Summary
Background
Early palliative care (EPC) leads to an improvement in quality of life and an unexpected survival benefit compared with oncological care for patients with metastatic lung cancer. The Early Palliative Integrated Care (EPIC) is aimed at examining whether EPC can improve overall survival in patients with metastatic upper gastrointestinal cancer.
Methods
We performed a multicentre, open-label, randomised phase-3 trial. Eligible patients were ≥18 years, had metastatic upper gastrointestinal cancer and a performance status of 0–2. Patients from 19 French centres were randomly assigned between 10/10/2016 and 17/12/2021 to receive EPC plus oncological care or standard oncological care (SOC) alone. EPC was provided by palliative care physicians and included five EPC visits scheduled every month, starting within 3 weeks after randomisation. The primary endpoint was overall survival, analysed by intention-to-treat. This study was registered at ClinicalTrials.gov (NCT02853474).
Findings
470 patients were randomised: 233 and 237 patients in the EPC and SOC groups, respectively. In the EPC group, 216/233 patients (92.7%) underwent ≥1EPC visit, with 159 EPC visits per protocol (68.2%). The median follow-up duration was 46 months. We did not observe any overall survival difference between the EPC (median = 7.0 months [95% confidence interval, 6.1–8.8]) and SOC groups (8.6 months [6.8–9.8]) (stratified hazard ratio = 1.04 [0.86–1.26], p = 0.68). No significant heterogeneity was found in primary tumour locations, performance status groups, sex, age groups, and inclusion periods.
Interpretation
Our findings suggested that receiving EPC did not improve the benefit of oncological care with regard to overall survival in patients with metastatic upper gastrointestinal cancer.
Funding
Programme Hospitalier de Recherche Clinique, Ligue Contre le Cancer, Conseil Régional du Nord-Pas-de-Calais.
Keywords: Early palliative care, Advanced cancer, Patient-centered care, Upper gastrointestinal cancer, Randomised trial, Survival
Research in context.
Evidence before this study
We searched PubMed for evidence regarding the effectiveness of early palliative care on survival and on patient related outcomes in the cancer setting in December 2010. The search was last updated in December 2023. We used the search terms “early palliative care”, “palliative care”, “integrated care”, “integrated palliative care”, “advanced cancer”, and “oncology”. The reference lists of all identified studies were examined for additional relevant studies. Randomised controlled trials on the effect of early palliative care on survival and patient-related outcomes in oncology were included as well as metaanalyses, editorials and comments. We restricted our search to articles published between Jan 1, 2005, and December 1, 2023. Our current study is based on evidence from one phase 3 randomised trial, in which patients with advanced lung cancers were offered palliative care in combination with standard oncological care early in the disease course, which showed that early integration of palliative care in oncology care led to significantly improved survival and other beneficial patient outcomes.
Added value of this study
To the best of our knowledge, EPIC is the first controlled trial ever designed to primarily estimate the overall survival benefit of early palliative care vs. standard oncologic care alone in patients with advanced malignancies. As a result, this large multicentre study in French patients with advanced upper gastrointestinal cancers failed to confirm the overall benefit reported earlier with early palliative care in a monocentric study in US patients with advanced lung cancers.
Implications of all the available evidence
Our negative findings highlight the interest of studies aiming at validating striking results through prospective clinical trials in various settings including various health care systems.
Introduction
The World Health Organization (WHO) defines palliative care as an approach that improves the quality of life of patients and their families who are facing problems associated with life-threatening illnesses.1 Palliative care is provided in conjunction with other therapies that are intended to prolong life. Metastatic malignancies are fatal diseases that benefit from palliative care. In the last century, palliative care focuses primarily on the end of life, and to date, international recommendations advocate its introduction as early as possible during the course of the disease.1, 2, 3
A randomised single-centre trial by Temel et al. published in 2010 was a landmark publication4 that allows experts to favourably consider the implementation of palliative care early in the course of advanced cancers.3,5,6 Among 151 patients newly diagnosed with metastatic lung cancer, Temel et al.4 showed that early palliative care (EPC) significantly improved the quality of life and depression symptoms from baseline to week 12 compared with standard oncological care (SOC). In addition, although patients receiving EPC had less aggressive care at the end of life, a survival benefit was reported for these patients (median survival: 11.6 months vs. 8.9 months, p = 0.02), with a hazard ratio (HR) for death estimated at 0.59 in the experimental group compared with the control group. Bakitas et al.7 earlier reported a significant improvement in the quality of life and mood of patients with advanced cancers who received EPC. However, they did not find any statistically significant differences in overall survival between the two patient groups. Bakitas et al.8 also investigated the effect of early vs. delayed palliative care visits on quality of life, with overall survival as a subsequent objective, in a series of 207 patients with various advanced cancers. Their trial failed to show any quality of life benefit; they observed no significant overall survival benefit favouring EPC, but reported a significant 1-year survival advantage.8 Pooled survival data analysis from the three aforementioned randomised prospective trials4,7,8 including 680 patients revealed a significant survival advantage for patients allocated to EPC compared with SOC with a significant 26% reduction in the risk of death.9 Maltoni et al.10 later studied the benefit of systematic vs. on-demand EPC on the quality of life in patients with advanced pancreatic cancer with overall survival as a secondary endpoint. They reported a significant improvement in the quality of life related to EPC, similar to that observed by Temel et al.,4 but with no significant overall survival benefit.
Notably, none of the four randomised studies that reported survival data4,7,8,10 were primarily designed to determine differences in overall survival. Therefore, we aimed to design the Early Palliative Integrated Care (EPIC) clinical trial to evaluate, for the first time, whether EPC integrated with SOC could increase overall survival as compared with SOC alone in patients with metastatic upper gastrointestinal cancer.
