Abstract
Background:
Giant cerebral aneurysms have a high rupture rate, are often difficult to treat, and have a poor prognosis. We report two cases in which good results were achieved with a short, two-stage operation using a combination of endovascular treatment (EVT) and direct surgery.
Case Description:
Case 1 - A 50-year-old man had become immobile due to truncal ataxia after nausea. Magnetic resonance imaging, computed tomography (CT), and angiography revealed a giant thrombosed aneurysm of the right vertebral artery 30 mm in diameter, which compressed medulla oblongata. He underwent endovascular parent artery occlusion (PAO) followed by direct surgical thrombectomy 3 days later.The patient’s outcome was modified Rankin score (mRS) 1 at 7 days after the operation and mRS 0 at 1 year. Case 2 - A 40-year-old man developed a progressive visual disturbance. CT showed a giant thrombosed aneurysm of 50 mm diameter in the C2 portion of the left internal carotid artery. A balloon test occlusion (BTO) and cerebral blood flow single-photon emission computed tomography under BTO suggested partial ischemic tolerance due to PAO. PAO followed by low flow bypass and thrombectomy of the aneurysm by direct surgery was performed on the same day.The patient’s vision was improved with the outcome of mRS 1.
Conclusion:
EVT in a short-term two-stage operation for a thrombosed giant cerebral aneurysm is effective for the purpose of hemostasis in the thrombectomy designed to decompress the suffered brain or nerve. Complete PAO and meticulous perioperative use of antithrombotic agents are necessary to avoid perforator failure and hemorrhagic complications in this technique.
Keywords: Endovascular therapy, Giant aneurysm, Thrombosed aneurysm, Two-staged operation

INTRODUCTION
Symptomatic giant thrombosed cerebral aneurysms have a poor prognosis due to subarachnoid hemorrhage or compressive neurological disorders.[1] Treatment requires not only prevention of rupture but also relief of pressure on surrounding tissues if the pressure is symptomatic, which is sometimes a difficult task due to serious complications.
In this report, we describe two cases of giant thrombosed aneurysms, which were treated with endovascular parental artery occlusion (PAO) embolization followed by craniotomy for thrombus removal in a short-term two-phase procedure with good outcomes.
CASE DESCRIPTION
Case 1
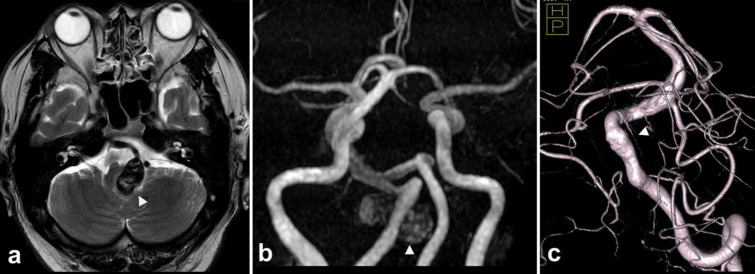
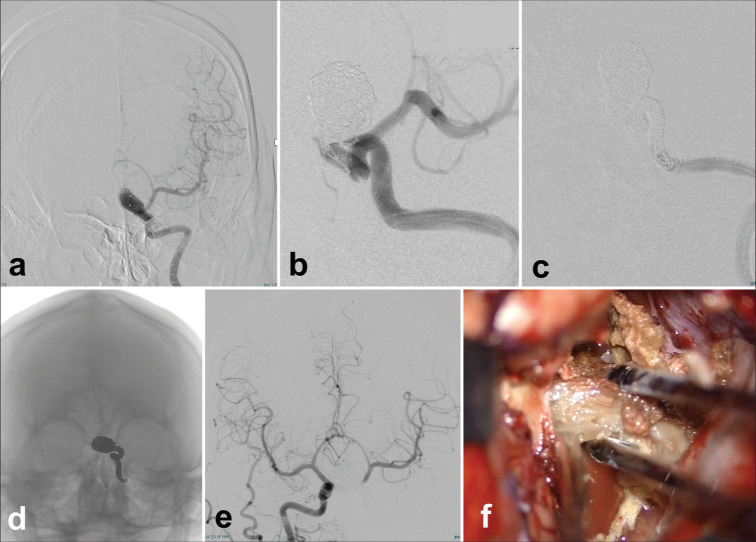
A 50-year-old man was transferred to the emergency department with loss of appetite, vomiting, and immobility. Neurologically, the patient developed a disorder of left dorsolateral medulla oblongata, and magnetic resonance imaging (MRI) showed a 30 mm mass located slightly left of center and heavily compressing the medulla oblongata [Figure 1]. Magnetic resonance angiography and three-dimensional computed tomographic angiography showed that this was a dolichoectatic aneurysm of the intracranial segment of the right vertebral artery (VA), and digital subtraction angiography (DSA) showed that the mass was a dolichoectatic aneurysm. On