Abstract
Background
Therapeutic hypothermia, or targeted temperature management (TTM), is a strategy of reducing the core body temperature of survivors of sudden cardiac arrest, cardiogenic shock (CS) or stroke. Therefore, a systematic literature review and meta-analysis were performed to tackle the question about whether the implementation of TTM is actually beneficial for patients with CS.
Methods
Study was designed as a systematic review and meta-analysis. PubMed, Cochrane Library, Web of Science and Scopus were searched from these databases inception to July 17, 2022. Eligible studies were those comparing TTM and non-TTM treatment in CS patients. Data were pooled with the Mantel-Haenszel method.
Results
Thirty-day mortality was reported in 3 studies. Polled analysis of 30-day mortality was 44.2% for TTM group and 48.9% for non-TTM group (risk ratio: 0.90; 95% confidence interval: 0.75 to 1.08; p = 0.27). Other mortality follow-up periods showed also no statistically significant differences (p > 0.05). The occurrence of adverse events in the studied groups also did not show statistically significant differences between TTM and non-TTM groups (p > 0.05 for myocardial infarction, stent thrombosis, sepsis, pneumonia, stroke or bleeding events).
Conclusions
The present analysis shows no significant benefit of TTM in patients with CS. Moreover, no statistically significant increase of the incidence of adverse effects was found. However, further randomized studies with higher sample size and greater validity are needed to determine if TTM is worth implementing in CS patients.
Keywords: targeted temeparature management, therapeutic hypothermia, cardiogenic shock, outcome, meta-analysis
Introduction
Cardiogenic shock (CS) is a life-threatening condition characterized by hypoxia and end-organ hypoperfusion, caused by severe impairment of the myocardium and diminished cardiac output [1]. With its mechanical complications, acute myocardial infarction (AMI) is responsible for most CS cases still burdened by significant mortality [2]. The persistence of high mortality rates, varying from 38% to 65% [3, 4] is very distressing despite the fact that technical treatment of AMI complicated by CS has improved over the last decades [5].
In this regard, targeted temperature management (TTM) through therapeutic hypothermia (32°C – 34°C for 12 to 24 hours by surface cooling or endovascular cooling) has been investigated in several studies [6–9]. This level of hypothermia has a potentially neuroprotective effect [10, 11]. It works by reducing the brain’s metabolism and thus the oxygen, adenosine triphosphate, and glucose consumption, which are associated with reducing reperfusion injury [6, 9]. The large randomised controlled trial brought more evidence of the beneficial use of TTM in patients after cardiac arrest (CA) with no shockable rhythm, leading to a higher percentage of patients with a favourable neurologic outcome at day 90 [12].
According to current recommendations, TTM is the standard of care in adult patients with return of spotnaeus circulation after out-of-hospital and in-hospital ventricular fibrillation CA [13–16]. A common complication after CA is fever, which has an incidence of 42%, and therefore TTM is effective in these patients [15, 16]. Randomized studies on porcine models showed possible benefits of TTM in CS with reduced acute mortality and hemodynamic parameter improvements [17, 18]. Unfortunately, this finding was not confirmed in humans [19].
Based on these assumptions, a systematic literature review and meta-analysis to tackle whether the implementation of TTM is beneficial for patients with CS was conducted herein.
Methods
The systematic review was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [20]. Due to study character (meta-analysis), ethical approval or patient consent was not suitable for this study.
Literature search
In this systematic review and meta-analysis, PubMed, Cochrane Library, Web of Science and Scopus was searched from these databases inception to July 17, 2022, for peer-reviewed original primary research articles, including observational or interventional studies, describing the outcomes of targeted temperature management in cardiogenic shock. For the search, the search term: “targeted temperature management” OR “TTM” OR “hypothermia” OR “therapeutic hypothermia” OR “mild hypothermia” AND “cardiogenic shock” OR “cardiogenic” OR “shock” was used. Additionally, manually checking the reference lists was done in each involved publication to identify eligible studies. Language and publication year restrictions were not applied. De-duplication and screening were carried out on EndNote software (X9; Claritive; Philadelphia, PA, USA).