Methods
Study design and participants
In this prospective, randomised, open-label, multicentre phase 3 trial, the patients were recruited in France by medical oncologists working in 19 secondary and tertiary public hospitals between October 10, 2016 and December 17, 2021. Key eligibility criteria were age ≥18 years, histologically proven oesophageal cancer, an oesogastric junction or stomach adenocarcinoma (including duodenum), or pancreatic or biliary tract adenocarcinomas, a European Cooperative Oncology Group (ECOG) performance status of 0–2, planned first-line chemotherapy for metastatic disease, and signed informed consent (detailed inclusion and exclusion criteria previously published in Hutt et al.11).
Ethics
The protocol was examined by the Patient Committee of the National League against Cancer. It was approved by an independent ethics committee and appropriate institutional review boards. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines. This study was registered at ClinicalTrials.gov (NCT02853474). The full protocol for this study has been previously published.11
Randomisation and masking
Randomisation 1:1 was performed using an online centralised randomisation software (CS-Randomization of Ennov Clinical), ensuring the concealment of the next patient allocation. It was based on a minimisation algorithm considering the centre, ECOG performance status (0–1 vs. 2), and primary tumour site (oesogastric/gastric vs. pancreas vs. biliary tract vs. oesophagus), with no random factor. The patients and investigators were not blinded to the treatment assignment.
Procedures
Medical oncologists were in charge of chemotherapy administration and supportive care in accordance with professional practices, regardless of the group assignment. Qualified palliative care physicians were responsible for palliative care outpatient visits. To match the standard practice in France, participants allocated to the SOC arm were not scheduled to receive palliative care, but a palliative care visit could be performed anytime upon request in case of worsening health status. In the experimental arm, a total of five EPC visits were scheduled. The first EPC visit was scheduled within the first 3 weeks after randomisation, followed by monthly EPC visits.
At the time of writing our protocol, there were no equivalents in the French context to the currently available recommendations of the American [2, 5, 6] or European guidelines3 regarding the content of palliative care visit. The content of each of the five EPC visits was described by the palliative care physician and recorded in a database following a specific checklist based on the available evidence,4 focusing on the following items: discussion with the patient, focusing on his/her understanding of the disease, its treatment and the palliative care process, evaluation of clinical status and symptoms, evaluation of psychological status, evaluation of the social environment, including lifestyle, and evaluation of stakeholder needs (psychologist, physiotherapist, dietician, social worker, or other health-care professionals). EPC visits could also include patient and family support, discussion about the identification of a ‘person of trust’ and advanced directives, and coordination of the continuum of care.
Outcomes
The primary outcome was the overall survival, which was defined as the time between the dates of randomisation and death, regardless of the cause. Patients alive at the cut-off date were censored at that time.
The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 items (EORTC QLQ-C30; version 3.0)12 measures the quality of life of patients with cancer. Questionnaires were completed by the patients at baseline, and then every 8 weeks thereafter. We investigated changes in quality of life throughout the course of the disease and used time until definitive deterioration (TUDD) curves.13 For the latter approach, the quality of life score was considered permanently deteriorated if it had decreased by more than 10 points compared with the score at baseline, without later improvement superior to 10 points as compared with baseline, or with no further evaluation available. Thus, the TUDD for quality of life scores was defined as the time from randomisation to the first observation of a definitive deterioration in the QLQ-C30 score or death.
We also studied changes in patients’ mood and anxiety using the Hospital Anxiety and Depression Scale (HADS)14 auto-evaluated at baseline and at 8 and 16 weeks thereafter. The HADS is designed to detect and quantify anxiety and depression. It contains 14 items scored from 0 to 3: seven items related to anxiety (score A) and seven items related to depression (score D). The maximum possible score was 21 points.
Finally, we recorded the number of patients treated with chemotherapy in the 30 days before death and the number of patients treated with advanced directives.
Statistical analysis
We anticipated a smaller effect (HR = 0.75) than that in the study by Temel et al. (HR = 0.59).4 Assuming proportional hazards over time; this was equivalent to an absolute difference of 10% in the 1 year overall survival (40.0% vs. 50.3%). A total of 381 deaths were required to ensure 80% power for the treatment effect, with a two-sided alpha of 5%. Assuming an exponential distribution of survival time with an accrual duration of 5 years, a 1-year minimum follow-up, and a final analysis at 6 years, 480 patients (240 in each group) were randomised. This calculation accounted for a 2% annual loss to follow-up. An interim analysis was planned after approximately 190 deaths had been recorded. The significance level was fixed at p = 0.003 for the interim analysis and p = 0.049 for the final analysis (Lan de Mets alpha-spending function with an O'Brien–Fleming efficacy stopping rule).15
Overall survival curves were estimated using the Kaplan–Meier method. The treatment effect of the EPC arm compared with that of the SOC arm was based on the estimation of the HR of death in a Cox model, controlling for primary site and performance status (stratification variables), and tested against the null hypothesis of no treatment effect with a two-sided alpha of 5%. The proportional hazards assumption underlying the HR estimate in the Cox models was evaluated using graphic methods and models, including the interaction with time. Heterogeneity of treatment effects by primary site and ECOG performance status (pre-planned analyses) as well as by sex, age group, period of inclusion (post hoc analysis) was evaluated using forest plots and interaction tests. The main analysis was performed on the intention-to-treat dataset, including all patients in the treatment group allocated by randomisation until their last follow-up visit, except for those who withdrew their consent immediately after randomisation. A sensitivity analysis was also planned on the per-protocol dataset where i) non-eligible patients were excluded, ii) patients in the standard arm who received more than one palliative care visit within the first 6 months of treatment since randomisation were censored at the date of their second palliative care visit if unrelated to the deterioration of clinical status, and iii) patients in the EPC arm who received less than five palliative care visits within the first 6 months since randomisation were censored at the date of the first missing palliative care visit.
