Abstract
Background
Novel pathways are needed to accommodate the increasing demand for transcatheter aortic valve implantation (TAVI) and ensure equitable access. A single Vancouver Facilitated TAVI program (VFTP) based at St. Paul's and Vancouver General Hospitals was established to streamline the assessment of remote patients with severe aortic stenosis using virtual technologies.
Methods
Remote patients with severe aortic stenosis who expressed difficulties traveling to complete their pre-TAVI workup were included and received prospective follow-up. Clinical and echocardiographic parameters were reported per the Valve Academic Research Consortium 3.
Results
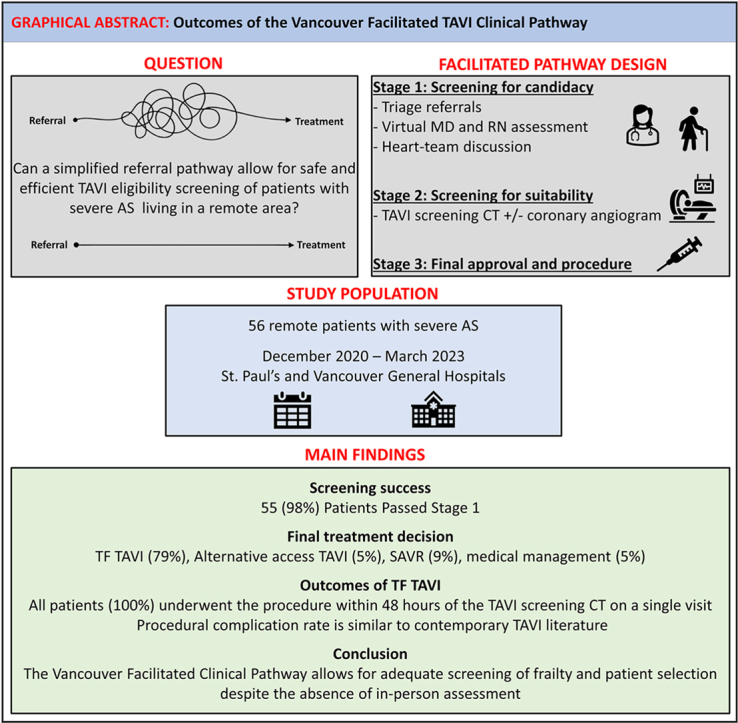
Between December 2020 and March 2023, a total of 56 remote patients were included in the VFTP. The mean patient age was 79.7 ± 9.1 years. A total of 55 patients (98%) passed the screening for candidacy; 45 patients (80%) were found suitable for transfemoral TAVI, 5 patients (9%) were directed toward surgical aortic valve replacement; 3 (5%) underwent alternative-access TAVI; and 2 patients (4%) were assigned to a watchful waiting strategy. No inpatient mortality, stroke, or major bleeding occurred in the transfemoral TAVI group, and the median hospital stay was 1 day (interquartile range, 1-2 days; range, 1-24 days). Two patients had an access-closure failure requiring surgical intervention; 1 patient had tamponade; and 4 patients had complete heart block requiring permanent pacemaker implantation. No hospital readmission had occurred at 30 days.
Conclusions
A simplified assessment pathway to assess TAVI candidacy using virtual technologies is safe and feasible. The VFTP potentially can increase access to TAVI and reduce inequity in TAVI care.
Graphical abstract
Résumé
Contexte
De nouvelles options sont nécessaires pour répondre à la demande croissante d'implantation valvulaire aortique par cathéter (IVAC) et en assurer un accès équitable. Un programme unique, à Vancouver, le Vancouver Facilitated TAVI Program (VFTP), a été mis en place dans les hôpitaux St. Paul et Vancouver General pour rationaliser l'évaluation des patients éloignés, atteints de sténose aortique sévère, en utilisant des technologies virtuelles.
Méthodes
Les patients éloignés atteints de sténose aortique grave, éprouvant des difficultés à se déplacer pour effectuer leur bilan pré-IVAC ont été inclus et ont fait l'objet d'un suivi prospectif. Les paramètres cliniques et échocardiographiques ont été rapportés selon le Valve Academic Research Consortium 3.