Inclusion and exclusion criteria
Two reviewers (M.P. and L.S.) independently screened the titles and abstracts against the agreed inclusion criteria and then extracted and relevant full-text records were selected. Discrepancies were resolved through discussion at each stage by consensus. Two additional reviewers (M.J.J. and A.G.) verified the eligibility of inclusion of the studies when necessary. Studies that were included in this meta-analysis had to fulfil the following PICOS criteria: (1) Participants, patients with 18 years old or older with cardiogenic shock; (2) Intervention, TTM; (3) Comparison, non-TTM; (4) Outcomes, detailed information for survival or mortality; (5) Study design, randomized controlled trials (RCT) and observational trials (non-RCT) comparing TTM and non-TTM care for their effects in patients with CS. Studies were excluded if they were reviews, observational studies, animal studies, case reports, letters, conference or poster abstracts, or articles not containing original data.
Data extraction
Two reviewers (L.S. and M.P.) independently extracted data which were then checked for accuracy by a third reviewer (J.S.). Extracted data included: year of study, country, study design, patient demographics, and study outcomes. Mortality (within 30-days) was evaluated as the primary outcome. The secondary endpoint was major adverse cardiovascular and cerebrovascular events, i.e. a composite of death, myocardial infarction, stent thrombosis or stroke during a long-term observation period.
Quality assessment
Two reviewers (A.G. and M.P.) independently evaluated studies for risk of bias and quality assessment. Any disagreements were discussed and resolved in a consensus meeting with the third reviewer (L.S.). The RoB 2 tool (revised tool for risk of bias in randomized trials) was used to assess the quality of randomized studies [21], and the ROBINS-I tool (tool to determine the risk of bias in non-randomized studies of interventions) was used to assess the quality of non-randomized trials [22]. The risk of bias assessments was visualized using the Robvis application [23].
Statistical analyses
All statistical analyses were performed using Review Manager 5.4 Software (RevMan; The Cochrane Collaboration, Oxford, UK). An alpha criterion of a p-value less than 0.05 was considered statistically significant. Depending on the reported effect size measures, pooled risk ratios (RR), odds ratios (OR) or mean difference (MD), and 95% confidence intervals (CI) were calculated. When the continuous outcomes were reported in a study as median, range, and interquartile range, means and standard deviations were estimated using the formula described by Hozo et al. [24]. A random-effects approach (inverse variance or Mantel-Haenszel) was chosen to allow expected heterogeneity across the studies. The degree of heterogeneity among studies was based on the Cochrane Q statistics and I2 statistics [25]. I2 values of 50% or less corresponded to low to moderate, and 75% or higher indicated large amounts of heterogeneity.
Results
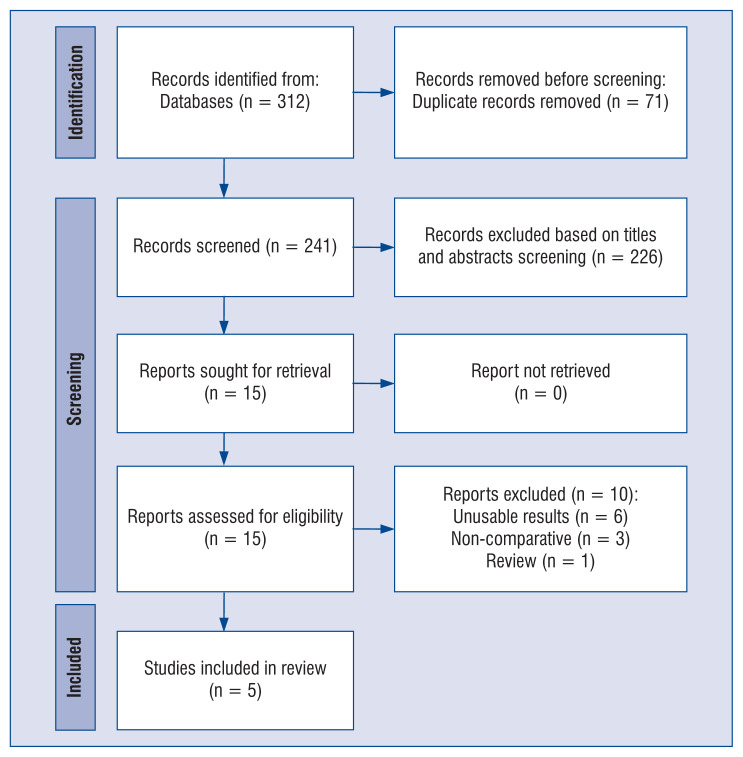
The flow diagram describing study selection is shown in Figure 1. A total of 5 studies [19, 26–29] comprising of 580 patients met the inclusion criteria. They included patients with cardiogenic shock between 2012 and 2022. Table 1 displays the baseline characteristics between patients with CS with or without TTM.