DSA, the frontal view of the right vertebral angiography on the affected side shows an anterior inferior cerebellar artery-posterior inferior cerebellar artery type aneurysm, and the left anterior oblique view shows that this irregular wall corresponds to the neck of the aneurysm, from which the main part of the thrombosed aneurysm dome protrudes medially and posteriorly, and an anterior spinal artery (ASA) was found 5 mm from the distal edge of the aneurysm. Our strategy was to reduce brainstem compression by removing the thrombus while ensuring blood flow control of the right VA, but the visibility and operationality of the distal side of the right VA would be difficult, so we decided to do an endovascular PAO followed by direct surgery. Fortunately, the origin of ASA had a 5 mm distance from the distal edge of the aneurysm. Double catheter technique was chosen to ensure a reliable PAO, which was completely achieved with the remaining flow of ASA through the distal radial artery approach [Figure 2]. After PAO, argatroban was administered continuously for 48 hours to prevent ischemia of the perforating branch, and after 24 hours of rest, craniotomy was performed to remove the thrombus.The patient had no complications, adequate decompression was achieved, and the outcome on day 7 was modified Rankin score 1 with no deficit at 1 year.
Figure 1:
Pre-treatment radiographical findings. (a) T2-weighted magnetic resonance image showed a mass lesion of 30 mm in diameter with hypo-signal intensity in the cerebellomedullary region (arrowhead) with surrounding hyperintensity suggesting brainstem. (b) Magnetic resonance angiography revealed that an aneurysm originated from the right vertebral artery (VA) (arrowhead). (c)Three-dimensional rotational digital subtraction angiography revealed that an anterior spinal artery (arrowhead) originated from 3 mm apart to the distal edge of the aneurysm neck on the right VA.
Figure 2:
Short-term two-stage operation for a patient with a thrombosed giant aneurysm of the right vertebral artery (VA). Endovascular parent artery occlusion of the affected artery (a and b) and decompressive partial removal of the giant thrombosed aneurysm through the suboccipital supracondylar fossa approach (c and d) and magnetic resonance imaging 1 year after the operation (e and f). (a)The right vertebral angiogram before the treatment showed an irregular arterial wall (arrow) that coincided with a giant aneurysm that is not described by internal thrombosis. An anterior spinal artery (ASA) originating from the V4 portion of the right VA is shown (arrowheads). (b)The left vertebral angiogram after the treatment shows complete obliteration of the right VA by coil embolization just before the origin of the ASA (arrowheads). (c)The intraoperative photograph shows the thrombosed aneurysm wall (asterisk). (d) Internal decompression of the thrombosed aneurysm. (e) T2WI 1 year after the surgery shows that the thrombosed aneurysm is reduced in size and brainstem compression is improved. (f)The right VA is not described on magnetic resonance angiography 1 year after the operation.
