ 60 years and were included in the analysis (Fig. 1). After applying selection and/or exclusion criteria, 42 of 122 patients (34%) were deemed eligible for HD. The remaining 80 potential donors were excluded based on the prespecified exclusion criteria. Of these, 53 had elevated cardiac enzymes (66%), 51 were active smokers (63%), and 27 had a known history of heart disease (34%). Six potential donors (8%) were excluded based on their having received inotropic support or high dosages of norepinephrine (≥ 0.2 mcg/kg/min) or epinephrine (> 0.05 mcg/kg/min) within 24 hours of initiation of procurement. Other criteria, including insulin-dependent diabetes, uncontrolled hypertension, and viral hepatitis, made less-selective exclusions, of 4 donors (5%) each. Only one donor (1%) had a predicted FWIT > 30 minutes.
Figure 1.
Selection of donors with death determination by circulatory criteria (DDCC) who are suitable for heart donation. Elevated cardiac enzyme levels were defined as follows: troponin-T ≥ 0.03 ng/mL, troponin-I ≥ 0.1 ng/mL, troponin-high sensitivity ≥ 14 ng/L, or creatine-kinase-myocardial band > 5%. Heart disease was defined as follows: left ventricular ejection fraction ≤ 50%, coronary artery disease, congenital heart disease, history of heart failure, or significant valvulopathy. Inotropic support was defined as follows: norepinephrine ≥ 0.2 mcg/kg/min or epinephrine > 0.05 mcg/kg/min or any dose of dobutamine or milrinone, within 24 hours of withdrawal of life-sustaining therapies.
Predonation investigations
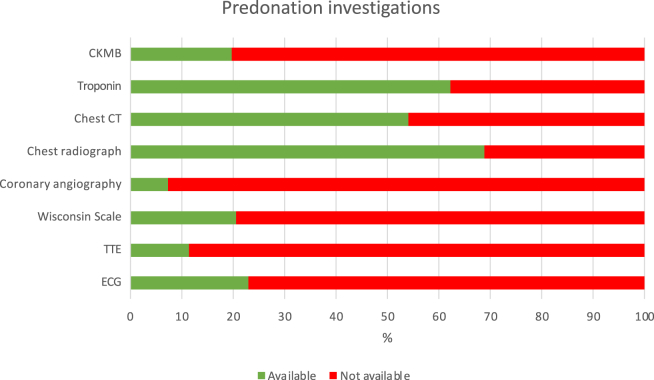
Information regarding smoking habits, viral hepatitis status, diabetes, and inotropic support in the 24 hours preceding the WLST was available for all donors (Fig. 2). Most donation records included chest computed tomography and chest radiograph reports, cardiac enzyme data, and a history of hypertension. Cardiac screening was included in only a minority of records. Overall, 7% of records included coronary angiography, 12% included a transthoracic echocardiogram, and 23% included an electrocardiogram. Similarly, data on vital signs immediately preceding WLST were present in only 40% of donation records. In 14 cases, detailed blood pressure monitoring was unavailable, preventing FWIT prediction.
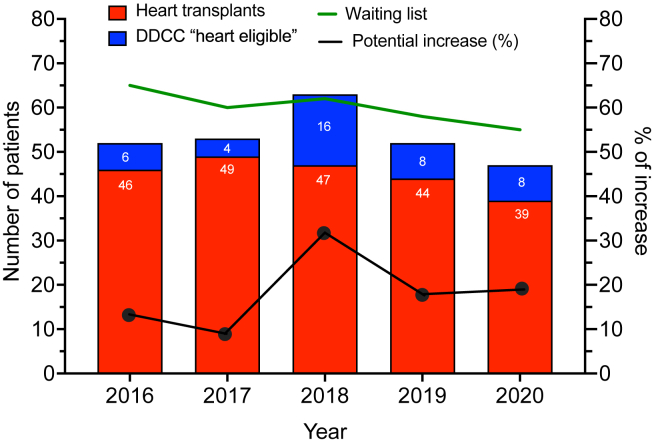
Figure 2.