Figure 1.
Flow diagram showing stages of the database search and study selection as per Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines
Table 1.
Patient characteristics among included trials
| Study | Country | Study design | TTM group | Non-TTM group | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| No. | Age | Sex, male | No. | Age | Sex, male | |||
| Blatt et al. 2015 | Israel | Prospective, open label | 8 | 69.6 ± 7.0 | 6 (75.0%) | 13 | 65.1 ± 10 | 9 (69.2%) |
| Fuernau et al. 2019 | Germany | RCT | 20 | 76.5 ± 2.3 | 12 (60.0%) | 20 | 76.3 ± 3.2 | 14 (70.0%) |
| Levy et al. 2022 | France | RCT | 168 | 57 ± 12 | 128 (76.2%) | 166 | 59 ± 12 | 125 (75.3%) |
| Orban et al. 2015 | Germany | RCT | 64 | 69.1 ± 13 | 55 (85.9%) | 81 | 70.4 ± 12.1 | 53 (65.4%) |
| Zobel et al. 2012 | Germany | Mached trial | 20 | 59.5 ± 15 | 16 (80.0%) | 20 | 59.3 ± 16.5 | 17 (85.0%) |
RCT — randomized controlled trial; TTM — targeted temperature management
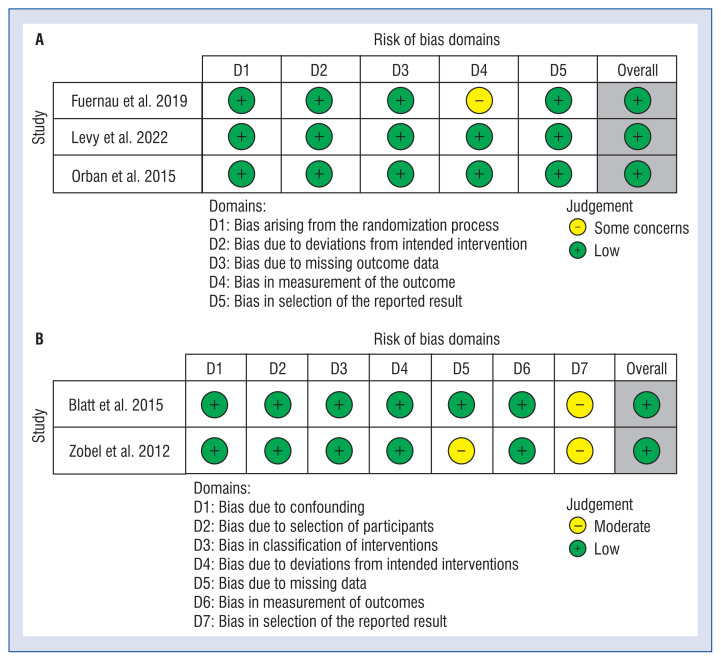
No significant differences between two patient cohorts were observed in the age (68.7 ± 12.8 vs. 69.1 ± 12.7 years, respectively; MD: 0.18; 95% CI: –135 to 1.72; p = 0.81) or male gender (79.5% vs. 69.4%, respectively; OR: 1.36; 95% CI: 0.54 to 3.41; p = 0.52). Polled analysis of patient characteristics between TTM and non-TTM groups is shown in Suppl. Table S1). The results of the assessment of risk of bias among the four included studies is provided in Figure 2.
Figure 2.
