Abstract
Background:
Cavernous sinus dural arteriovenous fistula (CSDAVF) is an abnormal arteriovenous connection involving the dura mater within or adjacent to the wall of the cavernous sinus. While cases with superior ophthalmic vein drainage and ocular symptoms are typical, we report a rare case of CSDAVF draining into the perimedullary vein of the medulla oblongata and spinal cord and causing cerebellar ataxia and myelopathy as the initial presentation.
Case Description:
A 73-year-old man presented with vertigo and rapidly progressing gait disturbance. Digital subtraction angiography revealed a left CSDAVF draining only into the spinal perimedullary veins, which was classified as Cognard type V. We performed a transvenous embolization through the occluded left inferior petrosal sinus and achieved a super-selective shunt occlusion using three platinum coils. The postoperative course was uneventful with immediate improvement of symptoms.
Conclusion:
CSDAVF should be considered as a differential diagnosis in a patient with venous congestion in the brainstem.
Keywords: Brainstem, Cavernous sinus, Dural arteriovenous fistula, Myelopathy, Venous congestion

INTRODUCTION
Cavernous sinus dural arteriovenous fistula (CSDAVF) is an abnormal arteriovenous connection involving the dura mater within or adjacent to the wall of the cavernous sinus. The clinical presentation depends on the pattern of venous drainage. When the drainage is diverted anteriorly into the superior ophthalmic vein, it causes ocular symptoms such as chemosis, proptosis, and ophthalmoplegia. Posterior drainage through the inferior petrosal sinus may cause pulsatile tinnitus, while critical venous infarction or hemorrhage may, in rare cases, result from cortical venous reflux through the superficial middle Sylvian, uncal, or petrosal veins.[1,8,14]
Reports of CSDAVF presenting with symptoms attributed to brainstem or spinal cord congestion and without ocular symptoms are rare. This report describes a rare case of CSDAVF draining into the perimedullary vein of the medulla oblongata and spinal cord alone, which caused cerebellar ataxia and myelopathy without any ocular symptoms.
CASE REPORT
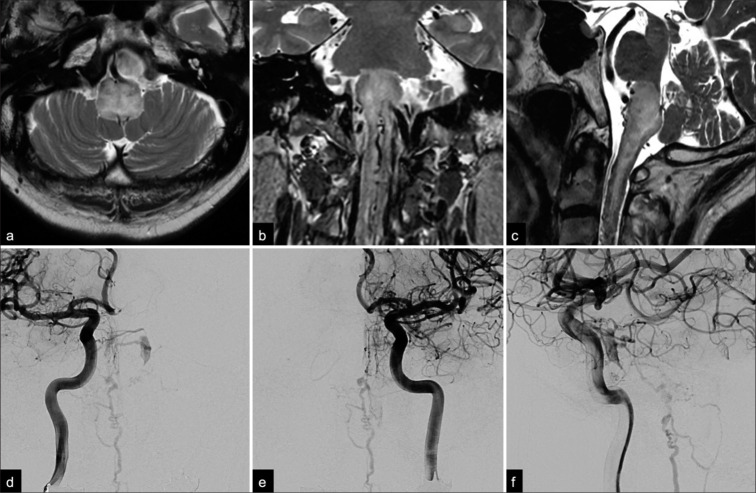
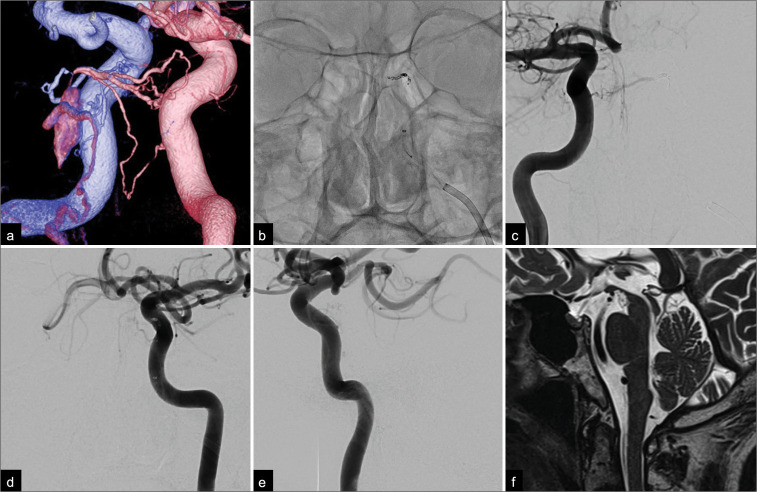
A 73-year-old man presented with vertigo and rapidly progressive gait disturbance. He had a previous medical history of cholecystectomy, hypertension, and dysuria and reported the onset of dysphagia 4 months earlier. Three months later, he developed nausea and vertigo followed by progressive left side-dominant paraparesis and bilateral lower limb paresthesia. Gait disturbance progressed rapidly within 2 weeks, and eventually, he required a wheelchair for daily activities. T2-weighted magnetic resonance imaging (MRI) showed an area of high-signal intensity in the medulla oblongata and the upper cervical spinal cord. Multiple flow voids were detected around the brainstem [Figures 1a-c]. Digital subtraction angiography revealed a small opacification, suggesting a left CSDAVF [Figures 1d-f]. A fused bilateral internal carotid three-dimensional digital subtraction angiogram clearly visualized the