Predicted increase in heart donation from donors with death determination by circulatory criteria (DDCC; “heart eligible”), compared to the recorded heart transplant activity and the number of patients on the heart transplant waitlist each year.
Portrait of potentially eligible donors with DDCC
Demographic and clinical characteristics for eligible and ineligible heart donors are reported in Table 1. Of the potentially eligible donors, 60% were female, compared to 25% in the ineligible group. Although donors aged > 50 years made up most of the potentially-eligible group, 8 (19%) were aged 41-50 years, 4 (10%) were aged 31-40 years, and 5 (12%) were aged < 31 years. The 3 diabetic donors in this group were on oral therapy. A total of 12 donors had a prior history of smoking, with half of these having ceased smoking for up to 10 years, and the other half having done so for > 10 years. Compared to ineligible donors, the group who were potentially eligible for HD tended to have a lower percentage of drug use, cocaine consumption, and history of cardiac arrest.
Table 1.
Characteristics of donors with death determination by circulatory criteria, and comparison between donors eligible vs ineligible for heart donation
| Characteristic | All (n = 122) | Eligible donors (n = 42) | Ineligible donors (n = 80) | P |
|---|---|---|---|---|
| Age, median (IQR) | 48 (18.8) | 52 (13.0) | 46 (20) | 0.155 |
| Gender | 0.003 | |||
| Female | 45 (36.9) | 25 (59.5) | 20 (25.0) | |
| Blood type | ||||
| A | 58 (47.5) | 18 (42.9) | 40 (50.0) | 0.587 |
| B | 11 (9.0) | 3 (7.1) | 8 (10.0) | 0.668 |
| AB | 4 (3.3) | 3 (7.1) | 1 (1.3) | 0.088 |
| O | 49 (40.2) | 18 (42.9) | 31 (38.8) | 0.734 |
| Body mass index, kg/m^2, median (IQR) | 24.9 (8.1) | 24.4 (7.8) | 25.7 (7.5) | 0.025 |
| Body surface area, m^2, median (IQR) | 1.9 (0.3) | 1.7 (0.3) | 1.9 (0.3) | 0.001 |
| Hypertension | 26 (21.3) | 7 (16.7) | 19 (23.8) | 0.421 |
| Hyperlipidemia | 14 (11.5) | 3 (7.1) | 11 (13.8) | 0.310 |
| Diabetes | 10 (8.2) | 3 (7.1) | 7 (8.8) | 0.768 |
| Chronic kidney disease | 1 (0.8) | 0 (0.0) | 1 (1.3) | 0.469 |
| Smoking | 75 (61.5) | 12 (28.6) | 63 (78.8) | 0.001 |
| Pack-year, median (IQR) | 15.0 (20.5) | 7.0 (8.6) | 18.8 (21.8) | 0.003 |
| Alcohol consumption | 99 (81.1) | 31 (73.8) | 68 (85.0) | 0.514 |
| Recreational drug use | 57 (46.7) | 12 (28.6) | 45 (56.3) | 0.033 |
| Cocaine | 16 (13.1) | 2 (4.8) | 14 (17.5) | 0.065 |
| Heart disease | 18 (14.8) | 0 (0.0) | 18 (22.5) | 0.002 |
| History of cardiac arrest | 44 (36.1) | 10 (23.8) | 34 (42.5) | 0.102 |
| Malignancy | 5 (4.1) | 3 (7.1) | 2 (2.5) | 0.229 |
| Cause leading to organ donation | ||||
| Cerebrovascular accident | 15 (12.3) | 2 (4.8) | 13 (16.3) | 0.086 |
| Anoxia | 41 (33.6) | 10 (23.8) | 31 (38.8) | 0.176 |
| Intracranial hemorrhage | 23 (18.9) | 7 (16.7) | 16 (20.0) | 0.687 |
| Traumatic brain injury | 26 (21.3) | 11 (26.2) | 15 (18.8) | 0.398 |
| Medical aid in dying | 15 (12.3) | 11 (26.2) | 4 (5.0) | 0.002 |
| Other | 2 (1.6) | 1 (2.4) | 1 (1.3) | 0.643 |
Values are n (%), unless otherwise indicated.