A summary table of review author judgements for each risk of bias item for randomized trials (A) and non--randomized trials (B)
Thirty-day mortality was reported in three studies. Polled analysis of 30-day mortality was 47.8% for TTM group and 46.5% for non-TTM group (RR: 1.04; 95% CI: 0.78 to 1.39; p = 0.86). Other mortality follow-up periods showed also no statistically significant differences (p > 0.05). The occurrence of adverse events in the studied groups also did not show statistically significant differences between TTM and non-TTM groups (p > 0.05 for myocardial infarction, stent thrombosis, sepsis, pneumonia, stroke or bleeding events). The detailed characteristics of the outcomes are presented in Table 2.
Table 2.
Pooled analysis of outcomes in targeted temperature management (TTM) and control groups
| Parameter | No. of studies | The frequency of occurrence | Events | Heterogeneity between trials | P-value for differences across groups | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| TTM | Control | RR | 95% CI | P-value | I2 statistic | |||
| Mortality: | ||||||||
| 30-days | 4 | 44.2% | 48.9% | 0.90 | 0.75 to 1.08 | 0.59 | 0% | 0.27 |
| 6-months | 1 | 52.4% | 57.8% | 0.91 | 0.75 to 1.10 | NA | NA | 0.32 |
| 1-year | 1 | 75.0% | 75.0% | 1.00 | 0.70 to 1.43 | NA | NA | 1.00 |
| 2-years | 1 | 65.0% | 60.0% | 1.08 | 0.67 to 1.75 | NA | NA | 0.74 |
| Myocardial injury | 1 | 6.3% | 6.2% | 1.01 | 0.26 to 3.94 | NA | NA | 0.98 |
| Stent thrombosis | 1 | 4.7% | 0.0% | 9.28 | 0.47 to 182.93 | NA | NA | 0.14 |
| Sepsis | 1 | 5.0% | 0.0% | 3.15 | 0.12 to 82.16 | NA | NA | 0.49 |
| Pneumonia | 1 | 45.0% | 30.0% | 1.91 | 0.52 to 7.01 | NA | NA | 0.33 |
| Stroke | 2 | 3.6% | 4.0% | 0.85 | 0.19 to 3.91 | 0.54 | 0% | 0.84 |
| Bleeding events or blood transfusion | 3 | 42.5% | 38.2% | 1.18 | 0.83 to 1.67 | 0.28 | 22% | 0.36 |
CI — confidence interval; NA — not applicable; RR — risk ratio
Discussion
Despite early revascularization strategy and advanced treatment, AMI complicated by CS is still burdened by the high mortality rate. Therapeutic hypothermia has shown its efficacy in the treatment of CA, but recent evidence has not provided significant effectiveness of TTM in case of proceeding AMI complicated by CS. This meta-analysis aims to summarize knowledge of the subject matter.
Out of the four studies included in this meta-analysis [19, 26–29], three showed no significant clinical advantages of TTM therapy, with no benefit in terms of 30-day survival (47.8% vs. 46.5; RR: 1.04 [0.78–1.39]). The usefulness of TTM was investigated in several trials, but most of these studies were performed on a small number of patients. Oddo et al. [30] showed that TTM might improve patient outcomes, particularly in the short duration of CA. On the contrary, Noc et al. [31] demonstrated that the intravascular cooling system favored a longer ischemic delay with increased adverse events rate and no benefit in myocardial tissue protection.
During AMI, TTM may reduce infarct size when performed before reperfusion [32, 33]. However, when CS complicates AMI, outcomes did not show a significant difference compared to a control group (6.3% vs. 6.2%; RR: 1.01; 95% CI: 0.26 to 3.94; p = 0.96). In the TTM group, the only mild (but statistically insignificant) trend toward reduction of biochemical markers (creatine kinase, troponin T) was observed [26]. This may be due to a delayed cooling start time or measuring infarct size with biomarkers only.