The Independent Data Monitoring Committee met when the results of the planned interim analysis were available (i.e. when 190 patients died) to review the results of the first efficacy interim analysis and re-estimate the sample size if the baseline overall survival rate differed from the protocol assumptions. No futility analysis was planned, as the proportional hazards assumption may not be respected, with a possibly larger treatment effect with a longer follow-up than in the first part of the survival curves.
The QLQ-C30 questionnaires were analysed according to the EORTC manual recommendations. For each dimension, patients with at least one score were included in the analysis. Changes over time were represented graphically, and the difference between the scores at baseline and 24 weeks was compared between the treatment groups using covariance analysis. The TUDD curves were estimated using the Kaplan–Meier method. The effect of the treatment group on TUDD was estimated using the HR of definitive deterioration in quality of life or death in a Cox model.
All statistical analyses were performed using Stata® software, version 17.0 (StataCorp LLC College Station, USA).
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all data in the study and had the final responsibility for the decision to submit for publication.
Results
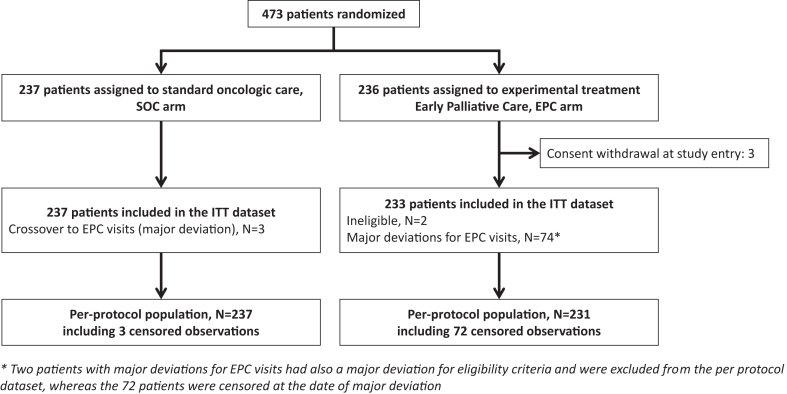
Overall, 473 patients were recruited and randomly assigned to either the EPC plus oncology care (n = 236) or SOC alone (n = 237) groups. Recruitment was stopped before a sample size of 480 patients was achieved, because the target number of deaths had already been reached. Because three patients from the EPC arm withdrew their consent immediately after consenting to the study, the intention-to-treat population was 470 (Fig. 1). The baseline clinical and treatment characteristics of the patients are shown in Table 1 and Supplementary Table S1, respectively. These baseline characteristics were well balanced between the two study groups, except for the proportion of patients without anxiety at baseline, which was lower in patients allocated to the EPC arm (129/221, 58%, in SOC arm vs. 95/222, 43%, in EPC arm).
Fig. 1.
Flowchart.
Table 1.
Baseline clinical characteristics.
| Characteristics | SOC |
EPC |
|---|---|---|
| N = 237 | N = 233 | |
| Sexa | ||
| Male | 142 (59.9%) | 131 (56.2%) |
| Female | 95 (40.1%) | 102 (43.8%) |
| Age–median (interquartile range) | 68 yrs (61–74) | 67 yrs (60–73) |
| 18–64 years | 91 (38.4%) | 95 (40.8%) |
| >64 years | 146 (61.6%) | 138 (59.2%) |
| ECOG—Performance status | ||
| 0 | 54 (22.8%) | 50 (21.5%) |
| 1 | 137 (57.8%) | 141 (60.5%) |
| 2 | 46 (19.4%) | 42 (18.0%) |
| Interval between diagnostic and randomization–median (interquartile range) | 0.4 mo (0.2–0.7) | 0.4 mo (0.2–0.7) |
| Primary tumour site | ||
| Oesophagus | 20 (8.4%) | 19 (8.2%) |
| Gastric and oesogastric junction | 25 (10.5%) | 23 (9.9%) |
| Pancreas | 149 (62.9%) | 148 (63.5%) |
| Biliary tract | 43 (18.1%) | 43 (18.5%) |
| Sites of metastases | ||
| Peritoneum | 60 | 50 |
| Liver | 154 | 162 |
| Lung | 57 | 55 |
| Other | 59 | 54 |
| HADS, anxiety (N) | N = 221 | N = 222 |
| Anxiety score–median (interquartile range), | 7 (4–10) | 8 (5–11) |
| No anxiety (score ≤7) | 129 (58.4%) | 95 (43.2%) |
| Doubtful anxiety (score between 8 and 10) | 46 (20.8%) | 54 (24.5%) |
| Definite anxiety (score ≥11) | 46 (20.8%) | 71 (32.3%) |
| HADS, depression (N) | N = 222 | N = 220 |
| Depression score–median (interquartile range) | 6 (3–10) | 7 (4–11) |
| No depression (score ≤7) | 133 (59.9%) | 125 (56.8%) |
| Doubtful depression (score between 8 and 10) | 48 (21.6%) | 30 (20.7%) |
| Definite depression (score ≥11) | 41 (18.5%) | 29 (20.0%) |
| Global Health Status (EORTC QLQ-C30), N | 225 | 219 |
| Mean (standard deviation) | 56.2 (22.1) | 53.7 (21.6) |
SOC, standard oncological care alone; EPC, early palliative care; N: number of patients; ECOG performance status, Eastern Cooperative Oncology Group performance status; HADS, hospital anxiety and depression scale; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire Core 30 items.
Sex: as reported by the patient upon admission to hospital.