shunting point, which consisted of the left inferolateral trunk and the right meningohypophyseal trunk [Figure 2a]. A pontine bridging vein originating from the dorsal aspect of the small compartment of the posterior cavernous sinus drained into the anterior pontomesencephalic vein (APMV) and then into the spinal perimedullary veins [Figures 1d-f] and was classified as Cognard type V. We performed a transvenous embolization through the occluded left inferior petrosal sinus under general anesthesia. The tip of a microcatheter was advanced successfully into the shunting point supported by a 4-French and 7-French coaxial guiding system. Transvenous embolization was then performed successfully with three platinum coils [Figure 2b]. The final angiograms of the internal carotid arteries showed complete obliteration of the CSDAVF and disappearance of the drainage veins around the brainstem [Figures 2c-e]. His symptoms improved immediately, and the postoperative course was uneventful. The ataxia in the left limb improved to the point that the patient could walk with minimal assistance by the time he was discharged on postoperative day 10. Follow-up sagittal T2-weighted MRI scans performed 6 months later showed complete resolution of the area of high-signal intensity in the brainstem and the spinal cord swelling [Figure 2f]. At the 1-year follow-up, the patient remains clinically asymptomatic.
Figure 1:
Preoperative (a) axial, (b) coronal, and (c) sagittal T2-weighted magnetic resonance images showing an area of high-signal intensity in the medulla oblongata and upper cervical spinal cord. Note that multiple flow void signs were detected on both the dorsal and ventral aspects of the brainstem. Anterior-posterior angiographic views of the (d) right and (e) left internal carotid arteries show a small opacification of the left cavernous sinus draining into the anterior pontomesencephalic vein through the pontine bridging vein. (f) A late arterial phase lateral view on a left internal carotid angiogram showed opacification of the posterior part of the cavernous sinus and tortuous congested veins around the medulla oblongata.
Figure 2:
(a) Posterior-anterior oblique view of a fused bilateral internal carotid three-dimensional digital subtraction angiogram showing the shunting point, which consisted of the left inferolateral trunk (blue) and the right meningohypophyseal trunk (pink). Note the small opacification in the posterior part of the cavernous sinus and the pontine bridging vein draining into the pontomesencephalic vein. (b) Anterior-posterior view on a non-subtracted craniogram obtained during embolization showed coils placed at the feeder as well as the shunting point. Postoperative anterior-posterior angiograms of both internal carotid arteries ((c) right; (d) left) and a (e) lateral angiogram of the left internal carotid artery showing complete obliteration of the fistula and the tortuous veins around the medulla oblongata. (f) A sagittal T2-weighted magnetic resonance image obtained 6 months after treatment showed a complete disappearance of the area of high signal intensity and flow voids around the brainstem and spinal cord.