IQR, interquartile range.
Fifteen of the donors with DDCC (15 of 122; 12%) received medical aid in dying (MAID), of whom 8 (50%) had amyotrophic lateral sclerosis. Others had advanced Parkinson’s disease (13%), multiple-system atrophy (13%), corticobasal degeneration (7%), multiple sclerosis (7%) or severe chronic obstructive pulmonary disease (7%). Eleven of these 15 patients who received MAID (73%) were identified as potential heart donors. In this subgroup, the median age was 56 years, and 73% were female. The median body mass index (20.4 kg/m2) was lower, compared to that in other groups. Overall, this subgroup presented a more favourable cardiovascular risk profile—none had a history of hypertension, hyperlipidemia, or diabetes, and none had smoked in the 10 years preceding organ donation.
The HDs of those 42 with DDCC who were identified as potentially eligible donors represent 4-16 additional cardiac offers per year that could have been provided (Fig. 2). Compared to the number of recorded HTs during the study period (n = 225), inclusion of those with DDCC in the pool of those with potential HD eligibility could have increased overall HT activity by 19% (range: 8%-34%). Had this practice been implemented, the highest increase in HD would have occurred in 2018.
Donation process and FWIT
Organs were retrieved in 28 centres. A total of 34% of all donations, and 45% of donations in the potentially-eligible group, took place in facilities with cardiac surgery capacity available onsite. Although nearly all donors donated at least one kidney, only a minority were lung or liver donors. Table 2 lists hospitalization characteristics (duration of intensive- or palliative-care stay and type of respiratory support required) and donation logistics, including location of WLST, use of invasive blood pressure monitoring, and premortem administration of heparin.
Table 2.
Organ donation by donors with death determination by circulatory criteria: logistics and timeline of organ procurement
| Logistic and timeline of organ donation after circulatory death | All (n = 122) | Eligible donors (n = 42) | Ineligible donors (n = 80) | P |
|---|---|---|---|---|
| Duration of ICU or PCU stay, median (IQR) | 7.0 (7.8) | 7.5 (7.8) | 7.0 (6) | 0.613 |
| Respiratory support | 105 (86.1) | 30 (71.4) | 75 (93.8) | 0.206 |
| Endotracheal intubation | 100 (82.0) | 25 (59.5) | 75 (93.8) | 0.050 |
| Tracheostomy | 5 (4.1) | 5 (11.9) | 0 (0.0) | 0.002 |
| Duration of mechanical ventilation support, d, median (IQR) | 7.0 (6.2) | 8.0 (7.1) | 6.3 (6) | 0.086 |
| Antemortem administration of heparin | 118 (96.7) | 42 (100.0) | 76 (95.0) | 0.790 |
| Invasive blood pressure monitoring | ||||
| Yes | 32 (26.2) | 8 (19.0) | 24 (30.0) | 0.261 |
| Unknown | 80 (65.6) | 28 (66.7) | 52 (65.0) | 0.914 |
| Location of WLST | ||||
| Operating room | 79 (64.8) | 24 (57.1) | 55 (68.8) | 0.450 |
| Other | 6 (4.9) | 5 (11.9) | 1 (1.3) | 0.011 |
| Unknown | 37 (30.3) | 13 (31.0) | 24 (30.0) | 0.928 |
| Wisconsin Scale, median (IQR) | 14 (3.0) | 12 (4.8) | 14 (2.0) | 0.186 |
| Distance to centre of organ transplantation, km, median (IQR) | ||||
| Distance to Montreal Heart Institute | 44.0 (224.8) | 14.0 (227.0) | 91.5 (200.2) | 0.664 |
| Distance to Québec Heart and Lung Institute | 241.5 (111.2) | 243 (60.5) | 240.0 (136) | 0.310 |
