Abstract
Background:
The specific objectives of this study are to identify the carotid cavernous fistula (CCF) type based on computerized tomography angiography (CTA) results, determine the cut-off diameter of the superior ophthalmic vein (SOV) and internal jugular vein (IJV) in CCF patients, and to evaluate the correlation between diameters of the right and left SOV and IJV with CCF type and location.
Methods:
A retrospective analysis of data from 35 CCF patients at our institution was conducted between January 2016 and October 2022. The analysis separated the vascular diameters of the right and left SOV and IJV, which were compared to 35 non-CCF patients. The non-CCF group consisted of individuals who underwent CTA for conditions unrelated to vascular abnormalities.
Results:
In 35 CCF patients, the dilatation of the left SOV was significantly correlated with direct CCF type with a cutoff of >0.5 cm and significantly associated with indirect CCF type with a cutoff of <0.5 cm (P = 0.017), while the right SOV was not significantly correlated (P = 0.187). There was no significant correlation between the right and left IJV with CCF type or location (right IJV, P = 0.996 and left IJV, P = 0.558). However, the analysis indicated that IJV size differences between CCF and non-CCF patients were significant.
Conclusion:
Dilation of the left SOV correlates with both direct and indirect CCF types, while the right SOV and IJV (both sides) do not show a significant correlation with CCF type or location. This suggests that left SOV dilation may serve as an early indicator of CCF type, particularly in cases involving the left side.
Keywords: Carotid cavernous fistula (CCF), Internal jugular vein, Superior ophthalmic vein (SOV)

INTRODUCTION
A carotid cavernous fistula (CCF) is an abnormal connection between the carotid artery and the cavernous sinus (CS), which is a venous structure in the intracranial area.[1] CCF is generally classified into direct and indirect.[8] Clinical symptoms and signs typically appear acutely in direct CCF cases and more slowly in indirect CCF cases.[1]
Craniocerebral trauma is the most common predisposing factor for direct CCF, accounting for over 75% of CCF cases. Previous research has shown that CCF occurs in 0.2% of patients with craniocerebral trauma and 4% with skull base fractures.[6] In Indonesia, the incidence of head trauma in East Java is 11.9%, resulting in a CCF prevalence rate of approximately 0.0022% in the province.
When a patient is suspected of having CCF, clinicians usually request a computerized tomography angiography (CTA) examination, followed by arteriography. If CCF is diagnosed, endovascular treatment remains the gold standard for CCF management, particularly for direct types.[15] However, in some cases, endovascular treatment is not performed after arteriography due to various reasons, such as cost or lack of availability of equipment, especially in indirect CCF cases. As a result, the costs and time required for treatment increase. Direct CCF requires immediate endovascular intervention to manage clinical symptoms and prevent long-term complications. Indirect CCF, on the other hand, can close spontaneously in more than 70% of cases.[1] Consequently, rapid CCF diagnosis and type determination are paramount for preempting potential complications. This study also addresses the value of its findings for regions with limited facilities that have access only to CT scans, offering guidance in diagnosing CCF types where angiography is unavailable.
MATERIALS AND METHODS
This retrospective study was conducted on 35 CCF patients at our institution from January 2016 to October 2022. The patient’s age ranges between 11 and 68 years. The average age of patients in the sample is 38 years. The study aimed to evaluate the correlation between the diameter of the superior ophthalmic vein (SOV) and internal jugular vein (IJV) with CCF type and lesion location.
The inclusion criteria for CCF cases that could be subjects of this study are as follows:
Patients who have undergone a CTA examination at our radiology department
Patients who have been confirmed to have CCF and its type are based on digital subtraction angiography (DSA) at the radiology department.
If there is a combination of direct and indirect CCF types, it will be concluded as direct CCF. The exclusion criteria were patients with the same medical record examined more than once, and the results of examinations other than the first will be excluded. However, it is important to note that in our patient cohort, specific conditions associated with SOV dilatation, such as increased intracranial pressure, Valsalva, Graves’ disease, and intra- or retrocavernous masses, were meticulously evaluated and excluded during the patient selection process.
