Abstract
Data on the C-reactive protein (CRP) flare response in patients with metastatic and unresectable urothelial carcinoma (mUC) are limited. The present study aimed to clarify the clinical significance of the CRP flare response in patients with mUC who received pembrolizumab. Between March 2018 and December 2022, patients with mUC who received pembrolizumab following chemotherapy were retrospectively reviewed. Patients were categorized into three groups based on the early kinetics of CRP: i) Flare-responders, in which CRP levels increased >2-fold from baseline (BL) within 1 month after pembrolizumab administration (CRP flare) and decreased to below-BL levels within 3 months; ii) responders, in which CRP levels decreased ≥30% from baseline within 3 months without CRP flare; and iii) non-responders, which included the remaining patients. Tumor response, survival and incidence of immune-related adverse events (AEs) were compared between the groups. Of the 108 eligible patients, 17 (16%), 27 (25%) and 64 (59%) were classified as CRP flare-responders, CRP responders and CRP non-responders, respectively. Objective response rate was higher in CRP flare-responders and CRP responders than in CRP non-responders. Progression-free survival and overall survival were longer in CRP flare-responders and CRP responders than in CRP non-responders. Among CRP flare-responders, patients with low BL CRP levels had a better tumor response and survival than patients with high BL CRP levels. Notably, there was no difference in the incidence of immune-related AEs. In patients with mUC who received pembrolizumab, CRP flare-responders showed favorable oncological outcomes; therefore, BL CRP levels could predict oncological outcomes in CRP flare-responders.
Keywords: CRP, baseline CRP level, flare response, pembrolizumab, urothelial carcinoma
Introduction
Urothelial carcinoma (UC) is a common urological malignancy, and UC of the urinary bladder is the ninth most common cancer in the world (1), furthermore, approximately 5–10% of patients have metastatic disease at the time of diagnosis (2,3). Although platinum-based chemotherapy has been performed as standard first line treatment in patients with metastatic and unresectable urothelial carcinoma (mUC), the prognosis was poor with a median overall survival of approximately 15 months (4). Furthermore, no survival-prolonging subsequent therapy following to the platinum-based chemotherapy was available for a long period.
In recent years, immune checkpoint inhibitors (ICIs) have been widely used to treat malignancies. Pembrolizumab demonstrated survival benefit in patients with mUC who had received systemic chemotherapy (5) and has been approved as subsequent treatment following prior chemotherapy in Japan. Longer survival was observed in patients with mUC treated in the recent era compared with those treated before pembrolizumab was approved (6).
Although pembrolizumab can be beneficial for patients with mUC, only a few patients experienced clinical responses (5). Additionally, some patients developed immune-related adverse events (irAEs), which can be serious. By contrast, a long duration of response was reported in patients with response (7); therefore, development of biomarkers that predict pembrolizumab response is expected.
An exploratory analysis for biomarkers using data from the randomized phase III KEYNOTE-045 trial revealed that patients with a higher tumor mutation burden and T-cell-inflamed gene expression demonstrated better response to pembrolizumab than those without (8). However, in clinical practice, these biomarkers cannot be measured easily.
C-reactive protein (CRP) is a representative acute phase reactant that is widely used to evaluate systemic inflammation. In addition, continued increasing of CRP levels been proven to be an indicator of chronic inflammation associated with many diseases such as obesity (9), cardiovascular disease (10), and also malignant tumors (11). Furthermore, chronic inflammation can contribute to initiation, promotion, growth, and invasion of tumors (12). Inflammation also affects the response to therapy, the association between the systemic inflammation and poor prognosis was reported in many cancers (13,14). In addition, the relation between serum CRP levels and oncological outcome has also been investigated in patients with several malignancies received ICI. Elevated the pretreatment CRP levels were found to be associated with the poor outcome in patients with many cancers treated with ICI, including UC, melanoma, lung cancer, renal cell carcinoma, and other cancers (15). Moreover, the relationship between changes in CRP levels with ICI treatment and oncological outcome have also been studied in patients with renal cell carcinoma and UC. Among patients with high pretreatment CRP levels, the patients with decreased CRP levels after initiation of ICI showed better survival comparing to those without (16,17).
In patients with mUC who were treated with pembrolizumab, both baseline (BL) CRP levels and post-treatment changes have been associated with the efficacy of pembrolizumab (17–19). The association between early CRP elevation (CRP flare phenomenon) and the efficacy of ICI therapy was first reported in non-small cell lung cancer (20). Patients with renal cell carcinoma who demonstrated a decrease in CRP levels below BL following flare phenomenon (CRP flare-response) showed favorable oncological outcome (21). We previously observed that patients with mUC treated with pembrolizumab showed a favorable outcome after the CRP flare-response (22). However, we could not perform detailed investigation for clinical significance of the CRP flare-response because of a small cohort, which included only three patients with CRP flare. Additionally, only one more study has investigated the association between CRP flare and oncological outcome in patients with mUC (23); thus, data on the clinical significance of CRP flares are limited. In this study, we used a multicenter cohort to investigate the impact of CRP flares on clinical outcome in patients with advanced UC treated with pembrolizumab.