Case 2
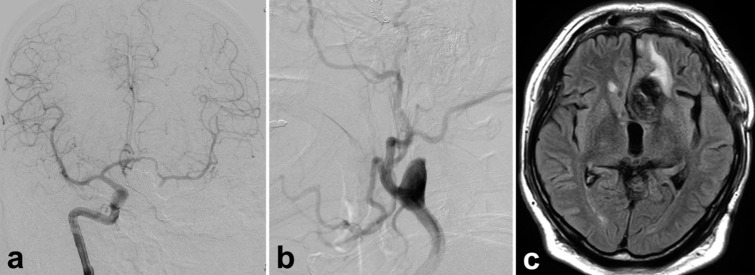
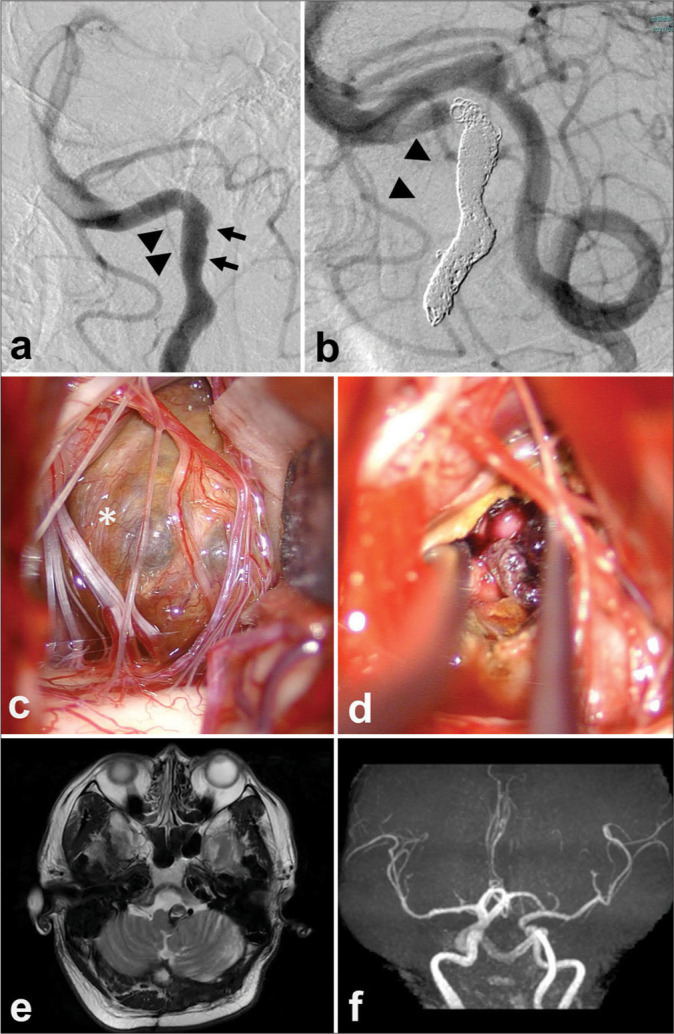
A 40-year-old man with rapidly deteriorating visual function had a 5 cm thrombosed aneurysm of the left internal carotid artery (ICA) [Figure 3]. On admission, he already had a light perception in the right eye and an exponential perception in the left eye. Immediate decompression of the thrombus was still desirable, but the thrombosed part and the residual blood flow were present in the aneurysm. Our strategy was to decompress the optic nerve by removing the thrombus after ensuring that the blood flow to the parent vessel was occluded, including the residual blood flow in the aneurysm. Direct surgery after endovascular PAO is planned [Figure 4] because it was difficult to secure the distal side of the affected ICA by only direct surgery. First, we studied the ischemic tolerance during PAO by balloon test occlusion. We found that the collateral pathway through the anterior communicating (Acom) artery was well developed. Still, there was a delay of 1.8 s on the occluded (affected) side in the venous phase, and an asymmetry index was 0.9 for the unoccluded side on single-photon emission computed tomography.The PAO technique was the same as in the first case through a distal radial approach. An anterior choroidal artery (Acho) is 7.5 mm distal to the neck of the aneurysm. After creating a solid frame at the site of the residual blood flow in the aneurysm, the aneurysm was completely occluded using the double catheter technique.The parent ICA was then completely occluded up to the petrous segment using the same double catheter technique.
