Abstract
Background:
There are very few reports of intradural disc herniations associated with achondroplasia described in the literature.
Case Description:
A patient with achondroplasia presented with progressive paraparesis attributed to a magnetic resonance-documented intradural disc herniation at the T12–L1 level occupying more than 90% of the spinal canal. It was successfully removed through a T12 laminectomy with durotomy; note a laminectomy would have been contraindicated if this had been an extradural anterior/anterolateral disc. Postoperatively, the patient progressively improved and, within 6 months, had 4/5 proximal/distal function and full sphincter control.
Conclusion:
A patient with achondroplasia and an intradural T12/L1 disc herniation (i.e., unlike an extradural anterior/anterolateral thoracic disc) successfully underwent a decompressive laminectomy with near full resolution of their preoperative paraparesis.
Keywords: Achondroplasia, Intervertebral disc displacement, Intradural disc herniation, Spinal stenosis

INTRODUCTION
Intradural disc herniations constitute only 0.26–0.3% of all herniated discs and are mainly found in the lumbar spine (92%) at the L4–L5 (55%) followed, followed by L3–L4 (16%) and L5–S1 (10%) levels.[2] Symptoms reflect disc locations and include radiculopathy, weakness, or sphincteric deficits. Patients with achondroplasia and degenerative spine disease, stenosis, and lumbar disc herniations may present with varying combinations of mechanical low back pain (28%) and/or neurogenic claudication/neurological deficits (21%).[5] Here, a 44-year-old male with achondroplasia presented with progressive paraparesis and sphincter dysfunction attributed to an acute magnetic resonance (MR)-documented intradural T12–L1 disc herniation that was successfully removed through a T12/L1 laminectomy/durotomy.
CASE PRESENTATION
A 44-year-old male with achondroplasia presented with 15 days of progressive, severe paraparesis (more right-sided) and 4 days of constipation. On examination, he had 1/5 strength in the right and/2/5 strength in the left leg (i.e., proximal/distal), asymmetric hypoesthesia in the lower extremities, and loss of both the patellar and Achilles reflexes. The enhanced magnetic resonance imaging of the thoracolumbar spine showed a disc herniation at the T12–L1 level, narrowing the canal by 90% [Figure 1]. As this was anticipated to be an intradural disc herniation, he successfully underwent an emergent T12/L1 laminectomy with durotomy for total intradural disc excision [Figure 2]. Immediately postoperatively, motor function improved to 3/5 bilaterally, and within 6 postoperative months, he regained 4/5 bilateral proximal/distal motor function and regained sphincter control.
Figure 1:
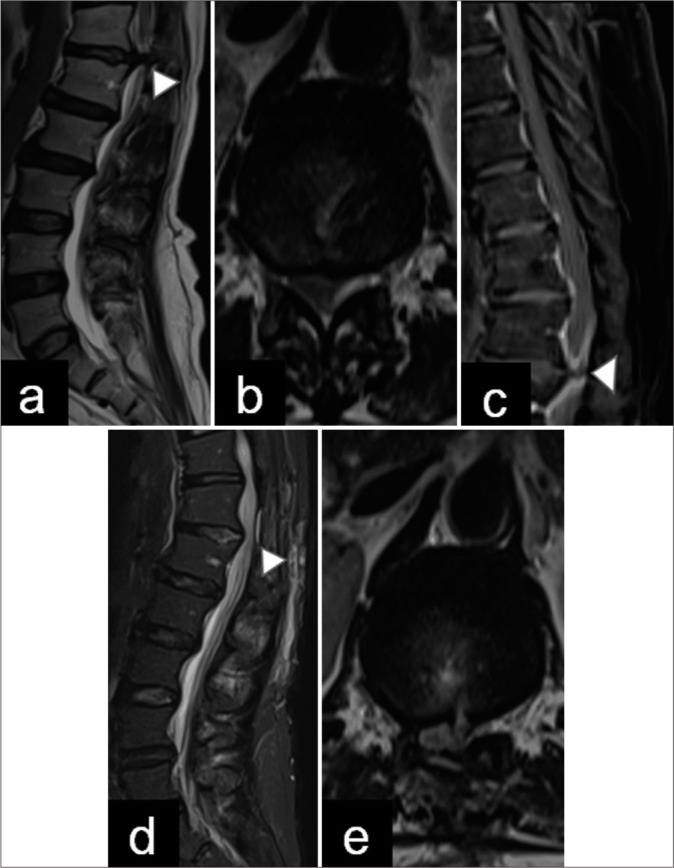
Pre- and post-operative magnetic resonance imaging (MRI). (a and b) Preoperative sagittal and axial T2-weighted images demonstrate a T12–L1 right-sided disc extrusion (arrowhead) occupying more than 90% of the spinal canal. (c) Post-contrast T1 MRI shows partial enhancement around the disc herniation. (arrowhead in image c is herniated disc), (d and e) Postoperative sagittal and axial T2-weighted images show an adequate decompression of the spinal canal. Postoperative changes in the laminectomy and discectomy are observed (arrowhead in image d is soft tissue inflamation).