In the EPC group, 216/233 patients (92.7%) received at least one palliative care visit, whereas 17/233 patients (7.3%) did not. The median time to the first palliative care visit was 7 days (95% CI: 7–9 days). The mean number of palliative care visits was 4.7 (standard deviation [sd] = 3.6; range: 0–16; median 4; interquartile range 2–6), but the mean number of EPC visits on the first 6 months after randomisation was 3.4 (sd = 1.7; range 0–5). Overall, in the EPC group, 159/233 patients (68.2%) had several EPC visits as planned by the protocol over their survival duration, whereas 74 patients (31.8%) did not and were classified as a major deviation.
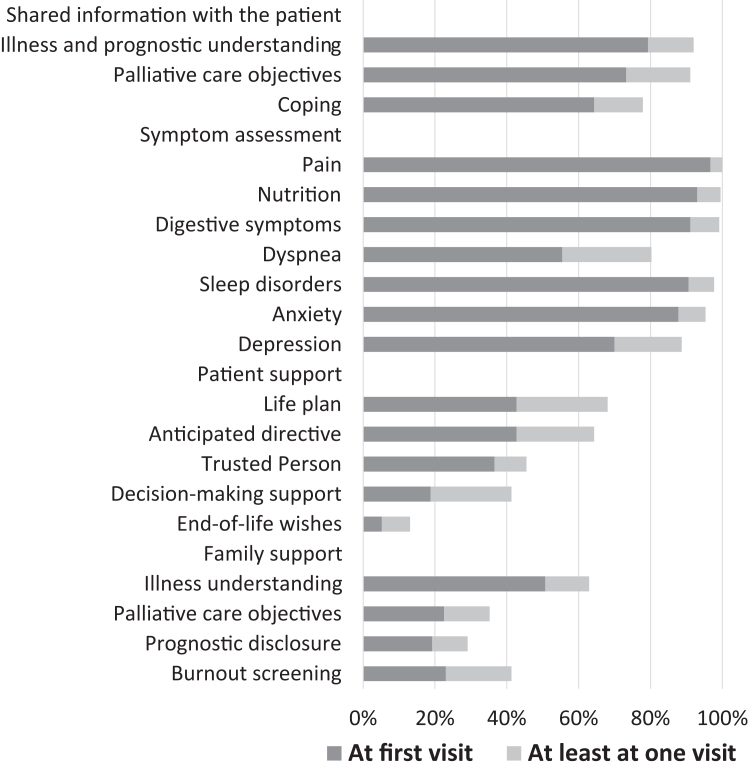
The EPC visits were outpatient visits led by a palliative care physician for patients allocated to the EPC arm (n = 213; missing data: 3). Other healthcare professionals, such as nurses (125/213, 58.7%) and psychologists (21/213, 9.9%), could have attended palliative care visits. At more than half of palliative care visits, patients' relatives were present. Following the EPC visits, some patients were referred to other healthcare professionals. In fact, 31/213 patients (14.6%) also met a pain doctor, 68/213 (31.9%) a social worker, 118/213 (55.4%) a dietician, 68/213 (31.9%) a nurse, 38/2131 (7.8%) a physiotherapist, 2/213 (0.9%) a chaplain, 110/213 (51.6%) a psychologist or psychiatrist, and 33/213 (15.5%) other healthcare professionals (39/213, 18.3%). In the SOC group (n = 237), only three patients (1.3%) had at least one palliative care visit within the first 6 months after randomisation for reasons other than worsening of their health condition and were classified as a major deviation (crossover to the EPC arm). In addition, as anticipated in the protocol, some patients allocated to the SOC arm (54/237, 22.7%) received at least one palliative care visit because of worsening health conditions. The mean number of palliative care visits was 0.6 (sd = 1.5; range: 0–10; median 0; interquartile range 0–0). The distribution of time to the first palliative care visit for both EPC and SOC patients is shown in Supplementary Figure S1. A graphical representation of the EPC content is shown in Fig. 2. For most patients, the EPC visits included information on illness, prognostic understanding, palliative care objectives, and coping strategies. Symptom management was addressed at least once in almost all patients, mainly at the first visit. EPC visits also include the patient's perspective. However, we observed that some topics, such as the identification of a trusted person, decision-making support, and end-of-life wishes, were addressed by a minority of patients. In terms of family support, illness understanding was frequently addressed, in contrast to palliative care objectives, prognostic disclosure, and burnout screening.
Fig. 2.
Description of content of EPC visits.
