Abstract
Background:
There has been no clear consensus on the clinical markers to distinguish alcohol-related seizures (ARSs) from epileptic seizures. We have reported the usefulness of gamma oscillation (30–70 Hz) regularity (GOR) analysis using interictal electroencephalography (EEG) data to evaluate epileptogenic focus. We conducted interictal GOR analysis using scalp EEG and susceptibility-weighted imaging (SWI) to visualize the epileptogenic focus in two cases initially suspected to have ARS.
Case Description:
In each case, a significantly high GOR area suggestive of epileptogenic focus was detected and that area was consistent with that where SWI showed hemosiderin deposit. In one patient, seizures were well controlled with the introduction of anti-seizure medication (ASM). In another patient, ASM was introduced but is refractory, and epilepsy surgery is being considered in the future.
Conclusion:
The interictal GOR analysis and SWI can successfully contribute to identify the patients suspected to have ARS who may have epileptogenic focus and can be treated with ASM and epilepsy surgery.
Keywords: Alcohol-related seizures, Anti-seizure medication, Epilepsy surgery, Gamma oscillation regularity, Susceptibility-weighted imaging

INTRODUCTION
Although alcohol-related seizure (ARS) is a significant symptom of alcohol abuse, little is known about its mechanism.[12] There has been no clear consensus on clinical markers to distinguish ARS from epileptic seizures.[1] Recently, we reported interictal gamma oscillation regularity (GOR) with electroencephalography (EEG), and could help determine the epileptogenic focus in epilepsy patients.[7-9] The GOR analysis calculates the average of gamma band sample entropy scores using multi-scale entropy analysis. Lower sample entropy scores represent a higher regularity of gamma oscillations, which we define as high GOR. One of the benefits of GOR analysis is that it may identify epileptogenic focus not very accurately detectable with conventional EEG findings.
We also focused on whether susceptibility-weighted imaging (SWI) can illustrate the unknown epileptogenic focus in patients with ARS. The SWI is an MRI technique that can distinguish hemosiderin deposit rich lesions, which often cause epilepsy. The SWI has been widely used as a standard protocol in addition to routine MRI after mild traumatic brain injury (TBI),[3] which is reported to be associated with alcohol abuse.[11] We hypothesized that some patients in the group of patients treated as ARS may have epilepsy triggered by TBI. For such patients, anti-seizure medication (ASM) and epilepsy surgery may improve the course of the disease.
In this report, we present our experience with patients with suspected ARS, where interictal GOR analysis and SWI allowed us to evaluate the epileptogenic focus and give appropriate treatment.
CLINICAL PRESENTATION
Case 1
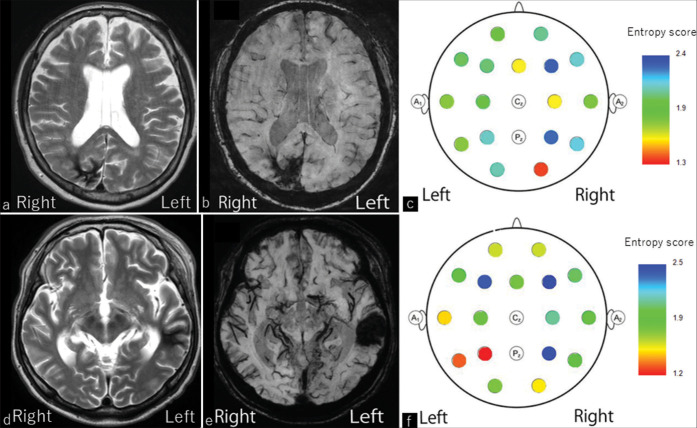
A 50-year-old female patient with alcohol abuse was admitted to our department with general convulsion and refractory seizures, such as an illusion of her left eye field and numbness in her left arm. There was a history of TBI in her 30s, and she often hit her left shoulder on the surroundings. T2-weighted image demonstrated low-signal intensity in the right occipital lobe [Figure 1a]. The SWI showed a hemosiderin deposit in the right occipital lobe [Figure 1b], which could explain the hemianopsia of frequent hitting of the left shoulder. In EEG, very small potential sharp transients were identified on the right occipital region. The interictal GOR analysis demonstrated significantly high GOR in the right occipital lobe [Figure 1c]. Oral levetiracetam of 1000 mg was started, but 2 months later, she was admitted to our department with general convulsion again. Since the addition of 100 mg of lacosamide orally, seizures have been well controlled.