Materials and methods
Study population
The inclusion criteria were patients aged 18 years or older with histologically confirmed mUC of the urinary tract. Patients had previously received systemic chemotherapy and subsequent pembrolizumab between March 2018 and December 2022 at one academic center (University of Occupational and Environmental Health) and six general hospitals (Kokura Memorial Hospital, Munakata Suikokai General Hospital, Shin-kokura Hospital, Shin-yukuhashi Hospital, Kitakyushu City Yahata Hospital, and Moji Medical Center). A total of 110 patients were included in this study and their median age and the range of age were 74 and 40 to 86 years old, respectively. Patients who developed infectious disease within 3 months of pembrolizumab administration and patients with insufficient data on CRP were excluded because evaluation of early CRP kinetics cannot be performed correctly. This retrospective, multicenter study was approved by the Ethics Committee of the University of Occupational and Environmental Health, Japan (UOEHCRB20-180), the ethics committee of Kokura Memorial Hospital (22092002E), the Ethics Committee of Munakata Suikokai General Hospital (22003), the Ethics Committee of Shin-kokura Hospital (2022–002), the Ethics Committee of Shin-yukuhashi Hospital (R3-35), the Ethics Committee of Kitakyushu City Yahata Hospital (20220510), and the Ethics Committee of Moji Medical Center (04–05). This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Study design
Patients received intravenous infusion of pembrolizumab once every 3 (200 mg) or 6 (400 mg) weeks and continued use until disease progression or unacceptable adverse events (AEs) were observed. Blood parameters, including CRP levels, were tested at BL and at least every 3 weeks within 3 months after starting pembrolizumab therapy. Chest and abdominal computed tomography scans were performed before initiation of pembrolizumab therapy and repeated every 8–12 weeks at the physician's discretion. Best overall response (BOR), progression-free survival (PFS), overall survival (OS), and incidence of irAEs were compared between the groups divided based on early CRP kinetics. BOR was defined as the best tumor response achieved during pembrolizumab treatment and assessed according to the Response Evaluation Criteria in Solid Tumor version 1.1. Objective response rate (ORR) was shown as the sum of complete and partial response rate. AEs were evaluated based on the Common Terminology Criteria for Adverse Events version 5.0. In addition, factors associated with PFS were investigated.
Definition of early CRP kinetics
According to the definition of CRP kinetics (21), patients were grouped as follows: i) Flare-responders, in which CRP levels increased to at least double from BL within the first month after initiation of pembrolizumab (CRP flare) and then decreased below BL within 3 months; ii) responders, in which CRP levels decreased by ≥30% from BL within 3 months without CRP flare; and iii) non-responders, which included the remaining patients.
Furthermore, CRP flare-responders were divided into two groups according to the median BL CRP value before pembrolizumab administration. Because several cutoff values of pretreatment CRP predicting the prognosis in patients with mUC who received pembrolizumab were reported and no established reference value was available (18,19,24–26), the median value was used.
Statistical analysis
Fisher's exact test was used for comparing BOR and incidence of irAEs among groups. OS and PFS were calculated from the first initiation of pembrolizumab until the last follow-up or death from any cause and disease progression, respectively. Survival analysis was performed using the Kaplan-Meier method and log-rank test. Multivariate analysis was performed using the Cox proportional hazards regression model to identify PFS-associated factors. P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (27).
Results
During the study, 110 patients with mUC received pembrolizumab as subsequent therapy following chemotherapy. One patient who had acute pyelonephritis 5 d after starting pembrolizumab treatment and a patient who had insufficient CRP data was excluded from the study cohort. Thus, 108 eligible patients were included in this study and 17 (16%), 27 (25%), and 64 (59%) patients were grouped as CRP flare-responders, CRP responders, and CRP non-responders, respectively. In CRP non-responders, more patients had histologic subtypes or divergent differentiation comparing to CRP flare-responders and CRP responders. No remarkable difference was shown in the other patient characteristics among the three groups (Table I).
Table I.