Bleeding events and blood transfusions were also included in the analysis. One study showed a higher risk of TIMI significant bleedings (p = 0.07). However, most of them were related to the arterial catheterization access for percutaneous coronary intervention [27]. It is known that hypothermia causes coagulopathy with increased clotting time; this event is called hypothermic coagulopathy [34]. One Meta-Analysis of Randomized Controlled Trials [35], which consisted of 43 trials and included 7,528 patients, did not find an increased risk of hemorrhage in patients treated with TTM in general, despite a higher risk of thrombocytopenia and transfusion requirement for patients treated with TTM, particularly in those cooled longer than 48 hours. Thus, TTM should be performed for a maximum period of 24 hours. One study [27], included in the pooled analysis, suggested a potential higher incidence of stent thrombosis. However, several studies have shown that TTM in AMI patients undergoing percutaneous coronary intervention is safe and is not related to increased incidence of stent thrombosis [36, 37]. However, those studies did not include patients who had CS. An extensive retrospective analysis [38], including 49,109 CA patients with AMI undergoing PCI, considered 1,193 patients treated with TTM. This analysis showed that patients undergoing therapeutic hypothermia, who developed CS, presented a greater incidence of stent thrombosis compared with no TTM group (OR: 1.3; 95% CI: 1.0 to 1.6; p = 0.04). No other significant differences in the TTM group regarding stent thrombosis, bleeding events [39], arrhythmias, infection, coagulopathy, or hypotension [40] were observed.
Furthermore, an increased risk of sepsis and pneumonia was not found in the present study. Still, considering the studies that showed an increased risk of these adverse effects in other disease units, such as CA, caution should be exercised [41, 42].
To sum up, the pooled analysis of all four studies showed that using TTM in CS patients is safe. No evidence of excessive adverse events was found in the TTM group. The safety and feasibility of TTM are described in the literature associated with CA’s treatment [12, 43] and CS [44].
Limitations of the study
The findings of this analysis have to be seen in light of some limitations. Firstly, it must be stressed that it included only one randomized control trial. Some studies comprise a retrospective control group, and that increases the risk of bias.
Meaningful drawbacks include a small number of patients in each study who were additionally enrolled by different inclusion and exclusion criteria. It has caused that a part of studies excluded patients who underwent CA, whereas one study included them. Furthermore, one study based its inclusion criteria on the availability of a platelet function assessment. The SHOCK-COOL Trial by Fuernau et al. [19] does not describe any criteria because the trial was started before introducing the data-sharing policy. Zobel et al. [28] presents an analysis of patients who suffered from AMI and had only moderately reduced ejection fraction. Therefore, results could be different in patients with more severe compromised left ventricular function.
Cooling methods were also not consistent. The desired temperature was not reached in all patients in the TTM group. The duration of cooling in the majority of the studies was 24 hours — however, the odd one comprised 12 hours of the cooling procedure. The meaningful fact is that the standardization of cooling procedures is relevant, which cannot be seen in this review.
Conclusions
In summary, the present analysis shows no significant benefit of TTM in patients with CS. Moreover, no statistically significant increase was found in the incidence of adverse effects. However, further randomized studies with higher sample sizes and greater validity are needed to determine if TTM is worth implementing in CS patients.
Supplementary Information
Acknowledgements
The study was supported by the ERC Research Net and by the Polish Society of Disaster Medicine.
Footnotes
This paper was guest edited by Prof. Togay Evrin
Conflict of interest: Adam Nieborek — work for BARD Poland; all other authors report no conflicts of interest.