Résultats
Entre décembre 2020 et mars 2023, un total de 56 patients éloignés ont été inclus dans le VFTP. L'âge moyen des patients était de 79,7 ± 9,1 ans. Au total, 55 patients (98 %) ont passé la sélection de candidatures; 45 patients (80 %) ont été jugés aptes à une IVAC transfémorale; 5 patients (9 %) ont été orientés vers un remplacement valvulaire aortique chirurgical; 3 (5 %) ont subi une IVAC par accès alternatif; et 2 patients (4 %) ont été assignés à une stratégie d'attente vigilante. Le groupe avec une IVAC transfémorale n'a pas connu de mortalité, d'accident vasculaire cérébral ou d'hémorragie majeure et la durée médiane d'hospitalisation a été de 1 jour (intervalle, 1 à 24 jours; intervalle interquartile, 1 à 2 jours). Deux patients ont eu un échec de la fermeture de l'accès nécessitant une intervention chirurgicale, un patient a eu une tamponnade et quatre patients ont eu un bloc cardiaque complet nécessitant l'implantation d'un stimulateur cardiaque permanent. Aucune réadmission à l'hôpital n'est survenue à 30 jours.
Conclusions
Un protocole simplifié pour évaluer la candidature à l'IVAC à l'aide de technologies virtuelles est sûr et réalisable. Le VFTP permettrait de potentiellement augmenter l'accès à l'IVAC et réduire les inégalités dans les soins liés à l'IVAC.
Transcatheter aortic valve implantation (TAVI) is now the default treatment strategy for a large proportion of patients with severe aortic stenosis (AS) regardless of the surgical risk profile.1, 2, 3, 4 Contemporary American College of Cardiology/American Heart Association practice guidelines give a class-1A recommendation for TAVI in patients aged ≥ 65 years with severe symptomatic AS, after shared decision-making and in the absence of anatomic contraindications to a transfemoral (TF) approach.5 This approach has increased demand for TAVI, as more patients are now being considered for this procedure. Consequently, by 2019, the volume for TAVI exceeded that for surgical aortic valve replacement (SAVR) in the US, and rates continue to increase.6 As TAVI becomes the chosen therapy for the majority of AS patients, healthcare systems increasingly are strained. Wait lists can be additive and lengthy, and can contribute to morbidity and mortality.7, 8, 9
Assessment of TAVI suitability is a comprehensive process that may require multiple visits involving cardiology, cardiac surgery, anesthesia, transthoracic echocardiography (TTE), cardiac computed tomography (CT), diagnostic coronary angiography, and frailty assessment. Remote patients inherently are disadvantaged, as the assessment for TAVI necessitating travel can be challenging, costly, and exhausting.
The Vancouver Facilitated TAVI program (VFTP) was built on previously established minimalist and accelerated reconditioning protocols,10,11 augmented by the streamlined assessment of remote patients using virtual technologies. Considering their remote distance, a simplified pathway was established to limit the number of visits and facilitate the completion of the pre-TAVI workup. The purpose of this study was to review the overall experience with the VFTP, assess short-term clinical and echocardiographic data, and validate the utility of virtual assessment in patients with severe AS considered for TAVI.
Methods
Study design and patient population
This was a single-centre prospective study of remote patients with severe AS referred to the VFTP in Vancouver (encompassing St. Paul’s and Vancouver General Hospitals) between December 2020 and March 2023. Remote patients were defined as those who lived outside Vancouver and expressed difficulties traveling to complete their pre-TAVI workup.
Eligible patients were identified, prospectively enrolled, and received follow-up. This study was approved by the local institutional ethics committee (University of British Columbia and Providence Health Care Research Ethics Board).