Patients characteristics stratified by early CRP kinetics.
| Characteristic | Non-responder | Responder | Flare-responder |
|---|---|---|---|
| Number of patients | 64 | 27 | 17 |
| Median age, years (IQR) | 73 (67–78) | 75 (49–81) | 75 (66–79) |
| Sex, n (%) | |||
| Male | 50 (78) | 20 (74) | 11 (65) |
| Female | 14 (22) | 7 (26) | 6 (35) |
| Median BMI, kg/m2 (IQR) | 23.3 (20.3–25.2) | 22.8 (20.4–25.5) | 20.6 (20.0–23.3) |
| ECOG-PS, n (%) | |||
| 0 | 40 (63) | 19 (70) | 10 (59) |
| ≥1 | 24 (37) | 8 (30) | 7 (41) |
| Primary lesion, n (%) | |||
| Upper tract | 32 (50) | 16 (59) | 9 (53) |
| Bladder | 28 (44) | 10 (37) | 7 (41) |
| Both | 4 (6.2) | 1 (3.7) | 1 (5.9) |
| Histological grade, n (%) | |||
| High grade | 64 (100) | 27 (100) | 17 (100) |
| G2 | 10 (16) | 3 (11) | 3 (18) |
| G2>G3 | 6 (9.4) | 4 (15) | 1 (5.9) |
| G3>G2 | 8 (13) | 2 (7.4) | 2 (12) |
| G3 | 40 (63) | 18 (67) | 11 (65) |
| Histologic type (%) | |||
| Pure UC | 44 (69) | 23 (85) | 14 (82) |
| Squamous differentiation | 15 (23) | 3 (11) | 1 (5.9) |
| Glandular differentiation | 1 (1.6) | 0 (0) | 1 (5.9) |
| Clear cell subtype | 1 (1.6) | 0 (0) | 0 (0) |
| Sarcomatoid subtype | 1 (1.6) | 1 (3.7) | 0 (0) |
| Giant cell subtype | 1 (1.6) | 0 (0) | 0 (0) |
| Nested subtype | 1 (1.6) | 0 (0) | 0 (0) |
| Plasmacytoid subtype | 0 (0) | 0 (0) | 1 (5.9) |
| Hemoglobin, n (%) | |||
| ≥10 g/dl | 36 (56) | 15 (56) | 10 (59) |
| <10 g/dl | 28 (44) | 12 (44) | 7 (41) |
| Liver metastasis, n (%) | |||
| Absence | 48 (75) | 23 (85) | 15 (88) |
| Presence | 16 (25) | 4 (15) | 2 (12) |
| Prior treatment line, n (%) | |||
| 1 | 54 (84) | 24 (89) | 16 (94) |
| ≥2 | 10 (16) | 3 (11) | 1 (5.9) |
| Radical surgery, n (%) | |||
| Absence | 28 (44) | 14 (52) | 7 (41) |
| Presence | 36 (56) | 13 (48) | 10 (59) |
| Time from prior chemotherapy, n (%) | |||
| ≥3 months | 22 (34) | 12 (44) | 8 (47) |
| <3 months | 42 (66) | 15 (56) | 9 (53) |
| Median baseline CRP, g/dl (IQR) | 0.80 (0.19–4.05) | 1.18 (0.35–3.08) | 0.32 (0.20–1.97) |
IQR, interquartile range; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; PS, performance status; UC, urothelial carcinoma; CRP, C-reactive protein.
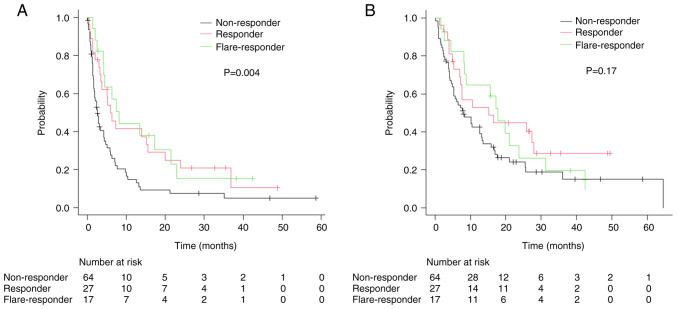
In BOR, ORR was 44, 48, and 16% in CRP flare-responders, CRP responders, and CRP non-responders, respectively (Fig. 1). The median follow-up was 8.6 months, 90 patients developed progression and 81 died during the study. Forty patients received subsequent therapy: 10, 2, and 28 patients received platinum-based chemotherapy, paclitaxel and gemcitabine therapy, and enfortumab vedotin, respectively, following pembrolizumab. In survival analysis, median PFS was 8.1 [95% confidence interval (CI) 4.1–21.5] months for CRP flare-responders, 5.9 (95% CI: 3.3–15.6) months for CRP responders, and 2.6 (95% CI: 1.7–4.2) months for CRP non-responders, with significant differences between the groups (Fig. 2A). Median OS for CRP flare-responders, CRP responders, and CRP non-responders was 17.8 (95% CI: 8.2–23.7) months, 15.2 (95% CI: 7.0–27.9) months, and 7.9 (95% CI: 5.3–13.2) months, respectively (Fig. 2B). Between the groups, difference in the incidence of irAEs, including severe cases (≥grade 3) was insignificant (Table II).