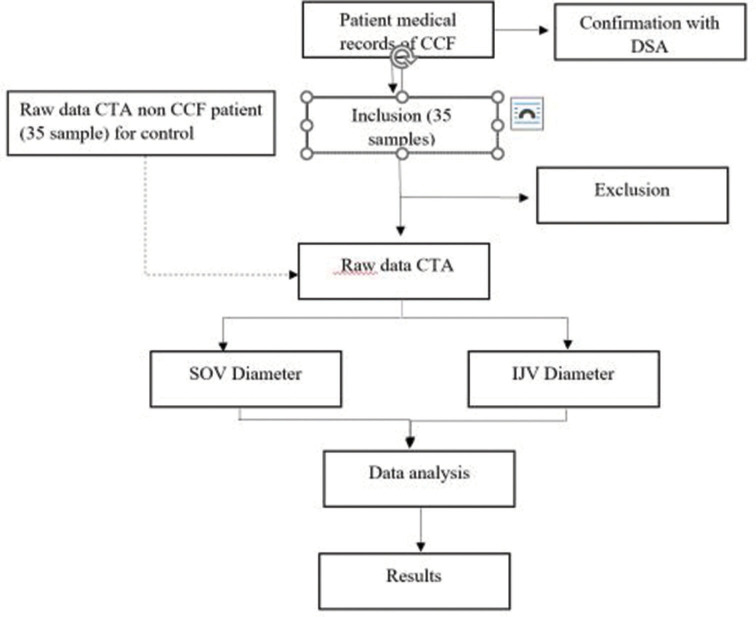
An interventional radiology expert re-evaluated the digital data of CTA. Clinical and imaging data were obtained from the medical records of CCF patients who met the inclusion and exclusion criteria and underwent a CTA examination on 16-slice Siemens Somatom Multislice CT scan and 128-slice Philips Ingenuity Core Multislice CT Scan machines. The obtained data were confirmed by DSA examination and then statistically analyzed using a correlation test, calculating the correlation between CCF type and the diameter of the SOV and IJV. The SOV and the IJV’s diameter in non-CCF patients were used as a comparison [Figure 1].
Figure 1:
Study flowchart. CCF: Carotid cavernous fistula, DSA: Digital subtractioangiography, CTA: Computed tomography angiography, IJV: Internal jugular vein, SOV: Superior ophthalmic vein
In the non-CCF group, patients were selected from those who had undergone CTA examinations for other conditions, excluding vascular disorders related to CCF. The baseline characteristics of the two groups are as follows:
CCF Group (n = 35): Patients aged 11–68 years, with a mean age of 38 years. Gender distribution was nearly equal, with 18 males (51.4%) and 17 females (48.6%)
Non-CCF Group (n = 35): Patients aged 15–70 years, with a mean age of 42 years. The gender distribution was 20 males (57%) and 15 females (43%).
The non-CCF patients were not healthy volunteers but rather individuals who underwent CTA due to other nonvascular conditions. These patients were selected based on their medical records, ensuring that none had any vascular abnormalities related to CCFs.
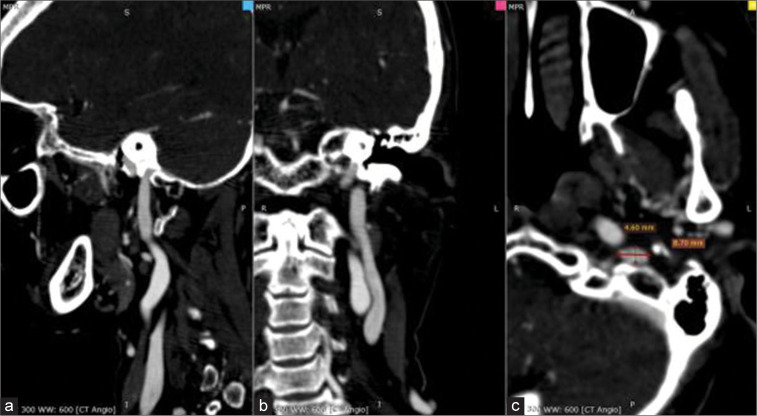
The diameter of the IJV and SOV were measured in two dimensions (craniocaudal, anteroposterior, and mediolateral) and divided by two [Figures 2 and 3].