Facilitated TAVI pathway
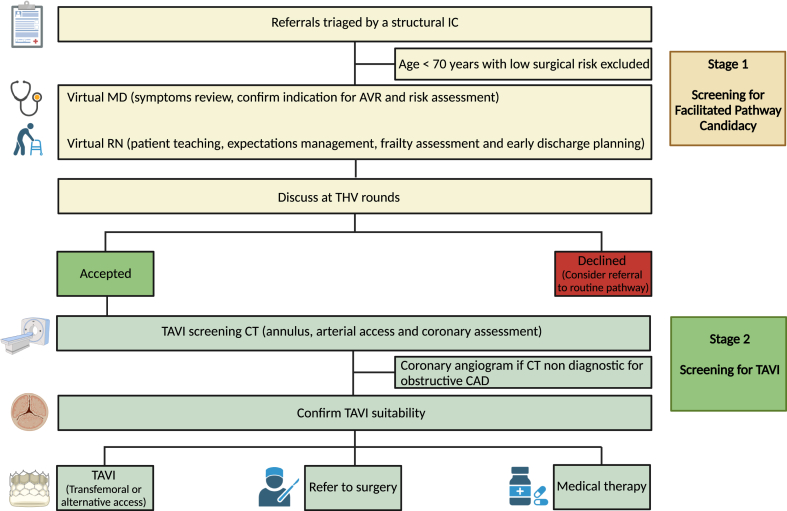
The program consists of a 2-stage process: screening for the facilitated pathway candidacy (provisional acceptance), and screening for TAVI (final acceptance and consent). The screening for candidacy stage included triaging TAVI referrals by a structural interventional cardiologist, a virtual physician (MD), and nurse (RN) assessment, and a review at the Transcatheter Heart Valve (THV) rounds. In the virtual MD clinic, a detailed clinical assessment was performed to assess AS-related symptoms, confirm indications, and address patient questions or concerns. The RN virtual assessment aimed to focus on patient teaching, detailed understanding of the facilitated protocol and management expectations, and early discharge planning. The nurse conducted a virtual frailty assessment (video call) using the Essential Frailty Toolset (EFT). The EFT, which has been shown to have superior predictive performance compared to other frailty-assessment tools, was used.12 The EFT is a practical and easy-to-perform assessment with a 5-point screen of 4 components (chair rise, cognitive impairment, serum hemoglobin, and serum albumin). When completed, all potential candidates for TAVI under the VFTP were reviewed at THV rounds. Patients who passed screening for candidacy (stage 1) were invited to Vancouver for combined coronary and pre-TAVI CT, with the goal of proceeding with TF TAVI within 48 hours on a single visit. Following analysis of the cardiac CT, patients were reviewed at THV rounds for the second time to confirm their suitability for TAVI (stage 2). If TF TAVI was found to be unfeasible, patients were referred for alternative access TAVI, SAVR, or conservative management (Fig. 1). A pre-TAVI coronary angiogram was not a requirement but could be considered on a case-by-case basis if the CT was not able to rule out severe proximal CAD.
Figure 1.
AVR, aortic valve replacement; CAD, coronary artery disease; CT, computed tomography; IC, interventional cardiologist; TAVI, transcatheter aortic valve implantation; THV, transcatheter heart valve. Created with BioRender.com.
Post-TAVI follow-up
Patients were given instructions on general post-TAVI care, including the need for infective endocarditis prophylaxis and driving restrictions, as per local guidelines. A post-TAVI baseline TTE was performed in all patients before discharge. A 30-day TTE was performed by the closest local hospital with echocardiography capabilities. Echocardiographic reports were sent to the THV clinic for review. The full TTE study was reviewed by the THV program if findings were unexpected. Patients were advised to contact the THV clinic if any issues occurred after discharge. Following TTE, a virtual 30-day follow-up meeting was arranged with an RN at the THV clinic.
Endpoints
The primary endpoint was a composite of inpatient mortality, stroke, major access-related complications, and new pacemaker implantation. Secondary endpoints were the individual components of the primary endpoint, and clinical and echocardiographic parameters, as per the Valve Academic Research Consortium 3, and hospital readmission rate at 30-day follow-up.
Statistical analysis
Assessment of procedural characteristics and outcomes involved collecting data from electronic medical records. Statistical analysis was performed using the Jamovi Project (2020; Jamovi Version 1.2, www.jamovi.org). The normality of distribution for each variable was evaluated through a Shapiro-Wilk test. Continuous variables were expressed as mean ± standard deviation (SD) for normally distributed values, or as median (interquartile range [IQR]) for those not adhering to a normal distribution. Categorical variables were depicted as counts (%).
Results
Study population and baseline characteristics
A total of 56 consecutive patients diagnosed with severe AS who were referred to the VFTP were enrolled between December 2020 and March 2023. At the time of referral, the mean age was 79.7 ± 9.1 years, and 61% of patients were male (Table 1). The mean left ventricular ejection fraction was 59% ± 11%, and the aortic valve mean gradient was 42.5 ± 13.4 mm Hg. The median European System for Cardiac Operative Risk Evaluation score (EuroSCORE II) mortality risk score was 2.1% (IQR, 1.5%-3.3%).
Table 1.