| Distance to McGill University Health Centre | 43.0 (226.8) | 17.0 (227.5) | 13.0 (200.2) | 0.994 |
| Distance to nearest transplant centre, km, median (IQR) | 13.0 (114.0) | 13.0 (126.0) | 13.0 (114.0) | 0.558 |
| Organs retrieved | ||||
| Kidney | 116 (95.1) | 39 (92.9) | 96.3 (n = 77) | 0.855 |
| Liver | 32 (26.2) | 14 (33.3) | 22.5 (n = 18) | 0.270 |
| Lung | 57 (46.7) | 24 (57.1) | 41.3 (n = 33) | 0.222 |
| Number of organs retrieved per donor | ||||
| Median (IQR) | 2 (1) | 2 (1) | 1 (1) | 0.010 |
| 1 | 58 (47.5) | 16 (38.1) | 42 (52.5) | 0.273 |
| 2 | 45 (36.9) | 17 (40.5) | 28 (35.0) | 0.636 |
| 3 | 19 (15.6) | 9 (21.4) | 10 (12.5) | 0.235 |
| WLST to SBP < 50 mm Hg, min, median (IQR) | 13 (10) | 11.5 (10) | 13.5 (8.75) | 0.408 |
| SBP < 50 mm Hg to asystole, min, median (IQR) | 2 (4.25) | 2.5 (5) | 2 (4) | 0.723 |
| Asystole to skin incision, min, median (IQR) | 5 (1) | 6 (2) | 5 (1) | 0.419 |
| Predicted FWIT duration, min, median (IQR) | 13 (5) | 14 (6.5) | 13 (4) | 0.362 |
Values are n (%), unless otherwise indicated.
FWIT, functional warm ischemic time; ICU, intensive-care unit; IQR, interquartile range; PCU, palliative-care unit; SBP, systolic blood pressure; WLST, withdrawal of life-sustaining therapies.
Table 2 also reports the median intervals for the various stages of the donation process, including the predicted duration of FWIT. The time of the first record of SBP being < 50 mm Hg was considered the start of the FWIT, although in many cases, SBP fluctuated, to < and > 50 mm Hg, until asystole. FWIT was able to be estimated for 108 of the 122 donors. SBP monitoring was unavailable for 10 donors who received MAID, but the time between WLST and asystole was available for all donors who received MAID and ranged from 4-23 minutes. For 62% of donors who did not receive MAID, the skin incision occurred immediately following the mandatory 5-minute standoff period. A 2-to-6-minute delay between declaration of death and skin incision was observed in all cases receiving MAID.
Discussion
Using strict selection criteria, one-third of those with DDCC who became donors and were aged ≤ 60 years (42 of 122; 34%) were deemed potentially eligible for HD in the province of Québec between 2016 and 2020. Depending on the years, this represents 4 (in 2017) to 16 (in 2018) additional hearts that would have been available for transplantation (Fig. 2). During the study period, 225 HTs were performed in the province of Québec.17 If all the additional hearts had been transplanted, this would have increased HT activity by 19%.
These findings are consistent with results of studies from some European countries and the US. Studies that focused on potentially-eligible donors estimated an increase in HT activity of 30%,15 40%,13 56%,16 or 100%.12 Osaki et al. expected a 17% rise in HD when including only those donor records with an electrocardiogram or a coronary angiography.11 Of these studies, 2 calculated the percentage of actual heart donors with DDCC, compared to the percentage of all donors with NDD. One reported a proportion of 24.3%-36.6%10; the other reported 37%.14 These results are in line with the proportion of potentially-eligible donors identified in the present study and suggest that the potential for HD may be comparable among patients with DDCC and those with NDD.