Figure 3:
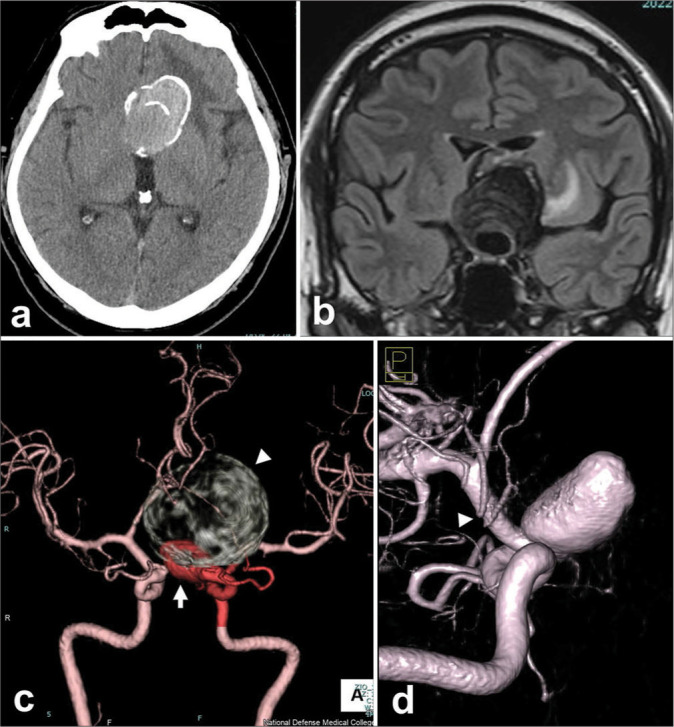
Pre-operative radiograms of a patient with a giant thrombosed aneurysm of the left internal carotid artery. (a and b) A large mass of 50 mm in diameter is shown at the left frontal region, which involves calcifications on computed tomography (CT) (a) and surrounding edema in the left frontal lobe on coronal magnetic resonance imaging of fluid-attenuated inversion recovery (b). (c)The whole configuration of the left carotid artery aneurysm, which consists of a large thrombosed and calcified part (arrowhead) and a flow-remnant part (arrow), is shown by the contrast-enhanced CT. (d)Three-dimensional rotational angiography revealed a 7.5 mm distance from the distal neck of the aneurysm to the origin of an anterior choroidal artery (arrowhead).
Figure 4:
Short-term two-stage operation for a patient with a thrombosed giant aneurysm of the left carotid artery. Endovascular parent artery occlusion of the affected artery (a-e) and decompressive partial removal of the giant thrombosed aneurysm through direct surgery (f). (a) A left internal carotid arteriogram shows a residual flow in the left carotid artery aneurysm and an upward-shifted left anterior cerebral artery (a). (b) Coil embolization was performed from the flow-residual part of the partially thrombosed aneurysm to the petrous portion of the left internal carotid artery (ICA). (c and d)The left ICA angiogram shows a complete occlusion from the aneurysm to the left proximal ICA. (e) A favorable distal flow of the left middle cerebral artery by the right ICA angiogram. (f) A decompressive removal of the aneurysmal thrombus was performed through left fronto-temporal craniotomy just after the low-flow bypass from the superficial temporal artery to the middle cerebral artery.
Just after PAO, protamine as a heparin antagonist was administered, and low-flow bypass and thrombus removal by direct surgery were performed.There were no postoperative complications [Figure 5]. His visual acuity has improved to 1.5 and 1.0 at 7 months, although homonymous hemianopsia persists.
Figure 5:
Post-operative course of the patient with giant thrombosed aneurysm of the right cerebral artery. (a) A right internal cerebral artery angiogram shows no dilation in the bilateral cerebral blood flow in the whole phase. (b) A left common carotid artery (CCA) angiogram shows a stump configuration at just distal the CCA bifurcation. (c) Fluid-attenuated inversion recovery magnetic resonance imaging 7 months after the surgery shows no regrowth of the aneurysm and further shrinking of the perifocal edema.
DISCUSSION
Large or giant cerebral thrombosed aneurysms
The 1-year mortality rate of giant thrombosed aneurysms is high, and prompt therapeutic intervention is considered.[1] In asymptomatic cases, observation may be chosen after careful consideration and discussion of the advantages and disadvantages of therapeutic intervention. However, in symptomatic aneurysms such as the present cases, surgical intervention is required for rapid decompression in addition to prevention of rupture.[3]
This study has two cases of thrombosed giant aneurysms as follows: one of which was a dolichoectatic VA aneurysm with brainstem compression, and another of which was an ICA aneurysm with optic nerve compression. Both cases required decompression of the suffered brain or nerve, but flow control during decompressive thrombectomy would be difficult only by direct surgery. It would be especially difficult to secure the distal part of the aneurysm safely.