Baseline characteristics of patients referred to the Vancouver Facilitated Transcatheter Aortic Valve Implantation Program (VFTP)
| Characteristics | All patients (n = 56) |
|---|---|
| BMI, kg/m2 | 28.4 ± 5.1 |
| Age, y | 79.7 ± 9.1 |
| Male | 34 (61) |
| LVEF, % | 59 ± 10.8 |
| MG, mm Hg | 42.5 ± 13.4 |
| Underlying heart disease | |
| CAD with prior PCI | 2 (4) |
| Prior CABG | 4 (7) |
| Prior SAVR | 1 (2) |
| Prior TAVI | 1 (2) |
| Comorbidities | |
| Anemia | 2 (4) |
| Chronic renal failure | 25 (45) |
| Hemodialysis | 0 (0) |
| Peripheral artery disease | 8 (14) |
| COPD | 6 (11) |
| Hypertension | 44 (79) |
| Dyslipidemia | 38 (68) |
| Diabetes mellitus | 16 (29) |
| Atrial fibrillation | 17 (30) |
| Prior stroke/TIA | 6 (11) |
| Prior pacemaker | 5 (9) |
Values are mean ± standard deviation, or n (%).
BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; MG, mean gradient; PCI, percutaneous coronary intervention; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; TIA, transient ischaemic attack.
The median number of days from referral to the VFTP until telehealth MD appointment for screening of candidacy was 47 (IQR, 29-64). The median number of days from provisional acceptance at THV rounds to the TF-TAVI procedure was 72 (IQR, 50-99).
Flowchart results of the VFTP
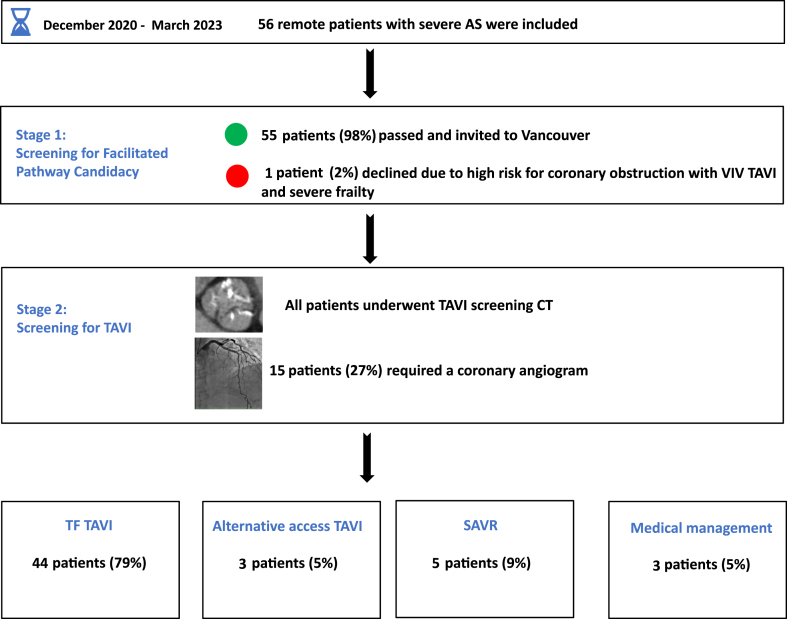
Of the 56 patients, 55 patients (98%) passed stage 1 of the program and were invited to Vancouver for a pre-TAVI screening CT with or without coronary angiogram. Only 15 patients (27%) underwent a coronary angiogram as part of the pre-TAVI workup. Patients who underwent a coronary angiogram were those with known CAD, prior percutaneous coronary intervention (PCI), suboptimal CT angiogram to diagnose CAD due to extensive coronary calcification or artifact, or when SAVR was recommended. Most patients (73%) did not require a coronary angiogram, as CT angiogram was sufficient to rule out proximal obstructive CAD.
A total of 45 patients (80%) were considered suitable and were accepted for TF TAVI. Among the remaining 10 patients, 5 (9%) were directed toward SAVR, owing to their having high-risk adverse root features; 3 (5%) were accepted for alternative-access TAVI due to prohibitive femoral access; and 2 (4%) were assigned to a watchful waiting strategy, as the degree of AS was considered moderate after repeated TTE. One patient from the TF-TAVI group declined the procedure, owing to advanced age and wishes for conservative therapy. Hence, 44 patients (79%) received TF TAVI (Fig. 2). No deaths occurred during the waiting period from referral to performance of the procedure.