Figure 2:
a: Sagittal, b:Coronal, and c: Axial. An axial view of an angiography computed tomography scan of a 60-year-old patient’s head shows the internal jugular vein in two-dimensional projection (anteroposterior and mediolateral).
Figure 3:
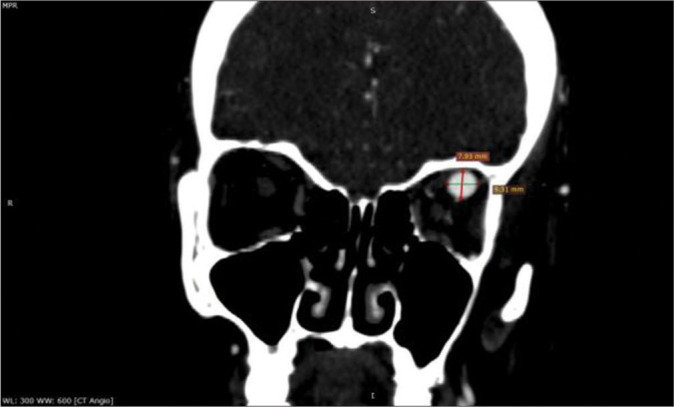
Coronal view of head angiography computed tomography scan of a 41-year-old patient shows the left superior ophthalmic vein in two-dimensional projection (craniocaudal and mediolateral).
The clinical parameters were the diameter of the SOV, the diameter of the IJV at the level of the inferior petrosal sinus, and the type of CCF, whether direct or indirect. The statistical analysis was conducted using the Chi-square test to investigate the relationship between gender and patient status with the use of CCF, Mann–Whitney U-test to determine the differences in Internal jugular vein right (IJVR), Internal jugular vein left (IJVL), superior ophthalmic vein right (SOVR), and superior ophthalmic vein left (SOVL) values between CCF patients with direct versus indirect, and the receiver operating characteristic test to find the cutoff for IJVR, IJVL, SOVR, and SOVL values between CCF patients with direct versus indirect [Figure 4].
Figure 4:
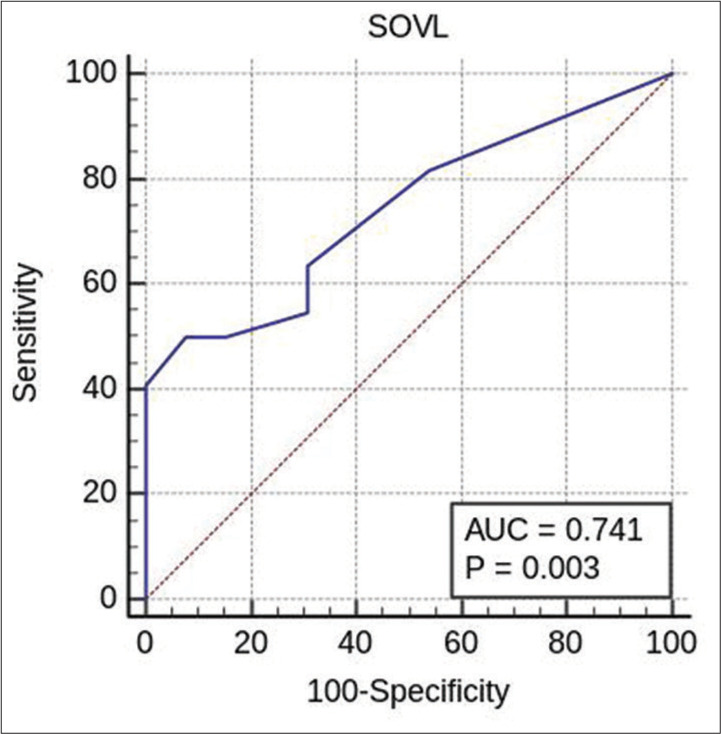
Receiver operating characteristic test for finding the cutoff of SOVL in carotid cavernous fistula patients based on direct versus Indirect. SOVL: Superior ophthalmic vein left, AUC: Area under curve.