The variability observed among studies may result from differences in the selection criteria, and from regional and annual differences in HT activity with donors with NDD. The upper age limit varies from 45-65 years.10, 11, 12, 13, 14, 15, 16 Although the definition of the FWIT has some variability, the consensus is that the maximum duration is 30 minutes.11, 12, 13, 14, 15, 16 Earlier studies relied on donation warm ischemic time, which began at the WLST, and ended at the time of aortic perfusion,13 reperfusion of other organs,11 or aortic cross-clamping.14 FWIT was used in recent studies and was defined as the time between recording of SBP at < 50 mm Hg and cardioplegic solution administration12 or abdominal aortic perfusion.16 We established FWIT using an estimated reperfusion time based on the time required in practice to perfuse the heart, as reported in the available literature 22,23 As previously highlighted by Messer et al,16 a significant delay between WSLT and hemodynamic instability was observed. Thus, the donation warm ischemic time is likely less representative of ischemic injury than is the FWIT, and it may underestimate the potential for HD following DDCC.
The selection of criteria for HD in our study were based on publications from experienced teams and are purposefully restrictive.17, 18, 19 Other combinations of exclusion criteria were tested. When only those aged ≤ 40 or ≤ 50 years were considered, 9 and 17, respectively, were potentially eligible for HD. Including donors with insulin-dependent diabetes did not affect the potential for HD among donors with DDCC. Removing the exclusion criteria of “elevated cardiac enzymes” or “active smoking” resulted in an overall 24% and 26% increase, respectively, in potential HT activity, compared to the number of HTs with donors with DDCC recorded for each year. When both criteria were removed, this increase reached 42% for the study period.
Another important finding of our study is that only a very small proportion of those with DDCC who became donors had cardiac imaging results (echocardiography, coronary angiogram, electrocardiogram) available.
This observation is consistent with findings in other reports and can be explained by the absence of use of the heart in this population. Given that HD after DDCC was not an option at the time of donation, assessing and compiling information on cardiac function would not have been of clinical interest. One study reported that only 17.3% of donor records included an electrocardiogram,10 and another that only 1% of donors with DDCC had undergone cardiac assessment.12 Authors responded to this limitation either by including only donors with complete records, or by opting for exclusion criteria instead of inclusion criteria to consider donors with missing information. Although the former option may select ideal heart donors with DDCC, the latter, presented here, aims to estimate the number of donors potentially eligible for HD.
With one exception, all ineligible donors were excluded from HD based on preexisting conditions and cardiovascular risk factors unrelated to donation after DDCC. Of the 108 records with detailed per-donation hemodynamic monitoring, only one presented with an estimated FWIT over the 30-minute limit. This donor was a patient who received MAID and who progressed from a SBP < 50 mm Hg to asystole in 18 minutes. The 5-minute standoff period was followed by a 6-minute delay before the skin incision, likely due to transportation to the operating room. Had this delay been avoided, this donor would have been included in the potentially-eligible group, and no donor would have been excluded from HD based on the FWIT. Furthermore, most of those with DDCC who became donors had an estimated FWIT of 10-15 minutes, and only 2% had an FWIT of 26-30 minutes.
In Canada and the province of Québec, the number of patients requiring MAID is increasing drastically. In the province of Québec, 494 deaths accompanied by MAID were recorded in 2016, and 2020 alone saw 2268 deaths accompanied by MAID.25 Of the 6453 patients who received MAID between 2016 and 2020, 31 (0.5%) became organ donors, and half were aged ≤ 60 years.25 Advanced age, underlying medical conditions preventing organ donation, lack of awareness of the possibility for organ donation, and factors related to the donation process, including hospitalization and extensive predonation investigations, may account for the small proportion of organ donation in patients who receive MAID. Moreover, donations from those who received MAID have since increased, accounting for 15% of organ donations.2 Although only 15 donors who received MAID were included in this study, a point worth noting is that 73% of these donors (11 of 15) were suitable for HD in this study.
Cardiovascular risk factors were less prevalent in donors who received MAID, which may account for the greater proportion of potentially-eligible heart donors identified in this group. Nonetheless, HD by patients who receive MAID may be complex, as premortem cardiac assessment likely would require invasive investigative procedures, such as coronary angiography, which patients may deem unacceptable. The need for invasive monitoring to establish the FWIT may be another barrier to HD. On the other hand, the possibility of becoming a donor may be appealing for some patients who receive MAID and their caregivers. These issues should be discussed thoroughly with patients who receive MAID.