The conditions of the collateral flow and perforating branch arteries
Conditions of collateral flow during PAO or the status of related perforating arteries are important for safety intervention. In our first case, the contralateral VA was well developed, and the ASA was far away from the distal neck of the aneurysm.Therefore, at first, PAO while preserving ASA was safely done, and 3 days later, the following decompressive surgery was completed with no bypass surgery. In our second case, the collateral path through the Acom artery was well developed but delayed cerebral blood flow was seen in the venous phase.The Acho is 7.5-mm distal to the neck of the aneurysm.Therefore, at first, aneurysm coiling with PAO was done, and after that, low-flow bypass and thrombectomy by direct surgery were completed.
Flow diversion for large or giant aneurysms
Recently, a new indication for endovascular treatment (EVT) with a flow diverter (FD) was added for wide-neck cerebral aneurysms with a maximum diameter of 5 mm or more in the VA (excluding the acute stage of rupture). In our cases, FD is also an option. FD is a breakthrough in that it prevents aneurysm rupture or enlargement without filling embolic substances. In particular, for aneurysms that develop with nerve compression symptoms, FD treatment has been reported to be highly effective in improving compression symptoms.[5,7] However, FD has not been elucidated for short- and medium- to long-term outcomes in such giant thrombosed aneurysms, including complications such as bleeding[8,10] and thrombosis,[2,4] and its natural history and superiority or inferiority to conventional therapies are not clear.[9] Furthermore, it is skeptical that FD can ameliorate neurological symptoms in thrombosed giant aneurysms such as our cases by reducing the water hammer effect. Surgical decompression under complete control of the parent artery would be required to improve neurological symptoms caused by compression of an organic hard thrombus.
Hybrid operation with combined EVT and direct surgery
Hybrid operation combining direct surgery and EVT has recently become common and favorable results,[6] indicating the usefulness of this strategy. Simultaneous hybrid surgery is useful[11] but requires intraoperative heparinization and carries the risk of complicated endovascular manipulation, especially in posterior circulation lesions, due to positioning. As in our case, after a thorough evaluation of the risk of cerebral ischemia associated with occlusion of the perforating branch or the mother vessel, it is useful to perform a short-term two-phase treatment to reduce the risks mentioned above. Our method of PAO by EVT before direct surgery was so effective in hemostasis during thrombectomy that desirable decompression was safely achieved. A further benefit of such a non-simultaneous combined way is not to undertake inconvenience of patients’ positioning compared to the simultaneous hybrid operation.
Antithrombotic use in short-term combined EVT and direct surgery
Robledo et al. reported a literature review including 17 studies on cranial aneurysms treated with endovascular embolization and arterial bypass; the overall success rate was 84%, with 16% of patients experiencing post-operative infarction.[6] Short-term two-stage EVT and direct surgery may reduce intraoperative bleeding, as the anticoagulants required for EVT are not needed for direct surgery. Furthermore, in the first case, there was concern about PAO-related ischemia in the perforating artery, but the use of antiplatelet therapy was helpful, and it was considered important to meticulous perioperative use such as antithrombotic therapy in this treatment.
CONCLUSION
Endovascular therapy in short-term two-stage operation for thrombosed giant cerebral aneurysms is effective for thrombectomy designed to decompress the suffered brain or nerve.
Meticulous perioperative use and control of antithrombotic agents are necessary to avoid perforator failure and hemorrhagic complications.
Footnotes
How to cite this article: Fujii K, Toyooka T, Yamamoto T, Nitta Y, Nakagawa M, Yoshiura T, et al. Efficacy of endovascular therapy in short-term two-stage operation for thrombosed giant cerebral aneurysm. Surg Neurol Int. 2024;15:374. doi: 10.25259/SNI_706_2024
Contributor Information
Kazuya Fujii, Email: ndmcfujii@yahoo.co.jp.