RESULTS
The general objective of this study was to determine the correlation between the diameter of the SOV and the IJV based on the type of CCF in patients who underwent CTA at our institution from January 2016 to October 2022. A total of 35 patients met the inclusion criteria for analysis in this study, while two patients were excluded from the study. Based on gender, it was found that there were nearly equal numbers of male and female patients, with 18 male patients (51.4%) and 17 female patients (48.6%) with CCF. The statistical analysis showed P > 0.05, indicating no significant difference in gender distribution between the CCF and non-CCF patient groups.
We conducted a separate analysis for the right and left sides of the SOV and IJV. The vascular diameters of the left and right SOVs and IJVs were compared for both CCF and non-CCF groups.
Analysis of CCF types based on CTA results
The distribution of CCF patients based on type was divided into two categories: direct and indirect. The study found that the majority of CCF patients were of the direct type, with 22 patients (62.9%), while 13 patients (37.1%) were of the indirect type.
The distribution of CCF patients based on location was divided into three categories: right, left, and bilateral. The study found that the majority of CCF patients were located on the left side, with 22 patients (62.9%), followed by 12 patients (34.3%) on the right side, and only one patient (2.9%) with bilateral CCF. In both CCF and non-CCF patients, the statistical analysis showed P > 0.05, indicating that the data were normally distributed. Normality tests were conducted for all CCF patients to determine the appropriate correlation test. The measurement of the right and left IJV was normally distributed, while the right and left SOV was not normally distributed. Pearson correlation tests were conducted for variable pairs that were normally distributed, while Spearman correlation tests were conducted for pairs of variables that were not normally distributed.
The diameter of the right and left SOV in CCF patients
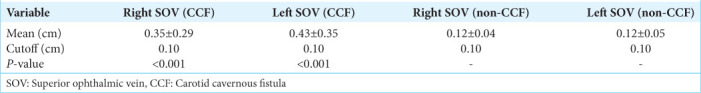
The average size of the right and left SOV in CCF patients was 0.35 ± 0.29 cm and 0.43 ± 0.35 cm, respectively. For patients with right-side CCF, the average diameter of the right SOV was 0.37 ± 0.28 cm, and the left SOV was 0.40 ± 0.31 cm. Although the right SOV showed slight enlargement, the correlation was not statistically significant (P = 0.156). In non CCF patients, the corresponding measurements were 0.12 ± 0.04 cm for the right SOV and 0.12 ± 0.05 cm for the left SOV [Table 1]. A significant difference in the size of the right and left SOV was found in CCF patients compared to non-CCF patients, with a cutoff value of 0.1 cm for both the right and left SOV (P < 0.05).
Table 1:
The average size of the right and left SOV in CCF patients.
The diameters of the right and left IJV in CCF patients
In our institution, the mean diameter of the right IJV was 0.92 ± 0.19 cm, while the mean diameter of the left IJV was 0.73 ± 0.17 cm. In non-CCF patients, the corresponding measurements were 0.78 ± 0.19 for the right IJV and 0.64 ± 0.16 cm for the left IJV [Table 2]. Compared to non-CCF patients, a significant difference was found in the size of the right and left IJV in CCF patients, with a cutoff value of 0.9 cm for the right IJV and 0.5 cm for the left IJV (P < 0.05).
Table 2:
The average size of the right and left IJV in CCF patients.