DISCUSSION
Symptoms of CSDAVF are thought to be associated with the type of drainage route, and retrograde cortical venous drainage into the superficial middle cerebral, basal vein, and posterior fossa has the potential to produce aggressive symptoms.[6] Drainage into both the basal cerebral vein and the posterior fossa is associated with a particularly high risk of aggressive symptoms, including brainstem and cerebellar edema or venous infarction. Kiyosue et al.[6] retrospectively analyzed their cases of CSDAVF and classified the basal cerebral venous and posterior fossa drainage routes as superolateral, posterolateral, or posteromedial. The superolateral type consists of the middle cerebral vein or uncal vein and/or the deep middle cerebral vein connecting to the basal cerebral vein. The posterolateral type consists of the superior petrosal sinus and petrosal vein connecting to the lateral mesencephalic vein and/or the transverse pontine vein connecting to the basal cerebral vein. The posteromedial type is characterized by drainage from the cavernous sinus through the prepontine bridging vein into the transverse pontine vein and/or the APMV connecting to the basal cerebral vein. Our case had only one pontine vein draining out from the cavernous sinus into the APMV and was classified as the posteromedial type. Although the pontine bridging veins are rarely noticed in daily clinical practice, Kiyosue et al. reported that they were the most common of the three types.[6]
CSDAVF causing venous congestion of the medulla oblongata and the spinal cord is rare, and very few cases have been reported. A review of 100 patients with progressive myelopathy caused by a Cognard type V dural arteriovenous fistula identified only 1 case of CSDAVF.[2] Furthermore, more than half of the patients were initially misdiagnosed as having another disease. The most common misdiagnoses were spinal or craniocervical junction arteriovenous fistula, myelitis, and brainstem tumor. Considering the median interval between onset of symptoms and diagnosis being 5 months, they emphasized the importance of the results of MRI, especially findings of abnormal vascular flow voids around the spinal cord, because delayed diagnosis was associated with a poor prognosis. Another review by Hou et al.[3] investigated 86 patients with intracranial dural arteriovenous fistula and brainstem edema. Consistent with the previous reviews, 40.2% of patients were misdiagnosed as having another disease. The location was confirmed to be the craniocervical junction in 27 patients (32.5%), the cavernous sinus in 11 (13.2%), the superior petrosal sinus in 9 (10.8%), the transverse and/or sigmoid sinus in 10 (12.0%), the tentorium in 21 (25.3%), and other sites in 5 (6.0%).
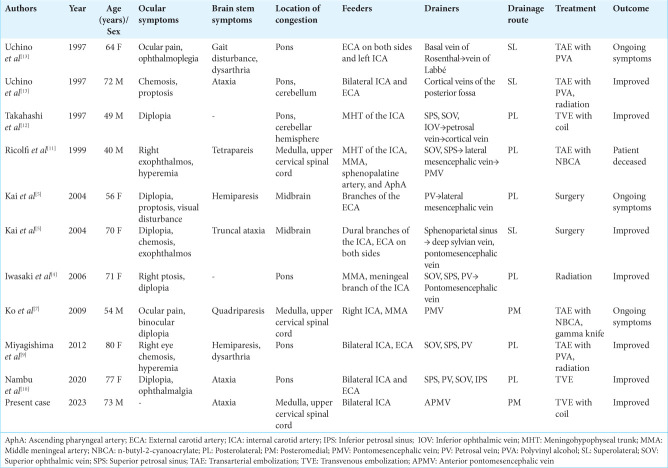
We conducted a systematic search for patients who had CSDAVF associated with brainstem edema and identified 10 cases (11, including our present case) with sufficient angiographic data for evaluation of the drainage route[4,5,7,9-13] [Table 1]. All 10 cases other than ours had ocular symptoms as the initial presentation; therefore, ours is the only case that presented with brainstem symptoms alone. In most of the cases, edema occurred in the pons, midbrain, and cerebellum. There were only three cases, including our case, in which edema was localized from the medulla oblongata to the upper cervical spinal cord. This may reflect the fact that our case did not have cranial nerve palsy, such as diplopia, as a result of brainstem edema. According to the drainage route classification mentioned above there were three cases of the superolateral type, six cases of the posterolateral type, and two cases of the posteromedial type. Retrograde leptomeningeal venous drainage routes that eventually drained into the brainstem were included in the superolateral type. The most common drainage route to the brainstem is the posterolateral type, which is understandable considering that the superior petrosal sinus, which is a natural drainage route for the cavernous sinus, is the gateway to the medullary drainage routes. Two patients, including our present case, were classified as the posteromedial type. We suspect that thrombosis of the cavernous sinus and staged occlusion of the typical drainage routes is the etiology of this type. In our case, the cavernous sinus was poorly visualized on MRI, making the diagnosis of CSDAVF extremely difficult. The initial diagnosis in our case was a craniocervical junction arteriovenous fistula, and careful interpretation of MRI findings was necessary to detect flow void signals around the brainstem.
Table 1:
Summary of reported cases of cavernous sinus dural arteriovenous fistula associated with brain stem congestion
CONCLUSION
Unlike typical cases of CSDAVF, our patient presented with symptoms attributable to venous congestion of the medulla oblongata without any ocular symptoms. CSDAVF should be borne in mind as a differential diagnosis in a patient with venous congestion in the brainstem.
Footnotes
How to cite this article: Goto H, Fujita A, Nakamura N, Kohta M, Sasayama T. Brainstem congestion as the initial presentation of cavernous sinus dural arteriovenous fistula without ocular symptoms. Surg Neurol Int. 2024;15:359. doi: 10.25259/SNI_637_2024
Contributor Information
Hiroki Goto, Email: hiro_ontheedge7@yahoo.co.jp.