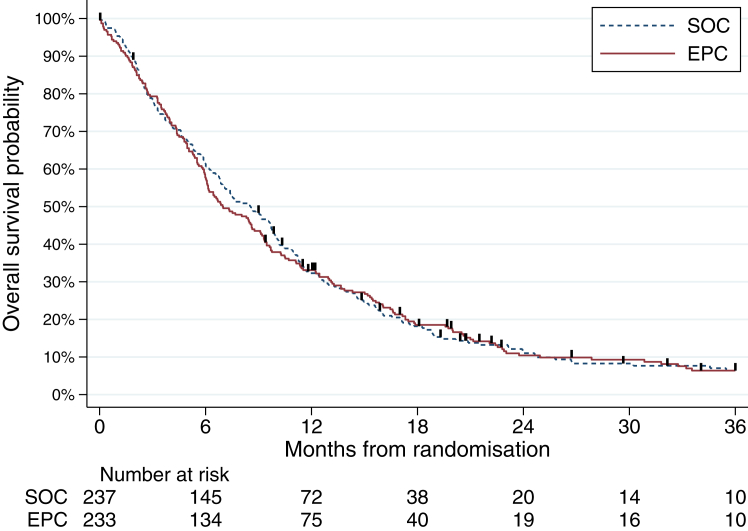
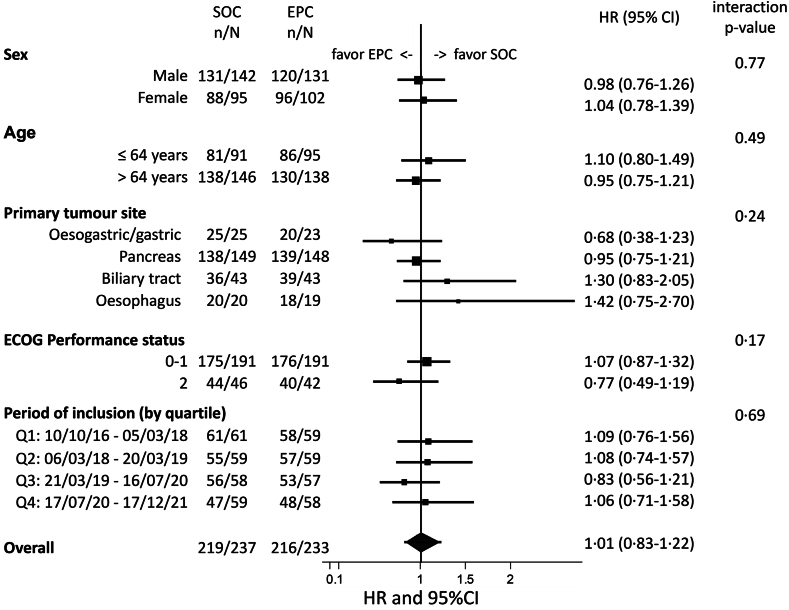
At the time of data cut-off (November 7, 2022), with a median follow-up of 46 months, 435/470 patients died. We did not observe any overall survival difference between arms (EPC arm: median overall survival = 7.0 months [95% CI: 6.1–8.8 months] and 1-year overall survival = 33.1% [27.1–39.2]; SOC arm: median overall survival = 8.6 months [6.8–9.8 months] and 1-year overall survival = 32.3% [26.4–38.3]; stratified HR = 1.04 [0.86–1.26], p = 0.68) in the intent-to-treat population (Fig. 3). As shown in Supplementary Figure S2, the survival findings were similar in the per-protocol dataset. We did not observe any significant heterogeneity in this finding across sexes (interaction test, p = 0.77), age groups (p = 0.49), primary tumour locations (interaction test, p = 0.24), ECOG performance status groups (p = 0.17), and period of inclusion (p = 0.69) (Fig. 4).
Fig. 3.
Kaplan–Meier survival curves for overall survival.
Fig. 4.
Heterogeneity of treatment according to stratification factors, sex, age and timing of inclusion (intention-to-treat analysis).
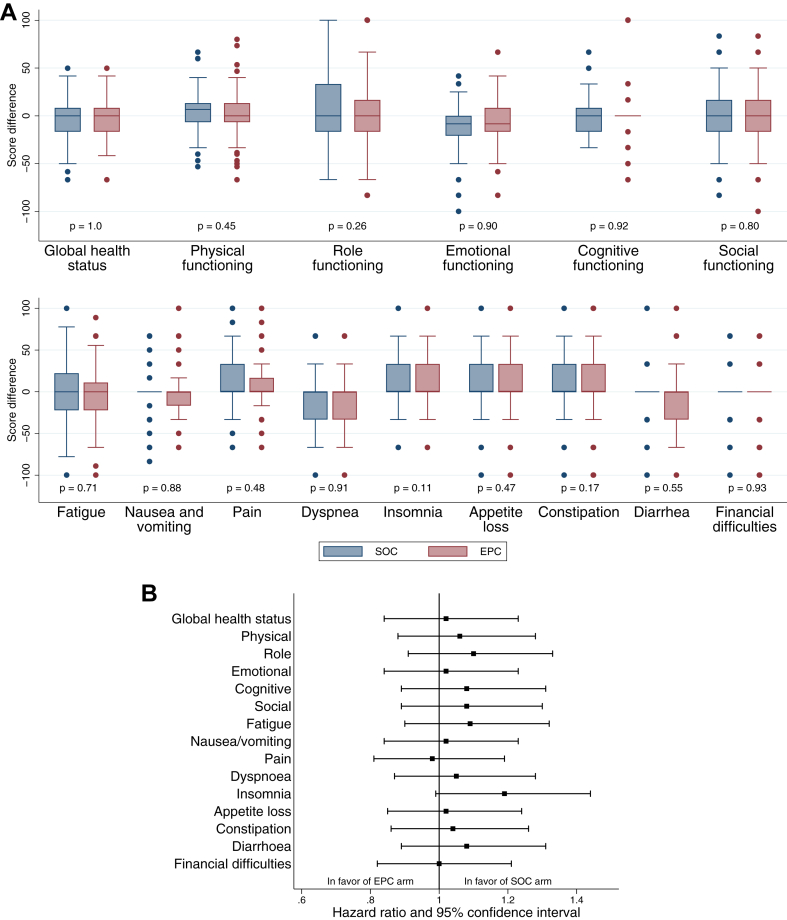
Of the 470 patients, 452 (96.2%) completed the EORTC QLQ-C30 questionnaire at baseline. Among the 286 patients still alive at 24 weeks, compliance with the quality of life questionnaires was 71.5% in both arms (98/137 in the EPC arm and 106/149 in the SOC arm). As shown in Fig. 5A for the evaluation at 24 weeks and detailed in Supplementary Figure S3 for all time points, quality of life did not seem to improve in patients allocated to the EPC compared with those allocated to the SOC group. Moreover, all dimensions of the EORTC QLQ-C30 functioning scores remained stable throughout the study course without any differences between the arms. Finally, the TUDD analysis did not reveal any difference between the treatment arms for any of the quality of life dimensions, as shown in the TUDD curve for global health status (Supplementary Figure S4) and summarised for the 15 dimensions in Fig. 5B. With regard to mood, as detailed in Supplementary Table S3, the percentage of patients without anxiety or depression showed a similar trend in both arms. When looking at markers of aggressiveness of care at the end of life, the percentage of patients who received chemotherapy in the last month of life (67/218, 30.7% in the SOC arm vs. 77/214, 36.0% in the EPC arm) and the percentage of patients who completed an advanced directive (26/215, 12.1% in the SOC arm vs. 38/212, 17.9% in the EPC arm) did not significantly differ between the study groups (p = 0.25 and p = 0.091, respectively).