Donors who received MAID tended to be older, with a median age of 56 years in this group, compared to a median age of 46 years in the group that did not receive MAID. This finding is not surprising, as the underlying conditions leading patients to request MAID as observed here (mainly degenerative neurologic diseases), become incapacitating at an older age. However, this change with age may reduce their eligibility for HD in practice, as the prevalence of cardiovascular diseases increases with age. On the other hand, 60% of donors who received MAID were female, and they therefore might present a more favourable cardiovascular profile. If deemed ethically acceptable, predonation cardiac investigations (coronary angiography and cardiac echocardiography) would help determine a donor’s suitability for HD.
Nonetheless, this group remains of interest, considering the particular context of use of MAID in Canada. As presented above, the country has seen an exponential increase in deaths accompanied by MAID since its legalization less than a decade ago. The province of Québec even holds the record for having the highest proportionate mortality of deaths accompanied by MAID, internationally.26 Canada has been at the forefront of discussions surrounding specific MAID-related issues, such as MAID for those with mental illness, MAID in the absence of a patient’s foreseeable natural death, and MAID advance requests. Therefore, the importance of MAID in Canada cannot be ignored, nor can its impact on organ donation.
Study Implications
The findings of our study support the potential for HD by those with DDCC in the province of Québec. Additionally, these results suggest that although FWIT always should be considered when evaluating cardiac graft viability, the limiting factor in HD by those with DDCC does not appear to be the dreaded inevitable cardiac ischemia, but rather characteristics that also would preclude HD in potential donors with NDD. Therefore, these results favour a revised protocol for multi-organ procurement from donors with DDCC. Based on current DDCC practice, this study also emphasizes the need for specific strategies targeting the perception of hearts donated by those with DDCC, to maximize HT of hearts from those with DDCC.
Finally, the potential for HD by those with DDCC appears to be greater in the subgroup of patients who receive MAID. Although only 15 donors who received MAID were included in the study, 11 (73%) were identified as potentially eligible for HD. This finding may be of particular interest to the province of Québec, due to the widespread acceptability of MAID in the province and the growing proportion of organ donations occurring among this group. Moreover, HD by those with DDCC may involve fewer ethical challenges in this subgroup, as consent could be obtained directly from the patients.
Limitations
This study carries all the limitations of a retrospective study. As predonation investigations were focused on other organs, cardiac screening information was available for only a minority of donors (Fig. 3). To avoid overestimating the potential for HD, we attempted to evaluate heart conditions using patients’ medical history. The use of cardiac enzyme levels as criteria to exclude a potential donor is arguable, but we chose conservative criteria. Therefore, in practice, although only a proportion of the 19% of donors identified as potentially eligible may become actual heart donors upon further cardiac evaluation, marginal donors (ie, donors other than the 42 donors presented here) could be considered for HD as more-extensive cardiac screening would be available.
Figure 3.
Available predonation investigations and clinical characteristics relevant to exclusion criteria. CKMB, creatinine kinase myocardial band; CT, computed tomography; ECG, electrocardiogram; TTE, transthoracic echocardiogram; Wisconsin Scale, University of Winsconsin Scale Donation after Cardiac Death Evaluation Tool.
Additionally, the median age of eligible donors (52 years), is higher than expected. The maximum age for HD eligibility in this study was set at 60 years, which is considered acceptable for HD by those with NDD. Although the threshold varies among centres and publications, it is probably too high for an HD made by a patient with DDCC. In the randomized trial of HD by those with DDCC, the donor mean age was 29 years, and the oldest age was 47 years. If only eligible donors aged ≤ 40 years and ≤ 50 years are considered, the percentages of suitable donors drop to 9.5% and 19%, respectively.
Another limitation is the absence of data regarding patients listed for HT during the study period. Thus, a simulation of the “real” increase is not feasible, and the level of potential for a decrease in waiting-list mortality remains unknown. Finally, the number of suitable hearts for transplantation from donors with NDD during the study period was unavailable. Therefore, we used the number of transplantations performed (accepted hearts) to calculate the proportion of the increase in cardiac offers, which may overestimate the results. Finally, a decrease in donations was observed during 2020, due to the coronavirus-19 pandemic, leading to potential underestimation of the number of donors with DDCC who are eligible for HD.