Figure 2.
AS, aortic stenosis; CT, computed tomography; TAVI, transcatheter aortic valve implantation; TF, transfemoral; SAVR, surgical aortic valve replacement; VIV, valve in valve.
Analysis of patient population accepted for TF TAVI
In the cohort of 44 patients who underwent TF TAVI, the mean age at the time of the procedure was 80.4 ± 6.0 years (range: 58-90). The median number of days from the referral to performance of the procedure was 134 (IQR, 106-179), ranging from 36 to 372 days.
In this group, 38 patients (86%) were implanted with a balloon-expandable SAPIEN 3 Ultra valve (Edwards Lifesciences, Irvine, CA), whereas 6 patients (14%) were selected to receive a self-expandable valve: for 5, the Evolut (Medtronic, Minneapolis, MN), and for 1, the ACURATE neo2 (Boston Scientific, Marlborough, MA). One patient underwent a valve-in-valve procedure with the SAPIEN 3 Ultra valve for a failed surgical aortic valve bioprosthesis.
No inpatient mortality, stroke, or major bleeding occurred. Two patients had an access closure failure requiring surgical intervention; 1 patient had tamponade that was likely related to right ventricular pacer perforation; and 4 patients had complete heart block requiring permanent pacemaker implantation. The median hospital stay, in days, was 1 (IQR, 1-2; range, 1-24). A total of 77% of patients (34 of 44) who underwent TF TAVI were discharged the following day. Factors contributing to a prolonged hospital stay included complete heart block, new or transient left bundle branch block, access closure issues requiring surgical intervention, planned post-TAVI PCI, and symptomatic atrial arrhythmia. At 30 days, 1 noncardiac mortality and no hospital readmission had occurred, and all patients had New York Heart Association class I-II symptoms (Table 2).
Table 2.
In-patient outcomes, adverse events, and 30-day follow-up in cohort of patients who underwent transfemoral transcatheter aortic valve implantation
| Characteristics | All patients (n = 44) |
|---|---|
| Inpatient outcomes | |
| All-cause death | 0 |
| Stroke | 0 |
| MI | 0 |
| Major bleeding | 0 |
| Complete heart block∗ | 4 (10) |
| Access closure complication† | 2 (5) |
| Pericardial effusion‡ | 1 (2) |
| Hospital LOS, d (median, IQR) | 1, 1–2 |
| MG at discharge, mm Hg | 9 ± 4 |
| 30-d outcomes | |
| Cardiovascular mortality | 0 |
| Noncardiovascular mortality | 1 (2) |
| Stroke | 0 |
| Rehospitalization rate | 0 |
| NYHA I–II at 30 d | 44 (100) |
| MG at 30-d, mm Hg | 12 ± 6 |
Values are n (%), or mean ± standard deviation, unless otherwise indicated.
IQR, interquartile range; LOS, length of stay; MG, mean gradient; MI, myocardial infarction; NYHA, New York Heart Association.
Requiring permanent pacemaker implantation.
Failure of closure devices to achieve hemostasis at the arteriotomy site, leading to surgical vascular repair.
Right ventricular pacing wire–related pericardial effusion (tamponade) necessitating pericardiocentesis.
Echocardiographic parameters pre-TAVI and during follow-up
All patients underwent TTE assessment before discharge, and at 30-day follow-up. The mean left ventricular ejection fraction postprocedure was 58% ± 11%, with a mean gradient of 9 ± 4 mm Hg. At 30-day follow-up, the values were 58% ± 12% and 12% ± 6%, respectively. One patient exhibited mild-moderate paravalvular leak post-TAVI, which remained unchanged at the 30-day follow-up and was treated conservatively.
Discussion
In this single-centre prospective study, we found the following: (i) a simplified assessment pathway using virtual technologies for TAVI candidacy and work-up is safe and feasible in older patients with AS; (ii) use of this approach allowed for adequate screening of frailty, despite the absence of in-person assessment; and (iii) this approach facilitated the treatment of remote patients with severe AS and was sufficient for successful performance of TF TAVI during the same visit in > 75% of the patients.
The use of telehealth is increasing in all areas of medicine, including cardiology.13 The COVID-19 pandemic strictly limited face-to-face contact between patients and medical staff, and forced the rapid development of telehealth.14 However, some limitations have been reported relating to use of telehealth in older patients who may have limited access to adequate technologies and some degree of cognitive impairment.15 In the present study, RN and MD virtual clinic consultations were possible in all patients, and this evaluation offered sufficient information to allow for heart-team decisions, with 55 of 56 patients (98%) passing the screening evaluation for candidacy.