Figure 2:
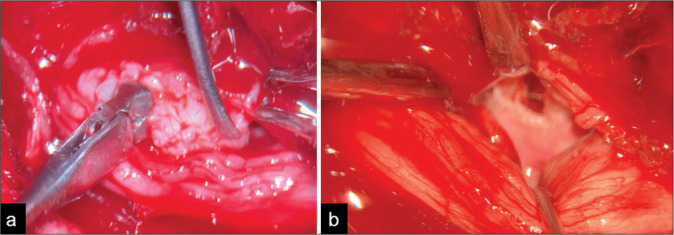
Intraoperative images of discectomy. (a) A lateral dissection of the lumbar roots is performed to resect the intradural disc herniation. (b) The post-discectomy surgical view is demonstrated. Roots are dissected and displaced laterally to explore the ruptured anterior dural wall.
DISCUSSION
Intradural disc herniations represent 0.26–0.3% of all disc herniations, which are typically located in the lumbar spine (i.e., 92% of the cases, with few being reported in the thoracic/thoracolumbar spine) [Table 1].[2,5] Patients with achondroplasia may be more susceptible to developing such thoracolumbar disc herniations due to their biomechanical alterations in their sagittal/pelvic inclination, lumbar lordosis, thoracic kyphosis, and spinal stenosis [Figure 3].[8] Here, a 44-year-old achondroplastic male presented with 15 days of progressive paraparesis and sphincter dysfunction. He successfully underwent a T12/L1 laminectomy with durotomy for resection of the acute MR-documented T12/L1 intradural disc herniation. Ducati et al., showed that[1-4,6,7,9] patients with intradural lumbar disc herniations who presented with motor impairment demonstrated better postoperative recoveries versus those exhibiting isolated sensory deficits.[2] Bonomo et al.,[1] and Schreiber and Rosenthal[8] patients with respective thoracic intradural discs versus routine disc herniations uniquely attributed to achondroplasia, which demonstrated partial or full recoveries of neurological function following appropriate and timely surgical intervention.
Table 1:
Summary of cases reported in the literature.
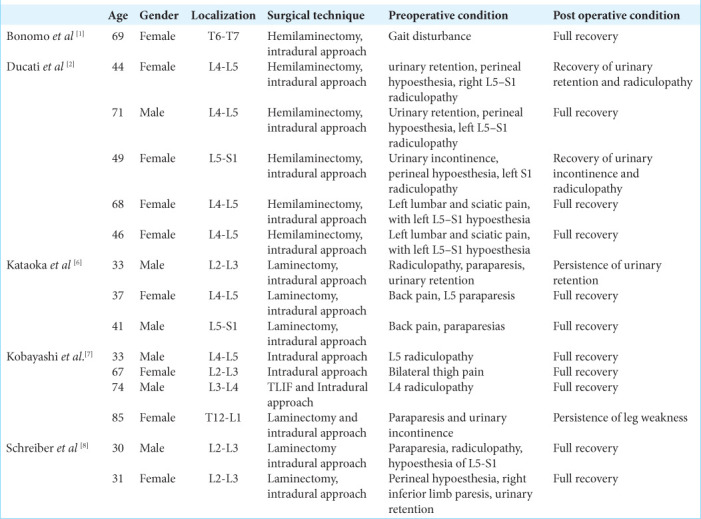
Figure 3:
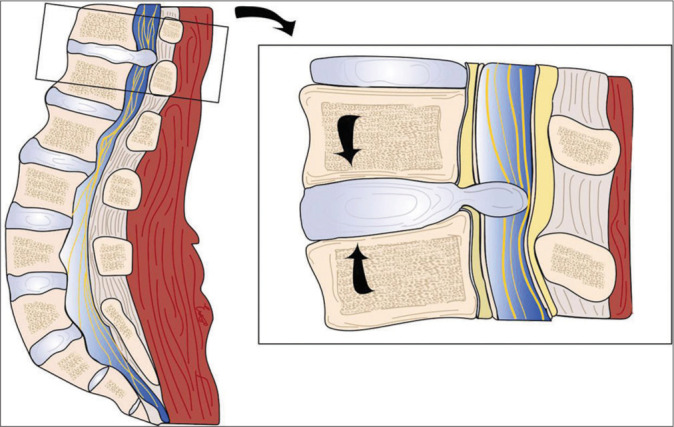
Graphic illustration of the thoracolumbar intradural disc herniation. Abnormal axial thoracolumbar forces are represented with arrows. A rupture of the annulus fibrosus, the posterior longitudinal ligament, and the anterior dura are demonstrated. Image credits: Edgar G Ordóñez-Rubiano, MD.