Fig. 5.
Quality-of-life analysis (QLQ-C30). A. Box plot of differences in QLQ-C30 scores between baseline and week 24 for the 15 dimensions of the QLQ-C30 questionnaire. B. Relative treatment effect on the time until definitive deterioration of quality of life for the 15 dimensions of the QLQ-C30 questionnaire (details in Supplementary Table S2).
Discussion
This large, multicentre, randomised trial of EPC integrated with SOC in patients with metastatic upper gastrointestinal cancers did not show any overall survival advantage compared with patients treated with SOC only in the intent-to-treat population or in the per-protocol population. In addition, EPC did not result in a higher quality of life throughout the course of the disease, delay quality-of-life deterioration, or reduce anxiety and depression. We also did not observe any differences between the two groups with regard to the percentage of patients who received chemotherapy in the last month of life or those who wrote advanced directives.
To the best of our knowledge, this is the first controlled trial designed to estimate the overall survival benefits of EPC vs. SOC alone in patients with advanced malignancies. This study was adequately powered to detect a 25% reduction in the risk of death with EPC compared with SOC, which was largely below the overall survival benefit reported in the Temel et al. trial (approximately 40%).4 Similar to most randomised trials which had reported survival data in this setting,7,10,16, 17, 18 our study failed to confirm overall survival improvement with EPC. The study by Temel et al. published in 2010 remains the only trial to clearly conclude a significant survival benefit of EPC, based on the analysis of 151 patients with advanced lung cancer.4 Hence, if Bakitas et al.8 reported a 15% difference at 1 year (EPC, 63% vs. delayed palliative care visits, 48%; p = 0.04) in patients with various advanced cancers, no significant difference was found when considering the entire overall survival (p = 0.14). Despite some uncertainties about the survival benefit, the trial by Temel et al.4 had a major impact on the oncology community, and major academics requested for the early integration of palliative care into the SOC.3,5,6
As Temel et al. reported,4 numerous studies have found improvements in the quality of life of patients receiving EPC7,10,19, 20, 21, 22; however, others did not,8,23 similar to ours. This heterogeneity of results could be related to the duration of the quality of life follow-up as suggested by Hoomani Majdabadi et al.24 In fact, Hoerger et al.9 reported that the allocation to EPC is associated with better quality of life in their meta-analysis on 1398 patients from five high-quality studies. This benefit disappeared when the follow-up period of time exceeded 10 months as observed by Hoomani Majdabadi et al.24 in a subsequent meta-analysis on 25 studies including 5160 patients. In our study, we did not observe a decrease over time in anxiety/depression scores, favouring the EPC arm, contrary to what was reported by Temel et al.4 and later by Huo et al.25 in their meta-analyses. However, when looking closely at the four clinical trials that were pooled into the meta-analysis of Huo et al., the overall benefit in decreasing depression scores was driven by only one study4 while one reported an increase in depression scores,8 and two were even.17,23 In fact, expecting a favourable effect on anxiety/depression scales from EPC is difficult when the quality of life is not enhanced by the intervention, as in our study and three others.8,17,23 Regarding markers of aggressiveness at the end of life or end-of-life planning, we observed no significant differences between randomised groups, similar to Vanbutsele et al.,22 Maltoni et al.10 and Scarpi et al.,18 but in contrast with the findings of another study.4
We acknowledge that some trial characteristics can be perceived as limitations and need to be discussed. First, the EPIC was an open-design trial, which may have induced some bias. If the risk of detection bias is certainly limited (overall survival as the primary endpoint with excellent follow-up), it may have led to a performance bias due to some deviations in the course of the interventions that we evaluated. However, the results were stable in our sensitivity analysis; the observations were censored at the date of deviation. In addition, masking the intervention is not feasible in such randomised trials, except when considering cluster randomised controlled trials in which patients can be informed about their own study group, ignoring the existence of another group [19]. Second, among the several parameters inherent to the design or conduct of our trial which could have had an impact on the results of the EPIC, we speculate that the parameter related to the definition of the EPC package should be key. No recommendations are available on the ideal number of EPC visits. We planned five EPC visits in EPIC and performed a mean number of 4.7 (median 4, interquartile range 2–6) and 3.3 visits (median 4, interquartile range 2–5) in the entire population of patients allocated to EPC arm and within the first 6 months since randomisation, respectively. The mean number of palliative care visits in the experimental group was within the range (4–6.54) of what was previously reported in similar situations.4,18,20,23 The percentage of patients receiving at least one palliative care visit in the SOC group was also rather similar to that in the EPIC (24.1%) and other studies (14%–34.3%). Moreover, we cannot ignore the fact that palliative care services, including those provided early in the course of cancer care can be characterised by a relative heterogeneity of practice, despite the publication of guidelines. Whether this translates to differences in outcomes remains unknown. We speculate that the multicentre design of our randomised trial, as compared with the single-centre design of the trial by Temel et al.,4 may enhance perceived heterogeneity in palliative care content. The content of palliative intervention, and more precisely of EPC, deserves a better definition, at least in the European context, although valuable efforts have already been expended.3,26
Beyond overall survival, we did not observe improvements in health-related quality of life in the current study. This finding may be attributed to the following arguments: assessment tool used (EORTC QLQ-C30 questionnaires vs. FACT tools), poor compliance in quality of life assessment, or the use of different means of comparison (baseline/12 weeks comparison vs. area-under-the-curve comparison with curves drawn from multiple quality of life data assessments). However, we do not share either of these arguments, as no striking differences have been previously reported with the use of either FACT or EORTC QLQ-C30 questionnaires.27 Moreover, our patients were rather compliant, as among patients alive at 24 weeks, 71.5% completed their quality of life questionnaires in both groups. Determining a meaningful difference in quality of life using the TUDD of quality of life analysis is difficult when no difference is observed in overall survival because the TUDD of quality of life analysis includes death as an event. In addition, we cannot rule out that the lack of quality of life improvement that we observed was related to the type of tumour included in this study. This has already been suspected by Temel et al.20 who were not able to reproduce in gastrointestinal malignancies the favourable impact on the quality of life of EPC visits observed in lung cancer. Finally, the relatively long duration of recruitment may have facilitated oncologists working in conjunction with palliative care specialists to develop their own expertise in supportive care as part of the palliative care package. However, these results remained stable over time (Supplementary Figure S2).