References
- 1.Vahdatpour C, Collins D, Goldberg S. Cardiogenic shock. J Am Heart Assoc. 2019;8(8):e011991. doi: 10.1161/JAHA.119.011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adrie C, Laurent I, Monchi M, et al. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10(3):208–212. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 3.Brener MI, Rosenblum HR, Burkhoff D. Pathophysiology and advanced hemodynamic assessment of cardiogenic shock. Methodist Debakey Cardiovasc J. 2020;16(1):7–15. doi: 10.14797/mdcj-16-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiele H, Ohman EM, de Waha-Thiele S, et al. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671–2683. doi: 10.1093/eurheartj/ehz363. [DOI] [PubMed] [Google Scholar]
- 5.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341(9):625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 6.Jones Q, Johnston B, Biola H, et al. Implementing standardized substance use disorder screening in primary care. JAAPA. 2018;31(10):42–45. doi: 10.1097/01.JAA.0000795888.70937.34. [DOI] [PubMed] [Google Scholar]
- 7.Stegman BM, Newby LK, Hochman JS, et al. Post-myocardial infarction cardiogenic shock is a systemic illness in need of systemic treatment: is therapeutic hypothermia one possibility? J Am Coll Cardiol. 2012;59:644–647. doi: 10.1016/j.jacc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Szarpak L, Filipiak KJ, Mosteller L, et al. Survival, neurological and safety outcomes after out of hospital cardiac arrests treated by using prehospital therapeutic hypothermia: A systematic review and meta-analysis. Am J Emerg Med. 2021;42:168–177. doi: 10.1016/j.ajem.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Deye N, Cariou A, Girardie P, et al. Endovascular versus external targeted temperature management for patients with out-of-hospital cardiac arrest: a randomized, controlled study. Circulation. 2015;132(3):182–193. doi: 10.1161/CIRCULATIONAHA.114.012805. [DOI] [PubMed] [Google Scholar]
- 10.Wieczorek W, Meyer-Szary J, Jaguszewski MJ, et al. Efficacy of targeted temperature management after pediatric cardiac arrest: a meta-analysis of 2002 patients. J Clin Med. 2021;10(7) doi: 10.3390/jcm10071389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szarpak L, Smereka J, Ruetzler K. Targeted temperature management: state of the art. Disaster Emerg Med J. 2019;4(2):68–73. doi: 10.5603/demj.2019.0014. [DOI] [Google Scholar]
- 12.Lascarrou JB, Merdji H, Le Gouge A, et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019;381(24):2327–2337. doi: 10.1056/NEJMoa1906661. [DOI] [PubMed] [Google Scholar]
- 13.Nolan J, Sandroni C, Böttiger B, et al. European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation. 2021;161:220–269. doi: 10.1016/j.resuscitation.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Scholefield BR, Silverstein FS, Telford R, et al. Therapeutic hypothermia after paediatric cardiac arrest: Pooled randomized controlled trials. Resuscitation. 2018;133:101–107. doi: 10.1016/j.resuscitation.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vargas M, Servillo G, Sutherasan Y, et al. Effects of in-hospital low targeted temperature after out of hospital cardiac arrest: A systematic review with meta-analysis of randomized clinical trials. Resuscitation. 2015;91:8–18. doi: 10.1016/j.resuscitation.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Bro-Jeppesen J, Jeppesen AN, Haugaard S, et al. TTM Investigators. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 17.Götberg M, van der Pals J, Olivecrona GK, et al. Mild hypothermia reduces acute mortality and improves hemodynamic outcome in a cardiogenic shock pig model. Resuscitation. 2010;81(9):1190–1196. doi: 10.1016/j.resuscitation.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzl M, Huber S, Maechler H, et al. Left ventricular diastolic dysfunction during acute myocardial infarction: effect of mild hypothermia. Resuscitation. 2012;83(12):1503–1510. doi: 10.1016/j.resuscitation.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuernau G, Beck J, Desch S, et al. Mild hypothermia in cardiogenic shock complicating myocardial infarction. Circulation. 2019;139(4):448–457. doi: 10.1161/CIRCULATIONAHA.117.032722. [DOI] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials BMJ 2019366l4898 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 22.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions BMJ 2016355i4919 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 24.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blatt A, Elbaz-Greener GA, Mizrachi A, et al. Adjunctive mild hypothermia therapy to primary percutaneous coronary intervention in patients with ST segment elevation myocardial infarction complicated with cardiogenic shock: A pilot feasibility study. Cardiol J. 2015;22(3):285–289. doi: 10.5603/CJ.a2014.0068. [DOI] [PubMed] [Google Scholar]