Analyzing the diameter of the right and left SOV based on CCF types
We analyzed the vascular diameters of the left and right SOVs separately for CCF patients. A significant correlation was found for left SOV diameters in patients with left-side CCF (P = 0.017) with a cutoff of >0.5 cm and significantly associated with indirect CCF type with a cut-off of <0.5 cm, while no significant correlation was found for the right SOV in right-side CCF cases (P = 0.187).
Analysis of the diameter of the right and left SOV based on the type of CCF showed a significant correlation on the left SOV between direct and indirect types with a cutoff of 0.5 cm and P < 0.05. However, there was no significant correlation on the right SOV between direct and indirect types with P > 0.05 [Figure 4]. The statistics of the SOV based on the type of CCF are presented [Table 3].
Table 3:
Statistical analysis of SOV based on CCF type.

Analysis of the right and left IJV diameter based on CCF type
In the case of the IJV, we observed no significant difference between the right and left sides (P > 0.05) in either direct or indirect CCF cases. This is likely because the IJV receives not only shunt blood flow but also cerebral venous drainage. As the disease progresses, the posterior drainage through the inferior petrosal sinus can become blocked, which may reduce blood flow to the IJV on the affected side [Table 4].
Table 4:
The correlation between right IJV and left IJV based on the location of CCF (P-value).

Analysis of right and left SOV diameter based on CCF type and location
The final analysis was performed by dividing patients based on the location of the lesion, presented overall, and by CCF type. The left side was the most affected by CCF, and significant correlations were found between the following variable pairs: left SOV with direct and indirect left CCF and right SOV with right CCF [Table 5]. There was only one patient with bilateral CCF; therefore, correlation analysis could not be performed on this patient.
Table 5:
Presents the correlation between right SOV and left SOV based on the location of CCF (P-value).

Analysis of right and left IJV diameter based on CCF location
No significant correlation was found between the diameter of the right and left IJV with the location of CCF (P > 0.05).
DISCUSSION
The drainage of CCF can be divided into five types: anterior drainage toward SOV, inferior drainage toward IPS and pterygoid plexus, contralateral drainage through intercavernous connections, posterior drainage through the deep venous system, superior petrosal sinus and cerebellar vein, and superior drainage through superficial middle cerebral vein.[2] Venous drainage was categorized into posterior/inferior drainage toward SPS and IPS, pterygoid and parapharyngeal plexus drainage, anterior drainage toward SOV and IOV, cortical drainage toward superficial middle cerebral vein, and perimesencephalic and cerebellar venous system.[19]
Of the total 35 CCF patients in this study, the majority had lesions on the left side, about one-third on the right side, and one patient had bilateral lesions. Similar findings were reported by Chen et al.[3] regarding the proportion of lesion locations, with the most on the left, followed by the right side, and only one patient with bilateral CCF. In other research, the left side had more CCF than the right side, with 26 patients on the left side, 23 patients on the right side, and 16 patients with bilateral CCF.[16] Bilateral CCF is rare, and according to the literature, most of them are post-traumatic CCF [5] while Lee et al. found that the right side had more CCF than the left side, and only one of 16 patients had bilateral CCF.[11] The difference is due to the location of the lesion, which can occur in various locations and lead to different results.