Terushige Toyooka, Email: terutoy0807@gmail.com.
Tetsuya Yamamoto, Email: ytetsuya1991@yahoo.co.jp.
Yuki Nitta, Email: myuon.yn@gmail.com.
Masaya Nakagawa, Email: nakagawamasaya19880215@gmail.com.
Toru Yoshiura, Email: torumiru.neurosurgery@gmail.com.
Satoru Takeuchi, Email: stake@ndmc.ac.jp.
Shunsuke Tanoue, Email: stanoue@ndmc.ac.jp.
Kojiro Wada, Email: stingray@ndmc.ac.jp.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management.The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Dengler J, Rufenacht D, Meyer B, Rohde V, Endres M, Lenga P, et al. Giant intracranial aneurysms: Natural history and 1-year case fatality after endovascular or surgical treatment. J Neurosurg. 2019;134:49–57. doi: 10.3171/2019.8.JNS183078. [DOI] [PubMed] [Google Scholar]
- 2.Fiorella D, Hsu D, Woo HH, Tarr RW, Nelson PK. Very late thrombosis of a pipeline embolization device construct: Case report. Neurosurgery. 2010;67(3 Suppl Operative):onsE313–4. doi: 10.1227/01.NEU.0000383875.08681.23. discussion onsE314. [DOI] [PubMed] [Google Scholar]
- 3.Linskey ME, Sekhar LN, Hirsch W, Jr, Yonas H, Horton JA. Aneurysms of the intracavernous carotid artery: Clinical presentation, radiographic features, and pathogenesis. Neurosurgery. 1990;26:71–9. [PubMed] [Google Scholar]
- 4.McAuliffe W, Wycoco V, Rice H, Phatouros C, Singh TJ, Wenderoth J. Immediate and midterm results following treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol. 2012;33:164–70. doi: 10.3174/ajnr.A2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon K, Albuquerque FC, Ducruet AF, Crowley RW, McDougall CG. Resolution of cranial neuropathies following treatment of intracranial aneurysms with the Pipeline Embolization Device. J Neurosurg. 2014;121:1085–92. doi: 10.3171/2014.7.JNS132677. [DOI] [PubMed] [Google Scholar]
- 6.Robledo A, Frank TS, O’Leary S, Kan P. Hybrid treatment for a giant fusiform partially thrombosed middle cerebral artery aneurysm with superficial temporal artery to middle cerebral artery bypass followed by endovascular vessel sacrifice: 2-dimensional operative video. Oper Neurosurg (Hagerstown) 2024;26:117–8. doi: 10.1227/ons.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 7.Szikora I, Marosfoi M, Salomvary B, Berentei Z, Gubucz I. Resolution of mass effect and compression symptoms following endoluminal flow diversion for the treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2013;34:935–9. doi: 10.3174/ajnr.A3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turowski B, Macht S, Kulcsar Z, Hanggi D, Stummer W. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent): Do we need to rethink our concepts? Neuroradiology. 2011;53:37–41. doi: 10.1007/s00234-010-0676-7. [DOI] [PubMed] [Google Scholar]
- 9.van Rooij WJ, Sluzewski M, van der Laak C. Flow diverters for unruptured internal carotid artery aneurysms: Dangerous and not yet an alternative for conventional endovascular techniques. AJNR Am J Neuroradiol. 2013;34:3–4. doi: 10.3174/ajnr.A3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velat GJ, Fargen KM, Lawson MF, Hoh BL, Fiorella D, Mocco J. Delayed intraparenchymal hemorrhage following pipeline embolization device treatment for a giant recanalized ophthalmic aneurysm. J Neurointerv Surg. 2012;4:e24. doi: 10.1136/neurintsurg-2011-010129. [DOI] [PubMed] [Google Scholar]
- 11.Xin C, Li Z, Zhang J, Xiong Z, Wu X, Zhao S, et al. Combined surgical and endovascular treatment of a complex posterior communicating artery aneurysm at one-stage in a hybrid operating room. World Neurosurg. 2018;116:383–6. doi: 10.1016/j.wneu.2018.05.033. [DOI] [PubMed] [Google Scholar]