Our study failed to demonstrate the benefits reported by Temel et al.4 in a monocentric study of a specific population (advanced lung cancer) and in a specific context (the United States of America). Is this related to the nature of the disease as suggested by Temel et al.?20 Upper gastrointestinal cancers have a major, rapid, and deep impact on nutritional status, and early nutritional support may produce significant overall survival.28 Is this associated with the differences in societal context and the healthcare system? In France, over the last few decades, due to successive national cancer plans, considerable efforts have been made to integrate supportive care (part of the palliative care package) into the practice of medical oncology. It is also likely that the current training of younger oncologists integrates a deeper education about palliative care than in the past. Undoubtedly, patients included in the SOC arm in the present study had access to supportive care. This is likely because, in France, the health system offers 100% cost coverage for all care. Our negative findings highlight the need for studies aimed at validating striking results through prospective clinical trials in various settings, including various healthcare systems.
In conclusion, we observed no effect of early integration of palliative care in patients with metastatic upper gastrointestinal cancers, either on overall survival or quality of life. However, this does not mean that the EPC has no value. In this context, to better understand why patients (and caregivers) still perceive EPC to be beneficial,19,29 future studies should focus on other endpoints, such as the emotional and cognitive perceptions of patients with advanced malignancies.
Contributors
Writing—original draft: AA, NP, MCLD.
Writing—review & Editing: AA, AdS, MBA, VB, AT, AE, AP, ES, EL, LT, NP, GC, MCLD.
Conceptualization: AA.
Formal analysis: AA, NP, MCLD.
Data collection: all;
Verification of the underlying data: AA, EB, MCLD.
Methodology: MCLD.
Project administration: MCLD.
Figures: EB, MCLD.
All authors confirm that they had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Data sharing statement
The clinical study report is available upon request, after approval by the corresponding author.
Declaration of interests
Antoine Adenis:
Grants or contracts from ARCUS, AstraZeneca, Bayer Pharma, BEIGENE, BMS, Roche; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from BMS and Novartis.
Support for attending meetings and/or travel from AstraZeneca, Bayer Pharma, BMS, MSD, Pierre Fabre, Servier.
Participation on a Data Safety Monitoring Board or Advisory Board from Astellas, Bayer Pharma, BMS, MSD.
Meher Ben Abdelghani:
Support for attending meetings and/or travel from Servier, Merck, Amgen, Roche.
Participation on a Data Safety Monitoring Board or Advisory Board from Incyte, Deciphera, Bayer, Pierre Fabre, Servier, BMS.
Vincent Bourgeois:
Grants or contracts from Servier.
Support for attending meetings and/or travel from Ipsen, Astra Zeneca, Sanofi.
Participation on a Data Safety Monitoring Board or Advisory Board from AstraZeneca, Pierre Fabre, Servier.
Anthony Turpin:
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Servier, BMS, MSD, Viatris, AstraZeneca, Daiichi.
Marie-Pierre Galais:
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Servier, BMS.
Support for attending meetings and/or travel from Servier, Bayer, AAA, Merck.
Emmanuelle Samalin:
Grants or contracts from Merck, Bayer, Servier.
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pierre Fabre Oncology; MSD, Amgen, BMS.
Support for attending meetings and/or travel from Pierre Fabre Oncology; Servier, MSD.
Participation on a Data Safety Monitoring Board or Advisory Board from Astellas, Servier, MSD.
Sahir Javed:
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca.
Support for attending meetings and/or travel from Lilly, Pfizer.
Delphine Cornuault-Foubert:
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from BMS.
Support for attending meetings and/or travel from Vifor, Amgen, Leopharma, Mundipharma.
Christine Belletier: Support for attending meetings and/or travel from Mundipharma, Servier and Viatris.
Nicolas Penel:
Grants or contracts from Bayer.
Acknowledgements
The authors would like to thank the patients and families who participated in the study; the staff members involved in study management, particularly Fanny Ben Oune, Victor Desteirdt, and Marie Vanseymortier from the sponsorship unit at Centre Oscar Lambret; Jennifer Wallet for help with statistical analyses; Stéphanie Delaine for funding acquisition; all investigators and their teams who participated in the study; Séverine Marchant for editorial assistance; Editage for assistance to ensure English language and grammar accuracy; the Patients' review committee from ‘La Ligue Nationale Contre le Cancer’ who reviewed the quality of the information letter and the monitoring plan and made some suggestions implemented into the protocol to improve patient comfort; Dr. Bernard Asselain, Dr. Vincent Berry, and Pr Ivan Krakowski for their participation in the Independent Data Monitoring Committee; the data managers from the Northwest Data Center (CTD-CNO) who managed the study data (The data centre is supported by grants from the French National League Against Cancer (LNC) and the French National Cancer Institute (INCa).); and Programme Hospitalier de Recherche Clinique (PHRC-K 16-180), Ligue Contre le Cancer, Conseil Régional du Nord-Pas de Calais for funding the study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102470.