In this study, CCF patients had an average size of the right SOV of 0.35 ± 0.29 cm and the left SOV of 0.43 ± 0.35 cm. These sizes exceeded the average diameter of the right-left SOV lumen in non-CCF patient populations with a cutoff of 0.12 cm. These sizes indicate the dilation of the SOV diameter in the study subjects, as the reported range of normal SOV diameters varies from 0.3 mm to 4.6 mm.[1] However, differences in race can lead to differences in normal values with the cutoff value of non-CCF patients in this study. Enlargement of the SOV on standard CT or magnetic resonance imaging (MRI) scans, whether unilateral or bilateral, has been reported to be associated with CCF.[9] Thisfinding is consistent with the literature and previous studies, which have reported that enlargement of the SOV on standard CT or MRI scans, whether unilateral or bilateral, is associated with CCF and indicates drainage to the anterior through the SOV.[4]
In this study, patients with CCF had an average right IJV size of 0.92 cm and a left IJV size of 0.73 cm. These measurements exceeded the average diameter of the IJV lumen in nonCCF patients with cutoffs of 0.78 cm and 0.64 cm for the right and left IJV, respectively. A significant correlation was found between the dilation of both the right and left IJV in CCF patients compared to non-CCF patients. The dilation of the IJV in CCF patients is consistent with the literature and what was reported, which is the drainage of blood from the inferior region through the plexus pterygoid and IPS.[4] The drainage system was also discussed, which divided drainage into four categories: superior to the Sylvian vein, anterior drainage to the SOV, posterior drainage to the petrosal sinus, and inferior drainage to the plexus pterygoid.[14] The right IJV was found to be larger than the left IJV, consistent with studies that measured the diameter of the IJV using CT scans.[13] The finding that the diameter of the right IJV is larger than the left IJV could be an additional argument for choosing access through the right IJV rather than the left IJV if the transarterial route is not possible.[17] Access to the IJV can be done through the IPS,[4] but contralateral access should be avoided even if there is occlusion on the ipsilateral side due to the possibility of severe drainage disturbance, and it is better to choose re-canalization of the occluded IPS to access the CS.[18]
Regarding the type of CCF, a significant correlation was found between the dilation of the left SOV and direct left CCF if it exceeded the cutoff of 0.5 cm and indirect left CCF if it was less than the cutoff of 0.5 cm. In direct CCF, anterior drainage on the ipsilateral left side causes dilation above the cutoff value of 0.5 cm, possibly due to higher pressure or high flow compared to indirect CCF.[4] In general, CCF has drainage to the SOV.[4] A previous study using color Doppler ultrasound found that CCF patients with higher pressure or high flow had larger SOV diameters than those with low pressure or low flow. [7] The right SOV did not significantly correlate with CCF type, possibly due to anatomical variations in the SOV. In 8.7% of patients, fenestration or division of the SOV was found, and all were found on the right side. The reason for finding SOV fenestration at a relatively high frequency is still unclear.[20]
Based on the CCF type, no significant correlation was found between the size of the right and left IJV and direct or indirect CCF. The right and left IJV dilation in CCF patients occurs due to drainage to the inferior side toward IPS, which can be found in both direct and indirect CCF types.[4] The absence of a significant correlation with CCF types can be explained by the study, which reported that among several drainage pathways, venous drainage is primarily to the ophthalmic vein in Thomas classification types 2 and 3.[12] In some existing studies, measurements were taken at different levels, such as at the level of the cricoid cartilage, thyroid gland, levels C2-C3, C5-C6, and C7-T1, which cannot represent the entire IJV.[10]
Based on the type of CCF, a significant correlation was found between direct left CCF and dilation of the ipsilateral SOV if it exceeds the cutoff of 0.5 cm and indirect left CCF if it is less than the cutoff of 0.5 cm. In direct CCF, drainage to the anterior on the ipsilateral left side leads to dilation above the cutoff of 0.5 cm, which may be due to higher pressure or high flow compared to indirect CCF. Contralateral drainage is known to be the rarest occurrence, generally as minor drainage, with the main drainage being ipsilateral – this is possible due to flow through intercavernous connections, which can be found in both direct and indirect types of CCF.[2]
One of the limitations of this study is the relatively small sample size, especially for patients with bilateral CCF, which are indeed rare cases. The limited number of samples did not allow for a more detailed analysis based on risk factors or patient characteristics. Furthermore, the retrospective study design and limited access to medical record data prevented the analysis of etiology and radiological characteristics. Therefore, the correlation found in this study needs further verification through more extensive prospective studies with more comprehensive data on etiology and risk factors to ensure generalizability to a more diverse population.