Figure 1.
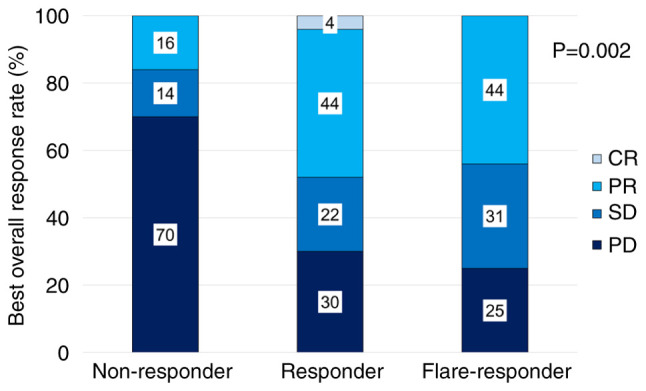
Best overall response for pembrolizumab in each group categorized by early C-reactive protein kinetics. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Figure 2.
Kaplan-Meier estimates of (A) progression-free survival and (B) overall survival in patients divided into three groups by early C-reactive protein kinetics.
Table II.
Immune-related adverse events in each group according to early C-reactive protein kinetics.
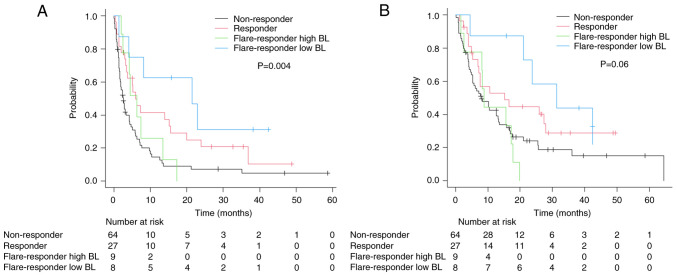
CRP flare-responders were further divided into two groups based on median BL (before initiation of pembrolizumab) CRP value (0.32 g/dl): CRP flare-responders low BL and CRP flare-responders high BL. In total, 8 and 9 patients were categorized as CRP flare-responders low and high BL, respectively. CRP flare-responders low BL included fewer patients with recent (<3 months) chemotherapy and low (<10 g/dl) hemoglobin compared with CRP flare-responders high BL (Table SI). Comparison between the groups revealed higher ORR in the CRP flare-responders low BL group than the CRP flare-responders high BL group (63% vs. 25%). Although CRP flare-responders low BL demonstrated the longest PFS (median 21.5 months, 95% CI: 1.3-not estimable), CRP flare-responders high BL showed a similar PFS (median 6.3 months, 95% CI: 2.1–13.4) as CRP responders (Fig. 3A). CRP flare-responders low BL demonstrated longer OS (median 31.4 months, 95% CI: 4.7-not estimable) compared with other groups, whereas CRP flare-responders high BL had similar OS (median 8.9 months, 95% CI: 1.7–17.8) as CRP non-responders (Fig. 3B). In multivariate analysis to predict PFS, CRP flare-responders low BL was a significant factor with lowest hazard ratio (Table III). Although severe (≥grade 3) irAEs were frequently observed in CRP flare-responders low BL compared with CRP flare-responders high BL (25% vs. 0%), the occurrence of all irAEs was similar between the groups (25% vs. 33%).
Figure 3.
Kaplan-Meier estimates of (A) progression-free survival and (B) overall survival in patients stratified by early CRP kinetics and BL CRP levels (only flare-responders). BL, baseline; CRP, C-reactive protein.
Table III.
Multivariate analysis to predict progression-free survival in patients with metastatic and unresectable urothelial carcinoma who received pembrolizumab.
| Factor | Hazard ratio | P-value |
|---|---|---|
| Age (continuous) | 1.00 (0.98–1.03) | 0.72 |
| Sex (female) | 1.81 (1.07–3.05) | 0.03 |
| Hemoglobin (<10 g/dl) | 1.33 (0.83–2.14) | 0.24 |
| ECOG-PS (≥1) | 1.25 (0.78–2.00) | 0.35 |
| Time from prior chemotherapy (<3 months) | 1.61 (1.00–2.60) | 0.05 |
| Liver metastasis (presence) | 1.54 (0.89–2.67) | 0.13 |
| CRP responder | 0.46 (0.26–0.79) | 0.01 |
| CRP flare-responder high BL | 0.46 (0.21–1.04) | 0.06 |
| CRP flare-responder low BL | 0.31 (0.12–0.80) | 0.02 |
ECOG, Eastern Cooperative Oncology Group; PS, performance Status; CRP, C-reactive protein; BL, baseline.