We believe the VFTP offers a unique opportunity for remote patients to benefit from a TAVI procedure, regardless of where they reside. A pan-Canadian evaluation of wait times showed that remarkable geographic inequity exists in regard to TAVI access.16 The same study demonstrated a > 3-fold difference in TAVI capacity among Canadian provinces. This finding has prompted calls for policymakers and clinicians to address inequalities in TAVI care and implement new pathways that ensure equal and equitable access to this procedure.17 A recent study comparing regional differences in outcomes for patients undergoing TAVI showed that greater access to TAVI was associated with better outcomes, including a lower mortality incidence.18 The VFTP has the potential to increase access to TAVI care while using current healthcare resources.
One way of achieving this increase is through optimization of patients' preprocedural workup. Our study showed that a strategy of routine pre-TAVI diagnostic coronary angiography may not always be necessary, as 73% of patients in the present cohort underwent TAVI without the need for a coronary angiogram. A coronary angiogram may, however, still be considered in patients with a known history of CAD, previous PCI, or when the cardiac CT quality is insufficient to rule out severe obstructive coronary disease due to extensive coronary calcification or suboptimal heart rate control. In this case, the angiogram can be performed at the beginning of the TAVI procedure.
Another important aspect of this study is that virtual appointments allowed for adequate screening for frailty, and patients in the present study had excellent outcomes, despite the absence of in-person evaluation. The virtual use of the EFT allowed for a simple remote assessment of frailty to be made by the RN, with no apparent issues in patient selection. Thus, no inpatient mortality occurred in our study, and the complication rate was comparable to that reported in contemporary practice studies.19 Moreover, the median length of hospital stay in patients undergoing TF TAVI in our program was 1 day, indicating that the virtual selection process did not result in the treatment of excessively frail patients with prolonged hospital stays.
Finally, despite the initial absence of information about anatomic suitability, most patients (79%) were found to be eligible for a TF approach and treatment during the same visit. Additionally, for patients who were unsuitable for a TF approach, another procedural strategy was offered for most of them, and only 5% of patients were assigned to conservative management. These results suggest that the amount of unnecessary travel is reduced drastically with this approach.
Limitations
This study has inherent limitations related to its small sample size and single-centre design. Although this simplified pathway worked adequately in the current cohort of patients, it remains unknown how many patients were not referred to the THV program or even diagnosed with AS due to limited contact with the healthcare system. We feel that the number of referrals to the facilitated program during the study period may not truly represent the number of remote patients with severe AS.
Additionally, whether the present approach allows for a reduction in wait times is unclear, as the study was conducted during the surge of the COVID-19 pandemic, which negatively influenced wait times for receipt of a TAVI procedure, owing to travel restrictions affecting those living remotely. We, however, believe that the widespread use of virtual technologies and streamlining assessment pathways could potentially reduce the time between diagnosis and treatment.
Conclusion
A simplified assessment pathway to assess TAVI candidacy using virtual technologies for remote patients with AS is safe and feasible. The VFTP can potentially increase access to TAVI, and it is a step forward toward reducing inequity in TAVI care.
Acknowledgments
Ethics Statement
The study has adhered to the relevant ethical guidelines.
Patient Consent
This study was approved by the local institutional ethics committee (University of British Columbia and Providence Health Care Research Ethics Board). Patient consent was not required, per the institutional research board, as data collection and analysis were conducted retrospectively.
Funding Sources
The authors have no funding sources to declare.
Disclosures
D.A.W. is a consultant to, and has received research funding from, Edwards Lifesciences and Abbott. J.G.W. is a consultant for Edwards Lifesciences and receives research funding from Edwards Lifesciences, Medtronic, and Boston Scientific. J.L. is supported by a Canadian Research Chair in Advanced Cardiopulmonary Imaging, consults for MVRX, Heartflow Inc., and Circle Cardiovascular Imaging; and provides CT core lab services for Edwards Lifesciences, Medtronic, Neovasc, Boston Scientific, and Tendyne Holdings, for which no direct compensation is received. J.S. has received speaking fees from Edwards Lifesciences; is a consultant for Edwards Lifesciences, Boston Scientific, NVT Medical, and Medtronic; and is an employee of Boston Scientific. S.S. is a consultant to Edwards Lifesciences, Anteris, Excision Medical, and Medtronic. J.S. and S.S. have received research support from Medtronic, Vivitro Labs, and Edwards Lifesciences. R.M. and R.H.B. are consultants for Edwards Lifesciences, and have received speaking fees from Edwards Lifesciences and Abbott Vascular. M.A. is a consultant for Medtronic and Edwards Lifesciences. S.B.L. is a consultant for Edwards and Medtronic. A.C. is a consultant for Edwards Lifesciences. All the other authors have no conflicts of interest to disclose.