CONCLUSION
The study found that direct CCF cases were more prevalent than indirect cases, and lesions were more commonly found on the left side. The cutoff diameter for both the superior ophthalmic and IJVs was 0.1 cm. Our findings indicate that the left SOV is more significantly associated with direct and indirect CCF cases, especially when the lesion is on the left side. However, no significant correlation was observed between the right SOV and CCF type or location. The IJV showed no significant differences in size between the right and left sides, regardless of CCF type or location.
Here are some recommendations based on the retrospective study:
In cases where CCF is suspected, unilateral or bilateral IJV dilation may provide supportive evidence for CCF diagnosis, although no significant difference was observed between the right and left sides
An observed dilation of the left SOV diameter exceeding the cutoff of 0.5 cm is an early indicator of direct CCF, especially in cases where there is evidence of direct left involvement
Conversely, a left SOV diameter below the cutoff of 0.5 cm is an early indicator of indirect CCF, especially in cases where there is evidence of direct left involvement
Given the larger size of the right IJV, it can serve as a primary access point for invasive CCF therapy in cases where arterial access is not feasible.
It should be noted that these findings are derived from a retrospective study with a relatively small sample size. Therefore, these observations may provide valuable insights in cases where the diagnosis of carotid-cavernous fistula is unclear or when dealing with challenging scenarios, such as indirect fistulas. Future studies should consider incorporating additional information, such as time interval, etiology, clinical presentation, and patient outcomes, particularly for direct CCF cases associated with head trauma.
Footnotes
How to cite this article: Sarastika HY, Ferriastuti W, Suwanto S, Mukherji S, Tripriyanggara A. Connecting the dots: Linking superior ophthalmic vein and internal jugular vein diameter to carotid cavernous fistula type and location. Surg Neurol Int. 2024;15:377. doi: 10.25259/SNI_601_2024
Contributor Information
Hartono Yudi Sarastika, Email: hartono.yudi.s@fk.unair.ac.id.
Widiana Ferriastuti, Email: widiana-f@fk.unair.ac.id.
Sidharta Suwanto, Email: sidhartasuwanto@gmail.com.
Suresh Mukherji, Email: sureshmukherji@hotmail.com.
Ardhi Tripriyanggara, Email: ardhi.t89.at@gmail.com.
Ethical approval
The research/study approved by the Institutional Review Board at General Academic dr Soetomo Hospital, number 1737/118/4/X/2022, dated October 31, 2022.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Adam CR, Shields CL, Gutman J, Kim HJ, Hayek B, Shore JW, et al. Dilated superior ophthalmic vein: Clinical and radiographic features of 113 cases. Ophthalmic Plast Reconstr Surg. 2018;34:68–73. doi: 10.1097/IOP.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 2.Aralasmak A, Karaali K, Cevikol C, Senol U, Sindel T, Toprak H, et al. Venous drainage patterns in carotid cavernous fistulas. ISRN Radiol. 2014;2014:760267. doi: 10.1155/2014/760267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CJ, Mastorakos P, Caruso JP, Ding D, Schmitt PJ, Buell TJ, et al. Transorbital approach for endovascular occlusion of carotid-cavernous fistulas: Technical note and review of the literature. Cureus. 2017;9:e976. doi: 10.7759/cureus.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi CT, Nguyen D, Duc VT, Chau HH, Son VT. Direct traumatic carotid cavernous fistula: Angiographic classification and treatment strategies: Study of 172 cases. Interv Neuroradiol. 2014;20:461–75. doi: 10.15274/INR-2014-10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docherty G, Eslami M, Jiang K, Barton JS. Bilateral carotid cavernous sinus fistula: A case report and review of the literature. J Neurol. 2018;265:453–9. doi: 10.1007/s00415-017-8657-y. [DOI] [PubMed] [Google Scholar]