CONCLUSION
A 44-year-old male with achondroplasia and a 15-day history of progressive paraparesis and sphincter loss successfully underwent a T12/L1 laminectomy/durotomy for excision of an acute MR-documented intradural disc herniation.
Footnotes
How to cite this article: Ordonez-Rubiano EG, Romo JA, Torres J, Troncoso SJ, Patiño J. Intradural T12–L1 disc herniation in a patient with achondroplasia: A case report. Surg Neurol Int. 2024;15:369. doi: 10.25259/SNI_347_2024
Contributor Information
Edgar G. Ordonez-Rubiano, Email: egordonez@fucsalud.edu.co.
Jorge Alberto Romo, Email: jaromo@fucsalud.edu.co.
Juan Torres, Email: juantorresb@gmail.com.
Santiago José Troncoso, Email: ssantiagotroncoso@gmail.com.
Javier Patiño, Email: javierpati@gmail.com.
Ethical Approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Bonomo G, Cusin A, Rubiu E, Iess G, Bonomo R, Boncoraglio GB, et al. Diagnostic approach, therapeutic strategies, and surgical indications in intradural thoracic disc herniation associated with CSF leak, intracranial hypotension, and CNS superficial siderosis. Neurol Sci. 2022;43:4167–73. doi: 10.1007/s10072-022-06059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducati LG, Silva MV, Brandao MM, Romero FR, Zanini MA. Intradural lumbar disc herniation: Report of five cases with literature review. Eur Spine J. 2013;22(Suppl 3):S404–8. doi: 10.1007/s00586-012-2516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredwall SO, Maanum G, Johansen H, Snekkevik H, Savarirayan R, Lidal IB. Current knowledge of medical complications in adults with achondroplasia: A scoping review. Clin Genet. 2020;97:179–97. doi: 10.1111/cge.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan Q, Xing F, Long Y, Xiang Z. Cervical intradural disc herniation: A systematic review. J Clin Neurosci. 2018;48:1–6. doi: 10.1016/j.jocn.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Kahanovitz N, Rimoin DL, Sillence DO. The clinical spectrum of lumbar spine disease in achondroplasia. Spine (Phila Pa 1976) 1982;7:137–40. doi: 10.1097/00007632-198203000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka O, Nishibayashi Y, Sho T. Intradural lumbar disc herniation. Report of three cases with a review of the literature. Spine (Phila Pa 1976) 1989;14:529–33. [PubMed] [Google Scholar]
- 7.Kobayashi K, Imagama S, Matsubara Y, Yoshihara H, Hirano K, Ito Z, et al. Intradural disc herniation: Radiographic findings and surgical results with a literature review. Clin Neurol Neurosurg. 2014;125:47–51. doi: 10.1016/j.clineuro.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber F, Rosenthal H. Paraplegia from ruptured lumbar discs in achondroplastic dwarfs. J Neurosurg. 1952;9:648–51. doi: 10.3171/jns.1952.9.6.0648. [DOI] [PubMed] [Google Scholar]
- 9.Thomalske G, Mohr G. Intradural sequestration of lumbal disk hernias with special reference to achondroplasia. Nervenarzt. 1974;45:376–9. [PubMed] [Google Scholar]