Figure 1:
Results of Cases 1 and 2. (a) In Case1, T2-weighted image demonstrates low signal intensity in the right occipital lobe. (b) SWI shows hemosiderin deposits in the right occipital lobe. (c) Interictal GOR analysis shows epileptogenic focus in the right occipital lobe. (d) In Case 2, T2-weighted image demonstrates low signal intensity in the left temporal lobe. (e) SWI shows hemosiderin deposits in the left temporal lobe. (f) Interictal GOR analysis shows epileptogenic focus in the left temporal lobe. Note that the lower the entropy score, the higher the GOR, and vice versa. GOR: Gamma oscillation regularity
Case 2
A 40-year-old male patient with a history of alcohol abuse was admitted to the outpatient clinic complaining of severe anxiety, lightning sensation in the visual field, and refractory loss of consciousness. The patient had been treated in other psychiatric clinics with a diagnosis of depression and had taken oral antidepressants for many years. He had refractory seizures, but those were diagnosed as ARS in other clinics. The patient had a history of several mild TBIs. T2-weighted image demonstrated low-signal intensity in the left temporal lobe and mild brain atrophy [Figure 1d]. The SWI clearly showed a hemosiderin deposit in the left temporal lobe [Figure 1e]. In EEG, interictal spike waves were observed mainly on the left temporal region. The interictal GOR analysis demonstrated significantly high GOR in the left temporal lobe [Figure 1f]. He was introduced to several ASMs but is refractory, and epilepsy surgery is being considered in the future.
EEG data recordings
EEG data were recorded using the Nihon-Kohden EEG system at 17 electrode sites (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, and Fz), in accordance with the international 10–20 system, with the two ear lobes jointly forming the reference. The EEG signals were recorded with a sampling rate of 500 Hz, a 1–60 Hz bandpass filter, and a time constant of 0.3 s. A 60-Hz notch filter was applied to all channels. All selected EEG data were inspected to ensure that artifacts did not contaminate them.
SWI protocol
For MRI for SWI sequence, the following parameters were used: TR 29 ms, TE 20 ms, Matrix 288 × 288, and flip angle 10°.
GOR analysis
The detailed algorithm for GOR analysis using the sample entropy method is described in our previous study.[7-9]
DISCUSSION
Previous reports show alcohol intake promotes iron accumulation in the brain.[4,10] Considering that mild TBI is associated with alcohol abuse,[11] we speculate that SWI should be performed for patients with ARS not only at the time of TBI onset but also at the time of admission of seizures even after a long period of their trauma. We suppose that SWI can be considered a routine MRI techniques to evaluate alcohol abuse patients because some pathological lesions may not be diagnosed with previous CT scans or routine MRI.
In this report, interictal GOR analysis could illustrate the epileptogenic focus in both two patients. To distinguish ARS from epileptic seizures, it may be desirable that those patients stay in the hospital for several days to be analyzed with video EEG monitoring and are observed for a sufficient period with ASMs in the outpatient department. However, alcoholic patients often refuse to be hospitalized or fail to follow their physicians’ advice. As interictal GOR analysis can be performed within 1 h in an outpatient department, we suppose that this new method may be highly compatible with such patients.
Although it is widely accepted that ASMs are not effective for patients with ARS,[5] we would like to emphasize that true epileptic patients, like the patients in this report, may be concealed among those diagnosed with ARS and that such epileptic patients should be appropriately treated with ASM, and epileptic surgery should be considered in the case of drug resistance. Furthermore, to accomplish this, we recommend that an interictal GOR analysis and SWI be performed on alcohol users with ARS. It is also reported that the mortality risk is very high in epilepsy patients who take alcohol.[6] Therefore, we assume that further analysis by interictal GOR analysis will be necessary among alcohol abuse patients with or without seizures.
In both two patients, the epileptogenic foci that were detected by interictal GOR analysis were directly related to the hemosiderin deposit lesion detected by SWI. Past mild TBI likely caused hemosiderin deposition and epileptogenic formation. Hemosiderin deposition and subsequent reactive gliosis can develop into epileptogenicity by facilitating neuronal synchrony.[2] We assume that the interictal GOR analysis may detect such neuronal synchrony as a significantly high GOR.