- 6.Ellis JA, Goldstein H, Connolly ES, Meyers PM. Carotid-cavernous fistulas. Neurosurg Focus. 2012;32:E9. doi: 10.3171/2012.2.FOCUS1223. [DOI] [PubMed] [Google Scholar]
- 7.Flaharty PM, Lieb WE, Sergott RC, Bosley TM, Savino PJ. Color doppler imaging a new noninvasive technique to diagnose and monitor carotid cavernous sinus fistulas. Arch Ophthalmol. 1991;109:522–6. doi: 10.1001/archopht.1991.01080040090035. [DOI] [PubMed] [Google Scholar]
- 8.Grumann AJ, Boivin-Faure L, Chapot R, Adenis JP, Robert PY. Ophthalmologic outcome of direct and indirect carotid cavernous fistulas. Int Ophthalmol. 2012;32:153–9. doi: 10.1007/s10792-012-9550-4. [DOI] [PubMed] [Google Scholar]
- 9.Henderson AD, Miller NR. Carotid-cavernous fistula: Current concepts in aetiology, investigation, and management. Eye (Lond) 2018;32:164–72. doi: 10.1038/eye.2017.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laganà MM, Pelizzari L, Scaccianoce E, Dipasquale O, Ricci C, Baglio F, et al. Assessment of internal jugular vein size in healthy subjects with magnetic resonance and semiautomatic processing. Behav Neurol. 2016;2016:9717210. doi: 10.1155/2016/9717210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JY, Jung C, Ihn YK, Kim DJ, Seong SO, Kwon BJ. Multidetector CT angiography in the diagnosis and classification of carotid-cavernous fistula. Clin Radiol. 2016;71:e64–71. doi: 10.1016/j.crad.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Leone G, Renieri L, Enriquez-Marulanda A, Dmytriw AA, Nappini S, Laiso A, et al. Carotid cavernous fistulas and dural arteriovenous fistulas of the cavernous sinus: Validation of a new classification according to venous drainage. World Neurosurg. 2019;128:e621–31. doi: 10.1016/j.wneu.2019.04.220. [DOI] [PubMed] [Google Scholar]
- 13.Lim CL, Keshava SN, Lea M. Anatomical variations of the internal jugular veins and their relationship to the carotid arteries: A CT evaluation. Australas Radiol. 2006;50:314–8. doi: 10.1111/j.1440-1673.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 14.Nishio A, Yoshimura M, Hara M, Komiyama M. Variation of the venous drainage of carotid cavernous sinus fistula. Osaka. 2003;6:1277. [Google Scholar]
- 15.Rupareliya C, Fraser JF, Sheikhi L. Simultaneous transarterial and transvenous contrast injection to reveal the fistulous point in carotid cavernous fistula: Illustrative case. J Neurosurg Case Lessons. 2022;3:CASE21456. doi: 10.3171/CASE21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen CC, Tsuei YS, Yang MY, You WC, Sun MH, Sheu ML, et al. Gamma knife radiosurgery for indirect dural carotid-cavernous fistula: Long-term ophthalmological outcome. Life (Basel) 2022;12:1175. doi: 10.3390/life12081175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tartière D, Seguin P, Juhel C, Laviolle B, Mallédant Y. Estimation of the diameter and cross-sectional area of the internal jugular veins in adult patients. Crit Care. 2009;13:R197. doi: 10.1186/cc8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theaudin M, Chapot R, Vahedi K, Bousser MG. Dural carotid-cavernous fistula: Relationship between evolution of clinical symptoms and venous drainage changes. Cerebrovasc Dis. 2008;25:382–4. doi: 10.1159/000120691. [DOI] [PubMed] [Google Scholar]
- 19.Thomas AJ, Chua M, Fusco M, Ogilvy CS, Tubbs RS, Harrigan MR, et al. Proposal of venous drainage-based classification system for carotid cavernous fistulae with validity assessment in a multicenter cohort. Neurosurgery. 2015;77:380–5. doi: 10.1227/NEU.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 20.Tsutsumi S, Ono H, Yasumoto Y, Ishii H. The superior ophthalmic vein: Anatomical perspective for transvenous access to the cavernous sinus. J Craniofac Surg. 2020;31:1153–6. doi: 10.1097/SCS.0000000000006303. [DOI] [PubMed] [Google Scholar]