Atsushi Fujita, Email: afujita@med.kobe-u.ac.jp.
Naoto Nakamura, Email: naoto.nakamura1988@gmail.com.
Masaaki Kohta, Email: kohta@med.kobe-u.ac.jp.
Takashi Sasayama, Email: takasasa@med.kobe-u.ac.jp.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Awad IA, Little JR, Akrawi WP, Ahl J. Intracranial dural arteriovenous malformations: Factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72:839–50. doi: 10.3171/jns.1990.72.6.0839. [DOI] [PubMed] [Google Scholar]
- 2.De Grado A, Manfredi C, Brugnera A, Groppo E, Valvassori L, Cencini F, et al. Watch brain circulation in unexplained progressive myelopathy: A review of Cognard type V arteriovenous fistulas. Neurol Sci. 2023;44:3457–80. doi: 10.1007/s10072-023-06870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou K, Li G, Qu L, Liu H, Xu K, Yu J. Intracranial dural arteriovenous fistulas with brainstem engorgement: An under-recognized entity in diagnosis and treatment. Front Neurol. 2020;11:526550. doi: 10.3389/fneur.2020.526550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwasaki M, Murakami K, Tomita T, Numagami Y, Nishijima M. Cavernous sinus dural arteriovenous fistula complicated by pontine venous congestion. A case report. Surg Neurol. 2006;65:516–8. doi: 10.1016/j.surneu.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Kai Y, Hamada JI, Morioka M, Yano S, Ushio Y. Brain stem venous congestion due to dural arteriovenous fistulas of the cavernous sinus. Acta Neurochir (Wien) 2004;146:1107–11. doi: 10.1007/s00701-004-0315-3. [DOI] [PubMed] [Google Scholar]
- 6.Kiyosue H, Mori H, Sagara Y, Hori Y, Okahara M, Nagatomi H, et al. Basal cerebral venous drainage from cavernous sinus dural arteriovenous fistulas. Neuroradiology. 2009;51:175–81. doi: 10.1007/s00234-008-0486-3. [DOI] [PubMed] [Google Scholar]
- 7.Ko SB, Kim CK, Lee SH, Yoon BW. Carotid cavernous fistula with cervical myelopathy. J Clin Neurosci. 2009;16:1350–3. doi: 10.1016/j.jocn.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Lasjaunias P, Chiu M, Ter Brugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986;64:724–30. doi: 10.3171/jns.1986.64.5.0724. [DOI] [PubMed] [Google Scholar]
- 9.Miyagishima T, Hara T, Inoue M, Terano N, Ohno H, Okamoto K, et al. Pontine venous congestion due to dural arteriovenous fistula of the cavernous sinus: Case report and review of the literature. Surg Neurol Int. 2012;3:53. doi: 10.4103/2152-7806.96076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nambu K, Misaki K, Yoshikawa A, Kamide T, Nambu I, Sasagawa Y, et al. Cavernous sinus dural arteriovenous fistula with an enhanced lesion in the brainstem mimicking a malignant tumor. World Neurosurg. 2020;140:13–7. doi: 10.1016/j.wneu.2020.04.165. [DOI] [PubMed] [Google Scholar]
- 11.Ricolfi F, Manelfe C, Meder JF, Arrué P, Decq P, Brugiéres P, et al. Intracranial dural arteriovenous fistulae with perimedullary venous drainage. Anatomical, clinical and therapeutic considerations. Neuroradiology. 1999;41:803–12. doi: 10.1007/s002340050846. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi S, Tomura N, Watarai J, Mizoi K, Manabe H. Dural arteriovenous fistula of the cavernous sinus with venous congestion of the brain stem: Report of two cases from the Departments of Radiology. AJNR Am J Neuroradiol. 1999;20:886–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Uchino A, Kato A, Kuroda Y, Shimokawa S, Kudo S. Pontine venous congestion caused by dural carotid-cavernous fistula: Report of two cases. Eur Radiol. 1997;7:405–8. doi: 10.1007/s003300050175. [DOI] [PubMed] [Google Scholar]
- 14.Viñuela F, Fox AJ, Debrun GM, Peerless SJ, Drake CG. Spontaneous carotid-cavernous fistulas: Clinical, radiological, and therapeutic considerations: Experience with 20 cases. J Neurosurg. 1984;60:976–84. doi: 10.3171/jns.1984.60.5.0976. [DOI] [PubMed] [Google Scholar]