Discussion
In this study, we demonstrated that 16% of the patients with mUC treated with pembrolizumab showed the CRP flare-response. A favorable tumor response and survival were observed in CRP flare-responders and responders compared with CRP non-responders. In CRP flare-responders, patients with low BL CRP demonstrated better tumor response and survival than those with high BL CRP. The CRP flare-response with low BL CRP levels was a significant favorable factor predicting PFS. Although no difference was shown in the occurrence of any grade of irAE, severe irAEs were most commonly observed in CRP flare-responders low BL.
The association between the CRP flare-response and oncological outcomes was reported in patients with several malignancies, such as renal cell carcinoma (21,28), non-small cell lung cancer (29,30), hepatocellular carcinoma (31), head and neck cancer (32), and urothelial carcinoma (22,23), treated with ICI. In patients who received ICI, the CRP flare-response was observed in approximately 10–25% of the patients. Consistent with our data, 17% of the patients with UC showed the CRP flare-response (23).
In the studies investigated the CRP flare-response, whereas the better tumor response and survival in patients with the CRP flare-response compared with those of the CRP responders and non-responders was reported (21,29,31), several studies showed that there was not much difference in oncological outcomes between CRP flare-responders and responders as well as our results (23,28,30,32). Interestingly, in this study, tumor response and survival were greatly different between the groups depending on BL CRP among CRP flare-responders. Although favorable oncological outcomes were observed in CRP flare-responders low BL, tumor response and survival in CRP flare-responders high BL, as well as non-responders, was low. In patient characteristics of CRP flare-responders, CRP flare-responders high BL had several poor prognostic factors; however, CRP flare-response with low BL was a significantly independent predictive factor associated with favorable PFS. These results suggest that patients with the CRP flare-response were an immunologically heterogeneous population. In patients with mUC who were categorized as CRP flare-responders, the duration to decrease below BL CRP levels was the predicting factor associated with efficacy of pembrolizumab (23). CRP BL levels were one of the biomarkers to classify CRP flare-responders according to ICI efficacy. In the tumor microenvironment, elevated BL CRP levels indicated chronic inflammation that causes or contributes to immunosuppression (33). High CRP levels induced an immunosuppressive microenvironment (34). Differences in the condition of the tumor microenvironment might have affected the oncological outcomes between CRP flare-responders low and high BL.
In this study, no significant association was observed between the incidence of irAE and the groups stratified by early CRP kinetics. The relationship between the CRP flare-response and development of AE has not been fully investigated; only one study reported no relation between them (23). The association between irAE development during ICI and favorable oncological outcomes was reported (35). Additionally, elevated levels of serum CRP during ICI were associated with the development of irAEs (36). Although the number of patients was small in our study, CRP flare-responders low BL most frequently showed severe irAEs. Therefore, careful observation performing blood tests or imaging might be important regarding the occurrence of irAE when patients with low BL CRP levels showed the CRP flare-response.
Furthermore, 0.32 g/dl was used as the cutoff of CRP value to further divide CRP flare-responders into two groups in this study. Several studies reported the usefulness of baseline CRP value as a biomarker predicting the prognosis in patients with mUC treated with pembrolizumab, however, various cutoff values were reported and optimal cutoff value has not been established (18,19,24–26). Although we used median as the cutoff value, approximately 0.5 g/dl was often used and not different from our cutoff value (18,19,24). Therefore, we believe our cutoff value was acceptable.
This study has several limitations. First, this study was retrospective; therefore, unavoidable selection biases and confounders may be included in the results. In fact, the histologic subtypes or divergent differentiation were more commonly observed in CRP non-responders comparing to CRP flare-responders and CRP responders. There are few studies have investigated the outcomes of systemic therapy for mUC with subtype of UC (SUC), defined here as UC with any histologic subtype or divergent differentiation. In patients with mUC treated with systemic chemotherapy, poor survival was observed in patient with SUC compared to those with pure UC (37,38). In contrast, studies investigated in patients with mUC treated with pembrolizumab reported that the tumor response and survival between patients with pure UC and patients with SUC were comparable (39,40). Although the impact on response to systemic therapies in patients with SUC remains unclear, the difference of histologic features among the groups may affect the results in our study. Moreover, we excluded patients who developed infectious disease within 3 months of pembrolizumab administration and patients with insufficient data on CRP. However, we believe there was little impact on the results because only two patients were excluded in our cohort. Additionally, molecular testing data, such as expression of programmed cell death ligand 1, and tumor mutation burden were not investigated. Although these data were important for investigating factors predicting oncological outcomes in patients treated with ICI, we could not collect the data because these immunohistochemical analyses are not covered by national insurance and cannot be routinely performed in daily practice in Japan.