Footnotes
See page 1225 for disclosure information.
References
- 1.Leon M.B., Smith C.R., Mack M., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Smith C.R., Leon M.B., Mack M.J., et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 3.Leon M.B., Smith C.R., Mack M.J., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 4.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 5.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 6.Carroll J., Mack M., Vemulapalli S., et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020;76:2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 7.Albassam O., Henning K.A., Qui F., et al. Increasing wait-time mortality for severe aortic stenosis: a population-level study of the transition in practice from surgical aortic valve replacement to transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2020;13 doi: 10.1161/CIRCINTERVENTIONS.120.009297. [DOI] [PubMed] [Google Scholar]
- 8.Elbaz-Greener G., Masih S., Fang J., et al. Temporal trends and clinical consequences of wait times for transcatheter aortic valve replacement: a population-based study. Circulation. 2018;138:483–493. doi: 10.1161/CIRCULATIONAHA.117.033432. [DOI] [PubMed] [Google Scholar]
- 9.Lauck S., Forman J., Borregaard B., et al. Facilitating transcatheter aortic valve implantation in the era of COVID-19: recommendations for programmes. Eur J Cardiovasc Nurs. 2020;19:537–544. doi: 10.1177/1474515120934057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauck S.B., Wood D.A., Baumbusch J., et al. Vancouver transcatheter aortic valve replacement clinical pathway: minimalist approach, standardized care, and discharge criteria to reduce length of stay. Circ Cardiovasc Qual Outcomes. 2016;9:312–321. doi: 10.1161/CIRCOUTCOMES.115.002541. [DOI] [PubMed] [Google Scholar]
- 11.Wood D.A., Lauck S.B., Cairns J.A., et al. The Vancouver 3M (multidisciplinary, multimodality, but minimalist) clinical pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers: the 3M TAVR study. Cardiovasc Interv. 2019;12:459–469. doi: 10.1016/j.jcin.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Afilalo J., Lauck S., Kim D.H., et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Creber R.M., Dodson J.A., Bidwell J., et al. Telehealth and health equity in older adults with heart failure: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2023;16 doi: 10.1161/HCQ.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang T., Mosier J., Subbian V., et al. Identifying barriers to and opportunities for telehealth implementation amidst the COVID-19 pandemic by using a human factors approach: a leap into the future of health care delivery? JMIR Hum Factors. 2021;8 doi: 10.2196/24860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Searcy R.P., Summapund J., Estrin D., et al. Mobile health technologies for older adults with cardiovascular disease: current evidence and future directions. Cardiovascular disease in the elderly. Curr Geriatr Rep. 2019;8:31–42. [Google Scholar]
- 16.Wijeysundera H.C., Henning K.A., Qiu F., et al. Inequity in access to transcatheter aortic valve replacement: a pan-Canadian evaluation of wait-times. Can J Cardiol. 2020;36:844–851. doi: 10.1016/j.cjca.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Wijeysundera H.C., Gaudino M., Qiu F., et al. Regional differences in outcomes for patients undergoing transcatheter aortic valve replacement in New York State and Ontario. Can J Cardiol. 2023;39:570–577. doi: 10.1016/j.cjca.2023.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sathananthan J., Gin K. How do we address health care inequalities for transcatheter aortic valve implantation in Canada? Can J Cardiol. 2020;36:797–798. doi: 10.1016/j.cjca.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Costa G., Saia F., Pilgrim T., et al. Transcatheter aortic valve replacement with the latest-iteration self-expanding or balloon-expandable valves: The Multicenter OPERA-TAVI Registry. JACC Cardiovasc Interv. 2022;15:2398–2407. doi: 10.1016/j.jcin.2022.08.057. [DOI] [PubMed] [Google Scholar]