- 27.Orban M, Mayer K, Morath T, et al. The impact of therapeutic hypothermia on on-treatment platelet reactivity and clinical outcome in cardiogenic shock patients undergoing primary PCI for acute myocardial infarction: Results from the ISAR-SHOCK registry. Thromb Res. 2015;136(1):87–93. doi: 10.1016/j.thromres.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 28.Zobel C, Adler C, Kranz A, et al. Mild therapeutic hypothermia in cardiogenic shock syndrome. Crit Care Med. 2012;40(6):1715–1723. doi: 10.1097/CCM.0b013e318246b820. [DOI] [PubMed] [Google Scholar]
- 29.Levy B, Girerd N, Amour J, et al. Effect of moderate hypothermia vs normothermia on 30-day mortality in patients with cardiogenic shock receiving venoarterial extracorporeal membrane oxygenation: a randomized clinical trial. JAMA. 2022;327(5):442–453. doi: 10.1001/jama.2021.24776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oddo M, Schaller MD, Feihl F, et al. From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006;34(7):1865–1873. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PubMed] [Google Scholar]
- 31.Noc M, Laanmets P, Neskovic AN, et al. A multicentre, prospective, randomised controlled trial to assess the safety and effectiveness of cooling as an adjunctive therapy to percutaneous intervention in patients with acute myocardial infarction: the COOL AMI EU Pivotal Trial. EuroIntervention. 2021;17(6):466–473. doi: 10.4244/EIJ-D-21-00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erlinge D, Götberg M, Grines C, et al. A pooled analysis of the effect of endovascular cooling on infarct size in patients with ST-elevation myocardial infarction. EuroIntervention. 2013;8(12):1435–1440. doi: 10.4244/EIJV8I12A217. [DOI] [PubMed] [Google Scholar]
- 33.Kohlhauer M, Berdeaux A, Ghaleh B, et al. Therapeutic hypothermia to protect the heart against acute myocardial infarction. Arch Cardiovasc Dis. 2016;109(12):716–722. doi: 10.1016/j.acvd.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Cho HJ, Kyong YY, Oh YM, et al. Therapeutic hypothermia complicated by spontaneous brain stem hemorrhage. Am J Emerg Med. 2013;31(1):266.e1–266.e3. doi: 10.1016/j.ajem.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Wang CH, Chen NC, Tsai MS, et al. Therapeutic hypothermia and the risk of hemorrhage: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94(47):e2152. doi: 10.1097/MD.0000000000002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosillo SO, Lopez-de-Sa E, Iniesta AM, et al. Is therapeutic hypothermia a risk factor for stent thrombosis? J Am Coll Cardiol. 2014;63(9):939–940. doi: 10.1016/j.jacc.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 37.Chisholm GE, Grejs A, Thim T, et al. Safety of therapeutic hypothermia combined with primary percutaneous coronary intervention after out-of-hospital cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2015;4(1):60–63. doi: 10.1177/2048872614540093. [DOI] [PubMed] [Google Scholar]
- 38.Shah N, Chaudhary R, Mehta K, et al. Therapeutic Hypothermia and Stent Thrombosis: A Nationwide Analysis. JACC Cardiovasc Interv. 2016;9(17):1801–1811. doi: 10.1016/j.jcin.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 39.Jacob M, Hassager C, Bro-Jeppesen J, et al. The effect of targeted temperature management on coagulation parameters and bleeding events after out-of-hospital cardiac arrest of presumed cardiac cause. Resuscitation. 2015;96:260–267. doi: 10.1016/j.resuscitation.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Batista LM, Lima FO, Januzzi JL, et al. Feasibility and safety of combined percutaneous coronary intervention and therapeutic hypothermia following cardiac arrest. Resuscitation. 2010;81(4):398–403. doi: 10.1016/j.resuscitation.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Geurts M, Macleod MR, Kollmar R, et al. Therapeutic hypothermia and the risk of infection: a systematic review and meta-analysis. Crit Care Med. 2014;42(2):231–242. doi: 10.1097/CCM.0b013e3182a276e8. [DOI] [PubMed] [Google Scholar]
- 42.Mongardon N, Perbet S, Lemiale V, et al. Infectious complications in out-of-hospital cardiac arrest patients in the therapeutic hypothermia era. Crit Care Med. 2011;39(6):1359–1364. doi: 10.1097/CCM.0b013e3182120b56. [DOI] [PubMed] [Google Scholar]
- 43.Bernard S. Hypothermia after cardiac arrest: expanding the therapeutic scope. Crit Care Med. 2009;37(7 Suppl):S227–S233. doi: 10.1097/CCM.0b013e3181aa5d0c. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt-Schweda S, Ohler A, Post H, et al. Moderate hypothermia for severe cardiogenic shock (COOL Shock Study I & II) Resuscitation. 2013;84(3):319–325. doi: 10.1016/j.resuscitation.2012.09.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.