In conclusion, patients with mUC who showed the CRP flare-response demonstrated favorable oncological outcomes, comparable with CRP responders. Furthermore, CRP levels before initiation of pembrolizumab could predict oncological outcomes in CRP flare-responders. Better tumor response and survival were expected in CRP flare-responders with low BL CRP levels than those with high BL CRP levels. However, careful follow-ups might be necessary to detect severe irAEs as early as possible for CRP flare-responders with low BL CRP levels.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AE
adverse event
- BL
baseline
- BOR
best overall response
- CRP
C-reactive protein
- ICI
immune checkpoint inhibitor
- mUC
metastatic and unresectable urothelial carcinoma
- ORR
objective response rate
- OS
overall survival
- SUC
subtype of urothelial carcinoma
- UC
urothelial carcinoma
- PFS
progression-free survival
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
IT was responsible for conceptualization, data curation, formal analysis, investigation, methodology, project administration and writing the original draft. SS, MT, RH, SH, HM and SA contributed to the study conception and design. MH, SS, MT, RH, SH, HM, SA, YN and AM were responsible for reviewing the manuscript and acquisition of data on patient backgrounds, clinical tests, including CRP levels, AEs and treatment efficacy at each participating institution. MH, YN and AM were responsible for interpretation of data and edited the manuscript. KH was responsible for analysis and interpretation of data, wrote and edited the manuscript. NF was responsible for analysis and interpretation of data, supervised the study, and wrote and edited the manuscript. IT and NF confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the University of Occupational and Environmental Health, Japan (approval no. UOEHCRB20-180), the Ethics Committee of Kokura Memorial Hospital (approval no. 22092002E), the Ethics Committee of Munakata Suikokai General Hospital (approval no. 22003), the Ethics Committee of Shin-kokura Hospital (approval no. 2022-002), the Ethics Committee of Shin-yukuhashi Hospital (approval no. R3-35), the Ethics Committee of Kitakyushu City Yahata Hospital (approval no. 20220510) and the Ethics Committee of Moji Medical Center (approval no. 04-05). An opt-out approach was used to obtain informed consent from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Flaig TW, Spiess PE, Abern M, Agarwal N, Bangs R, Buyyounouski MK, Chan K, Chang SS, Chang P, Friedlander T, et al. NCCN guidelines® insights: Bladder cancer, version 3.2024. J Natl Compr Canc Netw. 2024;22:216–225. doi: 10.6004/jnccn.2024.0024. [DOI] [PubMed] [Google Scholar]
- 3.Jones RJ, Crabb SJ, Linch M, Birtle AJ, McGrane J, Enting D, Stevenson R, Liu K, Kularatne B, Hussain SA. Systemic anticancer therapy for urothelial carcinoma: UK oncologists' perspective. Br J Cancer. 2024;130:897–907. doi: 10.1038/s41416-023-02543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 5.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taguchi S, Kawai T, Nakagawa T, Miyakawa J, Kishitani K, Sugimoto K, Nakamura Y, Kamei J, Obinata D, Yamaguchi K, et al. Improved survival in real-world patients with advanced urothelial carcinoma: A multicenter propensity score-matched cohort study comparing a period before the introduction of pembrolizumab (2003-2011) and a more recent period (2016-2020) Int J Urol. 2022;29:1462–1469. doi: 10.1111/iju.15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balar AV, Castellano DE, Grivas P, Vaughn DJ, Powles T, Vuky J, Fradet Y, Lee JL, Fong L, Vogelzang NJ, et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: Results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Ann Oncol. 2023;34:289–299. doi: 10.1016/j.annonc.2022.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Bellmunt J, de Wit R, Fradet Y, Climent MA, Petrylak DP, Lee JL, Fong L, Necchi A, Sternberg CN, O'Donnell PH, et al. Putative biomarkers of clinical benefit with pembrolizumab in advanced urothelial cancer: Results from the KEYNOTE-045 and KEYNOTE-052 landmark trials. Clin Cancer Res. 2022;28:2050–2060. doi: 10.1158/1078-0432.CCR-21-3089. [DOI] [PubMed] [Google Scholar]