This study had some limitations. First, it was a retrospective study. Second, the number of patients is small. Third, it is single institutional. Although the data obtained from these two cases were insufficient to convincingly suggest a role for interictal GOR in the identification of epileptogenic foci, it could highlight efficient use in isolating potential candidates who may be worthy of detailed evaluation for intractable seizures. We will continue to study this topic and provide more cases.
CONCLUSION
Interictal GOR analysis and SWI may be helpful in distinguishing some patients with ARS who may have epileptogenic lesions that can be treated with ASM or epilepsy surgery.
Footnotes
How to cite this article: Tsuji Y, Sato Y. Interictal gamma oscillation regularity analysis and susceptibility-weighted imaging on focal epilepsy cases with alcohol use disorders. Surg Neurol Int. 2024;15:361. doi: 10.25259/SNI_991_2023
Contributor Information
Yoshihito Tsuji, Email: tanunpas@gmail.com.
Yosuke Sato, Email: yanda2011@gmail.com.
Data availability statement
The raw data supporting this article will be made available by the authors without undue reservation.
Ethical approval
The research/study approved by the Institutional Review Board at the Department of Neurosurgery, Matsubara Tokushukai Hospital, Osaka, Japan, number #20–06, dated August 17, 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Bob P, Jasova D, Bizik G, Raboch J. Epileptiform activity in alcohol dependent patients and possibilities of its indirect measurement. PLoS One. 2011;6:e18678. doi: 10.1371/journal.pone.0018678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- 3.Hageman G, Hof J, Nihom J. Susceptibility-weighted MRI and microbleeds in mild traumatic brain injury: Prediction of posttraumatic complaints? Eur Neurol. 2022;85:177–85. doi: 10.1159/000521389. [DOI] [PubMed] [Google Scholar]
- 4.Juhás M, Sun H, Brown MR, MacKay MB, Mann KF, Sommer WH, et al. Deep grey matter iron accumulation in alcohol use disorder. Neuroimage. 2017;148:115–22. doi: 10.1016/j.neuroimage.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Lai JY, Kalk N, Roberts E. The effectiveness and tolerability of anti-seizure medication in alcohol withdrawal syndrome: A systematic review, meta-analysis and GRADE of the evidence. Addiction. 2022;117:5–18. doi: 10.1111/add.15510. [DOI] [PubMed] [Google Scholar]
- 6.Mclean B, Pace A, Gorton HC, Webb RT, Parisi R, Carr MJ, et al. Alcohol-specific mortality in people with epilepsy: Cohort studies in two independent population-based datasets. Front Neurol. 2021;1:623139. doi: 10.3389/fneur.2020.623139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato Y, Wong SM, Iimura Y, Ochi A, Doesburg SM, Otsubo H. Spatiotemporal changes in regularity of gamma oscillations contribute to focal ictogenesis. Sci Rep. 2017;7:9362. doi: 10.1038/s41598-017-09931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato Y, Ochi A, Mizutani T, Otsubo H. Low entropy of interictal gamma oscillations is a biomarker of the seizure onset zone in focal cortical dysplasia type II. Epilepsy Behav. 2019;96:155–9. doi: 10.1016/j.yebeh.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Tsuji Y, Yamazaki M, Fujii Y, Shirasawa A, Harada K, et al. Interictal high gamma oscillation regularity as a marker for presurgical epileptogenic zone localization. Oper Neurosurg. 2022;23:164–73. doi: 10.1227/ons.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Martins-Bach AB, Alfaro-Almagro F, Douaud G, Klein JC, Llera A, et al. Phenotypic and genetic associations of quantitative magnetic susceptibility in UK Biobank brain imaging. Nat Neurosci. 2022;25:818–31. doi: 10.1038/s41593-022-01074-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch KA, Derry C. Mild traumatic brain injury and epilepsy: Alcohol misuse may underpin the association. J Neurol Neurosurg Psychiatry. 2014;85:593. doi: 10.1136/jnnp-2013-306267. [DOI] [PubMed] [Google Scholar]
- 12.Woo KN, Kim K, Ko DS, Kim HW, Kim YH. Alcohol consumption on unprovoked seizure and epilepsy: An updated meta-analysis. Drug Alcohol Depend. 2022;232:109305. doi: 10.1016/j.drugalcdep.2022.109305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting this article will be made available by the authors without undue reservation.