Conclusion
The present study suggests significant potential to have increased HD offers by an average of 19% during the 4 years of the study in the province of Québec. Implementing a program of HD by those with DDCC in Québec may help reduce HD waiting time and increase the number of HTs.
Acknowledgements
The authors thank Mrs. Marie-Josée Simard and the Transplant Québec personnel for supporting and contributing to this project.
Ethics Statement
The research reported has adhered to the relevant ethical guidelines.
Patient Consent
The authors confirm that consent does not apply to this article. This is a retrospective study using de-identified data about deceased patients. Therefore, consent is not feasible, and our institutional review board did not require it.
Funding Sources
P.-E.N. received a research grant ($CAD 2500) from the Société Québécoise d’Insuffisance Cardiaque. T.H.F. received a research grant ($4600) from the PRogramme d’Excellence en Médecine pour l’Initiation En Recherche (PREMIER). The other authors have no funding sources to declare.
Disclosures
N.N. and P.-E.N. received a grant from the Heart & Stroke Foundation of Canada, unrelated to this work: GIA #G-23-0034193 ($300.000)— Title: Creating new opportunities for the transplantation of heart donated after circulatory death by combining normothermic regional perfusion and innovative strategies. The other authors have no conflicts of interest to disclose.
Footnotes
See page 1048 for disclosure information.
References
- 1.Khush K.K., Hsich E., Potena L., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-eighth adult heart transplantation report—2021; focus on recipient characteristics. J Heart Lung Transplant. 2021;40:1035–1049. doi: 10.1016/j.healun.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Transplant Quebec Statistiques officielles 2022. https://www.transplantquebec.ca/sites/default/files/bilan_2022_public_final_1.pdf Available at:
- 3.Shemie S.D., Torrance S., Wilson L., et al. Heart donation and transplantation after circulatory determination of death: expert guidance from a Canadian consensus building process. Can J Anesth Can Anesth. 2021;68:661–671. doi: 10.1007/s12630-021-01926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khush K.K., Cherikh W.S., Chambers D.C., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report—2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1056–1066. doi: 10.1016/j.healun.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon J.H., Ghannam A.D., Shorbaji K., et al. Early outcomes of heart transplantation using donation after circulatory death donors in the United States. Circ Heart Fail. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.122.009844. [DOI] [PubMed] [Google Scholar]
- 6.Institut canadien d’information sur la santé Statistiques annuelles sur les transplantations d’organes au Canada: dialyse, transplantation et don d’organes, 2009 à 2018. https://secure.cihi.ca/free_products/CORR-snapshot-2019-fr.pdf Available at:
- 7.Anguela-Calvet L., Moreno-Gonzalez G., Sbraga F., et al. Heart donation from donors after controlled circulatory death. Transplantation. 2021;105:1482–1491. doi: 10.1097/TP.0000000000003545. [DOI] [PubMed] [Google Scholar]
- 8.Honarmand K., Parsons Leigh J., Basmaji J., et al. Attitudes of healthcare providers towards cardiac donation after circulatory determination of death: a Canadian nation-wide survey. Can J Anesth. 2020;67:301–312. doi: 10.1007/s12630-019-01559-6. [DOI] [PubMed] [Google Scholar]
- 9.Honarmand K., Parsons Leigh J., Martin C.M., et al. Acceptability of cardiac donation after circulatory determination of death: a survey of the Canadian public. Can J Anesth Can Anesth. 2020;67:292–300. doi: 10.1007/s12630-019-01560-z. [DOI] [PubMed] [Google Scholar]
- 10.Farr M., Truby L.K., Lindower J., et al. Potential for donation after circulatory death heart transplantation in the United States: retrospective analysis of a limited UNOS dataset. Am J Transplant. 2020;20:525–529. doi: 10.1111/ajt.15597. [DOI] [PubMed] [Google Scholar]