- 9.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Burger PM, Koudstaal S, Mosterd A, Fiolet ATL, Teraa M, van der Meer MG, Cramer MJ, Visseren FLJ, Ridker PM, Dorresteijn JAN, UCC-SMART study group C-reactive protein and risk of incident heart failure in patients with cardiovascular disease. J Am Coll Cardiol. 2023;82:414–426. doi: 10.1016/j.jacc.2023.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Zhu M, Ma Z, Zhang X, Hang D, Yin R, Feng J, Xu L, Shen H. C-reactive protein and cancer risk: A pan-cancer study of prospective cohort and Mendelian randomization analysis. BMC Med. 2022;20:301. doi: 10.1186/s12916-022-02506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes JV, Cobucci RN, Jatobá CA, Fernandes TA, de Azevedo JW, de Araújo JM. The role of the mediators of inflammation in cancer development. Pathol Oncol Res. 2015;21:527–534. doi: 10.1007/s12253-015-9913-z. [DOI] [PubMed] [Google Scholar]
- 13.Mosca M, Nigro MC, Pagani R, De Giglio A, Di Federico A. Neutrophil-to-lymphocyte ratio (NLR) in NSCLC, gastrointestinal, and other solid tumors: Immunotherapy and beyond. Biomolecules. 2023;13:1803. doi: 10.3390/biom13121803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DSJ, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer. A glasgow inflammation outcome study. Eur J Cancer. 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Han CL, Meng GX, Ding ZN, Dong ZR, Chen ZQ, Hong JG, Yan LJ, Liu H, Tian BW, Yang LS, et al. The predictive potential of the baseline C-reactive protein levels for the efficiency of immune checkpoint inhibitors in cancer patients: A systematic review and meta-analysis. Front Immunol. 2022;13:827788. doi: 10.3389/fimmu.2022.827788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishihara H, Takagi T, Kondo T, Fukuda H, Tachibana H, Yoshida K, Iizuka J, Okumi M, Ishida H, Tanabe K. Predictive impact of an early change in serum C-reactive protein levels in nivolumab therapy for metastatic renal cell carcinoma. Urol Oncol. 2020;38:526–532. doi: 10.1016/j.urolonc.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Kijima T, Yamamoto H, Saito K, Kusuhara S, Yoshida S, Yokoyama M, Matsuoka Y, Numao N, Sakai Y, Matsubara N, et al. Early C-reactive protein kinetics predict survival of patients with advanced urothelial cancer treated with pembrolizumab. Cancer Immunol Immunother. 2021;70:657–665. doi: 10.1007/s00262-020-02709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiwara M, Yuasa T, Urasaki T, Komai Y, Fujiwara R, Numao N, Yamamoto S, Yonese J. Effectiveness and safety profile of pembrolizumab for metastatic urothelial cancer: A retrospective single-center analysis in Japan. Cancer Rep 4. Cancer Rep (Hoboken) 2021;4:e1398. doi: 10.1002/cnr2.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Yatsuda J, Shimokawa M, Fuji N, Aoki A, Sakano S, Yamamoto M, Suga A, Tei Y, Yoshihiro S, et al. Prognostic value of pre-treatment risk stratification and post-treatment neutrophil/lymphocyte ratio change for pembrolizumab in patients with advanced urothelial carcinoma. Int J Clin Oncol. 2021;26:169–177. doi: 10.1007/s10147-020-01784-w. [DOI] [PubMed] [Google Scholar]
- 20.Ozawa Y, Amano Y, Kanata K, Hasegwa H, Matsui T, Kakutani T, Koyauchi T, Tanahashi M, Niwa H, Yokomura K, Suda T. Impact of early inflammatory cytokine elevation after commencement of PD-1 inhibitors to predict efficacy in patients with non-small cell lung cancer. Med Oncol. 2019;36:33. doi: 10.1007/s12032-019-1255-3. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda S, Saito K, Yasuda Y, Kijima T, Yoshida S, Yokoyama M, Ishioka J, Matsuoka Y, Kageyama Y, Fujii Y. Impact of C-reactive protein flare-response on oncological outcomes in patients with metastatic renal cell carcinoma treated with nivolumab. J Immunother Cancer. 2021;9:e001564. doi: 10.1136/jitc-2020-001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomisaki I, Harada M, Tokutsu K, Minato A, Nagata Y, Kimuro R, Matsumoto M, Fujimoto N. Impact of C-reactive protein flare response in patients with advanced urothelial carcinoma who received pembrolizumab. In Vivo. 2021;35:3563–3568. doi: 10.21873/invivo.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klümper N, Sikic D, Saal J, Büttner T, Goldschmidt F, Jarczyk J, Becker P, Zeuschner P, Weinke M, Kalogirou C, et al. C-reactive protein flare predicts response to anti-PD-(L)1 immune checkpoint blockade in metastatic urothelial carcinoma. Eur J Cancer. 2022;167:13–22. doi: 10.1016/j.ejca.2022.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Yasuoka S, Yuasa T, Nishimura N, Ogawa M, Komai Y, Numao N, Yamamoto S, Kondo Y, Yonese J. Initial experience of pembrolizumab therapy in japanese patients with metastatic urothelial cancer. Anticancer Res. 2019;39:3887–3892. doi: 10.21873/anticanres.13539. [DOI] [PubMed] [Google Scholar]