Appendix A. Supplementary data
References
- 1.WHO Definition of palliative care. https://www.who.int/news-room/fact-sheets/detail/palliative-care/en/
- 2.Ferrell B.R., Temel J.S., Temin S., et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:96–112. doi: 10.1200/JCO.2016.70.1474. [DOI] [PubMed] [Google Scholar]
- 3.Jordan K., Aapro M., Kaasa S., et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol. 2018;29:36–43. doi: 10.1093/annonc/mdx757. [DOI] [PubMed] [Google Scholar]
- 4.Temel J.S., Greer J.A., Muzikansky A., et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 5.Ferrell B.R., Twaddle M.L., Melnick A., Meier D.E. National consensus project clinical practice guidelines for quality palliative care guidelines, 4th edition. J Palliat Med. 2018;21:1684–1689. doi: 10.1089/jpm.2018.0431. [DOI] [PubMed] [Google Scholar]
- 6.Dans M., Kutner J.S., Agarwal R., et al. Palliative care, version 2.2021. Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2021;19:780–788. [Google Scholar]
- 7.Bakitas M.A., Lyons K.D., Hegel M.T., et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakitas M.A., Tosteson T.D., Li Z., et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33:1438–1445. doi: 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoerger M., Wayser G.R., Schwing G., Suzuki A., Perry L.M. Impact of interdisciplinary outpatient specialty palliative care on survival and quality of life in adults with advanced cancer: a meta-analysis of randomized controlled trials. Ann Behav Med. 2019;53:674–685. doi: 10.1093/abm/kay077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maltoni M., Scarpi E., Dall'Agata M., et al. Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer. 2016;65:61–68. doi: 10.1016/j.ejca.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Hutt E., Da Silva A., Bogart E., et al. Impact of early palliative care on overall survival of patients with metastatic upper gastrointestinal cancers treated with first-line chemotherapy: a randomised phase III trial. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-015904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 13.Anota A., Hamidou Z., Paget-Bailly S., et al. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015;24:5–18. doi: 10.1007/s11136-013-0583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;286:171–173. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 15.Lan K.G., DeMets D.L. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 16.Groenvold M., Petersen M.A., Damkier A., et al. Randomised clinical trial of early specialist palliative care plus standard care versus standard care alone in patients with advanced cancer: the Danish Palliative Care. Trial Palliat Med. 2017;31:814–824. doi: 10.1177/0269216317705100. [DOI] [PubMed] [Google Scholar]
- 17.Brims F., Gunatilake S., Lawrie I., et al. Early specialist palliative care on quality of life for malignant pleural mesothelioma: A randomised controlled trial. Thorax. 2019;74:354–361. doi: 10.1136/thoraxjnl-2018-212380. [DOI] [PubMed] [Google Scholar]
- 18.Scarpi E., Dall'Agata M., Zagonel V., et al. Systematic vs. on-demand early palliative acre in gastric cancer patients: a randomized clinical trial assessing patient and healthcare service outcomes. Support Care Cancer. 2019;7:2425–2434. doi: 10.1007/s00520-018-4517-2. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann C., Swami N., Krzyzanowska M., et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383:1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 20.Temel J.S., Greer J.A., El-Jawahri A., et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol. 2017;35:834–841. doi: 10.1200/JCO.2016.70.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanbutsele G., Pardon K., Van Belle S., et al. Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial Lancet. Oncol. 2018;19:394–404. doi: 10.1016/S1470-2045(18)30060-3. [DOI] [PubMed] [Google Scholar]
- 22.Vanbutsele G., Van Belle S., Surmont V., et al. The effect of early and systematic integration of palliative care in oncology on quality of life and health care use near the end of life: a randomised controlled trial. Eur J Cancer. 2020;124:186–193. doi: 10.1016/j.ejca.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Temel J.S., Sloan J., Zemla T., et al. Multisite, randomized trial of early integrated palliative and oncology care in patients with advanced lung and gastrointestinal cancer: alliance A221303. J Palliat Med. 2020;23:922–929. doi: 10.1089/jpm.2019.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoomani Majdabadi F., Ashktorab T., Ilkhani M. Impact of palliative care on quality of life in advanced cancer. A meta-analysis of randomised controlled trials. Eur J Cancer Care. 2022;31 doi: 10.1111/ecc.13647. [DOI] [PubMed] [Google Scholar]
- 25.Huo B., Song Y., Chang L., Tan B. Effects of early palliative care on patients with incurable cancer: a meta-analysis and systematic review. Eur J Cancer Care. 2022;31 doi: 10.1111/ecc.13620. [DOI] [PubMed] [Google Scholar]
- 26.Kaasa S., Loge J.H., Aapro M., et al. Integration of oncology and palliative care: A Lancet Oncology Commission. Lancet Oncol. 2018;19:e588–e653. doi: 10.1016/S1470-2045(18)30415-7. [DOI] [PubMed] [Google Scholar]
- 27.Luckett T., King M.T., Butow P.N., et al. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: issues, evidence and recommendations. Ann Oncol. 2011;22:2179–2190. doi: 10.1093/annonc/mdq721. [DOI] [PubMed] [Google Scholar]
- 28.Lu Z., Fang Y., Liu C., et al. Early interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: a phase III randomized controlled trial. J Clin Oncol. 2021;39:748–756. doi: 10.1200/JCO.20.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borelli E., Bigi S., Potenza L., et al. Changes in cancer patients' and caregivers' disease perceptions while receiving early palliative care: A qualitative and quantitative analysis. Oncologist. 2021;26:e2274–e2287. doi: 10.1002/onco.13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.