- 11.Osaki S., Anderson J.E., Johnson M.R., Edwards N.M., Kohmoto T. The potential of cardiac allografts from donors after cardiac death at the University of Wisconsin Organ Procurement Organization. Eur J Cardiothorac Surg. 2010;37:74–79. doi: 10.1016/j.ejcts.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Roest S., Kaffka Genaamd Dengler S.E., Van Suylen V., et al. Waiting list mortality and the potential of donation after circulatory death heart transplantations in the Netherlands. Neth Heart J. 2021;29:88–97. doi: 10.1007/s12471-020-01505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noterdaeme T., Detry O., Hans M.F., et al. What is the potential increase in the heart graft pool by cardiac donation after circulatory death?: potential of cardiac donation after circulatory death. Transpl Int. 2013;26:61–66. doi: 10.1111/j.1432-2277.2012.01575.x. [DOI] [PubMed] [Google Scholar]
- 14.Singhal A.K., Abrams J.D., Mohara J., et al. Potential suitability for transplantation of hearts from human non–heart-beating donors: data review from the Gift of Life Donor Program. J Heart Lung Transplant. 2005;24:1657–1664. doi: 10.1016/j.healun.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 15.Jawitz O.K., Raman V., DeVore A.D., et al. Increasing the United States heart transplant donor pool with donation after circulatory death. J Thorac Cardiovasc Surg. 2020;159:e307–e309. doi: 10.1016/j.jtcvs.2019.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messer S., Page A., Rushton S., et al. The potential of heart transplantation from donation after circulatory death donors within the United Kingdom. J Heart Lung Transplant. 2019;38:872–874. doi: 10.1016/j.healun.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Messer S., Cernic S., Page A., et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2020;39:1463–1475. doi: 10.1016/j.healun.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Smith D.E., Kon Z.N., Carillo J.A., et al. Early experience with donation after circulatory death heart transplantation using normothermic regional perfusion in the United States. J Thorac Cardiovasc Surg. 2022;164:557–568.e1. doi: 10.1016/j.jtcvs.2021.07.059. [DOI] [PubMed] [Google Scholar]
- 19.Copeland H., Knezevic I., Baran D.A., et al. Donor heart selection: evidence-based guidelines for providers. J Heart Lung Transplant. 2023;42:7–29. doi: 10.1016/j.healun.2022.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louca J., Öchsner M., Shah A., et al. The international experience of in-situ recovery of the DCD heart: a multicentre retrospective observational study. eClinicalMedicine. 2023;58 doi: 10.1016/j.eclinm.2023.101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayer A., Schroder J.N., Casalinova S., et al. The future of heart procurement with donation after circulatory death: current practice and opportunities for advancement. J Heart Lung Transplant. 2022;41:1385–1390. doi: 10.1016/j.healun.2022.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman J.R.H., McMaster W.G., Rali A.S., et al. Early US experience with cardiac donation after circulatory death (DCD) using normothermic regional perfusion. J Heart Lung Transplant. 2021;40:1408–1418. doi: 10.1016/j.healun.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Miñambres E., Royo-Villanova M., Pérez-Redondo M., et al. Spanish experience with heart transplants from controlled donation after the circulatory determination of death using thoraco-abdominal normothermic regional perfusion and cold storage. Am J Transplant. 2021;21:1597–1602. doi: 10.1111/ajt.16446. [DOI] [PubMed] [Google Scholar]
- 24.Iyer A., Dhital K. Cardiac donation after circulatory death. Curr Opin Organ Transplant. 2020;25:241–247. doi: 10.1097/MOT.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 25.Government of Canada Second annual report on medical assistance in dying in Canada 2020. https://www.canada.ca/en/health-canada/services/publications/health-system-services/annual-report-medical-assistance-dying-2020.html Available at:
- 26.Duong D., Vogel L. What’s the status of medical assistance in dying in Canada? Can Med Assoc J. 2023;195:E488–E489. doi: 10.1503/cmaj.1096045. [DOI] [PMC free article] [PubMed] [Google Scholar]