- 25.Nagasaka H, Yamamoto S, Suzuki A, Usui K, Terao H, Nakaigawa N, Kishida T. C-reactive protein is a prognostic factor for survival in metastatic upper tract urothelial carcinoma patients receiving pembrolizumab. In Vivo. 2024;38:1823–1828. doi: 10.21873/invivo.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura D, Jinnouchi N, Abe M, Ikarashi D, Matsuura T, Kato R, Maekawa S, Kato Y, Kanehira M, Trakata R, Obara W. Prognostic outcomes and safety in patients treated with pembrolizumab for advanced urothelial carcinoma: Experience in real-world clinical practice. Int J Clin Oncol. 2020;25:899–905. doi: 10.1007/s10147-019-01613-9. [DOI] [PubMed] [Google Scholar]
- 27.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoeh B, Garcia CC, Banek S, Klümper N, Cox A, Ellinger J, Schmucker P, Hahn O, Mattigk A, Zengerling F, et al. Early CRP kinetics to predict long-term efficacy of first-line immune-checkpoint inhibition combination therapies in metastatic renal cell carcinoma: An updated multicentre real-world experience applying different CRP kinetics definitions. Clin Transl Immunology. 2023;12:e1471. doi: 10.1002/cti2.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klümper N, Saal J, Berner F, Lichtensteiger C, Wyss N, Heine A, Bauernfeind FG, Ellinger J, Brossart P, Diem S, et al. C reactive protein flare predicts response to checkpoint inhibitor treatment in non-small cell lung cancer. J Immunother Cancer. 2022;10:e004024. doi: 10.1136/jitc-2021-004024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saal J, Bald T, Eckstein M, Ritter M, Brossart P, Ellinger J, Hölzel M, Klümper N. Early C-reactive protein kinetics predicts immunotherapy response in non-small cell lung cancer in the phase III OAK trial. JNCI Cancer Spectr. 2023;7:pkad027. doi: 10.1093/jncics/pkad027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin Q, Kou X, Zheng Y, Zhou F, Zhang X, Liu H. Early C-reactive protein kinetics predict response to immune checkpoint blockade in unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2023;10:2009–2019. doi: 10.2147/JHC.S432054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas M, Lein A, Fuereder T, Schnoell J, Brkic FF, Liu DT, Kadletz-Wanke L, Heiduschka G, Jank BJ. Early on-treatment C-reactive protein and its kinetics predict survival and response in recurrent and/or metastatic head and neck cancer patients receiving first-line pembrolizumab. Invest New Drugs. 2023;41:727–736. doi: 10.1007/s10637-023-01388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakayama T, Saito K, Kumagai J, Nakajima Y, Kijima T, Yoshida S, Kihara K, Fujii Y. Higher serum C-reactive protein level represents the immunosuppressive tumor microenvironment in patients with clear cell renal cell carcinoma. Clin Genitourin Cancer. 2018;16:e1151–e1158. doi: 10.1016/j.clgc.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T, Ichikawa J, Giuroiu I, Laino AS, Hao Y, Krogsgaard M, Vassallo M, Woods DM, Stephen Hodi F, Weber J. C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J Immunother Cancer. 2020;8:e000234. doi: 10.1136/jitc-2019-000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, Fernandes R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors-a systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. doi: 10.1016/j.ctrv.2020.102134. [DOI] [PubMed] [Google Scholar]
- 36.Abolhassani AR, Schuler G, Kirchberger MC, Heinzerling L. C-reactive protein as an early marker of immune-related adverse events. J Cancer Res Clin Oncol. 2019;145:2625–2631. doi: 10.1007/s00432-019-03002-1. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh MC, Sung MT, Chiang PH, Huang CH, Tang Y, Su YL. The prognostic impact of histopathological variants in patients with advanced urothelial carcinoma. PLoS One. 2015;10:e0129268. doi: 10.1371/journal.pone.0129268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minato A, Murooka K, Okumura Y, Takaba T, Higashijima K, Nagata Y, Tomisaki I, Harada K, Fujimoto N. Efficacy of platinum-based chemotherapy in patients with metastatic urothelial carcinoma with variant histology. In Vivo. 2024;38:873–880. doi: 10.21873/invivo.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi M, Narita S, Matsui Y, Kanda S, Hidaka Y, Abe H, Tsuzuki T, Ito K, Kojima T, Kato M, et al. Impact of histological variants on outcomes in patients with urothelial carcinoma treated with pembrolizumab: A propensity score matching analysis. BJU Int. 2022;130:226–234. doi: 10.1111/bju.15510. [DOI] [PubMed] [Google Scholar]
- 40.Minato A, Furubayashi N, Harada M, Negishi T, Sakamoto N, Song Y, Hori Y, Tomoda T, Tamura S, Kuroiwa K, et al. Efficacy of pembrolizumab in patients with variant urothelial carcinoma: A multicenter retrospective study. Clin Genitourin Cancer. 2022;20:499.e1–499.e8. doi: 10.1016/j.clgc.2022.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.