Abstract
Bone metastasis (BM) is a common complication of cancer and contributes to a higher mortality rate in patients with cancer. The treatment of BM remains a significant challenge for oncologists worldwide. The colony-stimulating factor (CSF) has an important effect on the metastasis of multiple cancers. In vitro studies have shown that CSF acts as a cytokine, promoting the colony formation of hematopoietic cells by activating granulocytes and macrophages. Other studies have shown that CSF not only promotes cancer aggressiveness but also correlates with the development and prognosis of various types of cancer. In recent years, the effect of CSF on BM has been primarily investigated using cellular and animal models, with limited clinical studies available. The present review discussed the composition and function of CSF, as well as its role in the progression of BM across various types of cancer. The mechanisms by which osteoclast- and osteoblast-mediated BM occur are comprehensively described. In addition, the mechanisms of action of emerging therapeutic agents are explored for their potential clinical applications. However, further clinical studies are required to validate these findings.
Keywords: macrophage colony-stimulating factor, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor bone metastasis, osteoclast, osteoblast
1. Introduction
Cancer is the leading cause of mortality worldwide and has become a major concern with increasing age and changing lifestyle habits. The predominant cause of mortality by cancer is the occurrence of distant metastases and for most cancers, bone is a common site for metastasis. For example, it is estimated that ~70% of patients with advanced breast cancer and over 70% of patients with advanced prostate cancer develop bone metastases (BM). In addition, in patients with metastatic prostate cancer, the probability of BM is as high as 90% (1). BM not only increases the economic burden on healthcare systems and patients but also significantly reduces patient quality of life and survival rates. In addition, it frequently affects the spine, ribs, pelvis and femur, leading to a higher incidence of spinal cord compression, pathological fractures and intractable pain (2). In addition, complications related to BM are significant, typically occurring every 3–6 months on average. These complications reduce patient quality of life and mortality is frequently associated with unresolved skeletal complications. The prognosis of metastatic bone disease varies based on the primary cancer site, with patients with breast and prostate cancer often surviving for years, while patients with lung cancer typically survive only a few months (3). However, current clinical treatments for BM are limited, highlighting the need to evaluate new therapeutic targets.
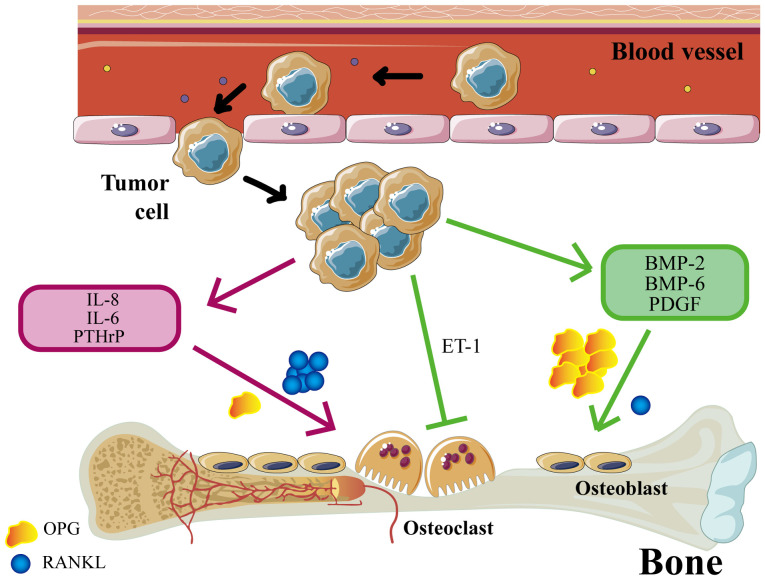
For tumor metastasis, primary tumor cells must first undergo epithelial-mesenchymal transition (EMT) to invade surrounding tissues and enter the microvasculature of the blood or lymphatic system (4). Cancer cells in the bloodstream can spread to distant organs, settling in the metastatic microenvironment. They may become dormant or proliferate there, eventually forming secondary tumors (5). The occurrence of BM is associated with osteoblasts and osteoclasts (OCs) in the bone microenvironment (Fig. 1). BM can be categorized into osteolytic and osteogenic subtypes. In addition, NF-κB ligand receptor-activating factors (RANKL) are key mediators of osteoclastogenesis (6). Tumor cells in osteolytic BM can stimulate OCs and promote their differentiation by secreting cytokines such as TNF, RANKL, prostaglandins, leukemia inhibitory factor and IL-6, −8, −11, −15 and −17. Tumor cells can upregulate OC function by increasing the ratio of RANKL to osteoprotegerin (OPG) (7). OCs-mediated bone matrix degradation releases various cytokines and growth factors that promote tumor cell growth (7). Colony-stimulating factors (CSF) are a group of cytokines responsible for hemopoiesis, blood cell function regulation and maintaining homeostasis and overall immunity (8). CSF plays a crucial role in regulating blood cell function, given the instability and short lifespan of these cells (9). The CSFs are of a number of types and mainly include granulocyte-CSF (G-CSF), macrophage-CSF (M-CSF), granulocyte-macrophage CSF (GM-CSF) and multipotent CSF (multi-CSF) (10). In addition, CSF has profound effects on the development of granulocytes, macrophages and lymphocytes. The present article reviews the CSF family, its relationship to BM, the underlying mechanisms involved and the preclinical applications of CSF.
Figure 1.
Bone metastasis occurs as a gradual process. First, a change in the pre-metastatic bone microenvironment facilitates the attraction of more tumor cells. Second, cancer cells are extravasated from the blood and are finally colonized into bone tissue. There are two main types of bone metastasis: Osteolytic bone metastasis and osteoblastic bone metastasis. The mechanism of osteolytic bone metastasis is that cancer cells can promote the secretion of RANKL and inhibit the secretion of OPG through the secretion of IL-8, IL-6 and PTHrP, among others, so that the activity of osteoclasts is enhanced. The mechanism of osteoblastic bone metastasis is as follows: Bone morphogenetic protein (BMP)-2, BMP-6 and PDGF, produced by tumor cells, can promote the secretion of OPG and inhibit the secretion of RANKL while enhancing the activity of osteoblasts. In addition, cancer cells can directly inhibit osteoclast activity by secreting ET-1. RANKL, NF-κB ligand receptor-activating factors; OPG, osteoprotegerin; PTHrP, parathyroid hormone-related peptide; BMP, bone morphogenetic protein; PDGF, platelet derived growth factor; ET-1, endothelin-1.
2. Physiological functions of CSF and its influential role in bone metastasis in various types of cancer
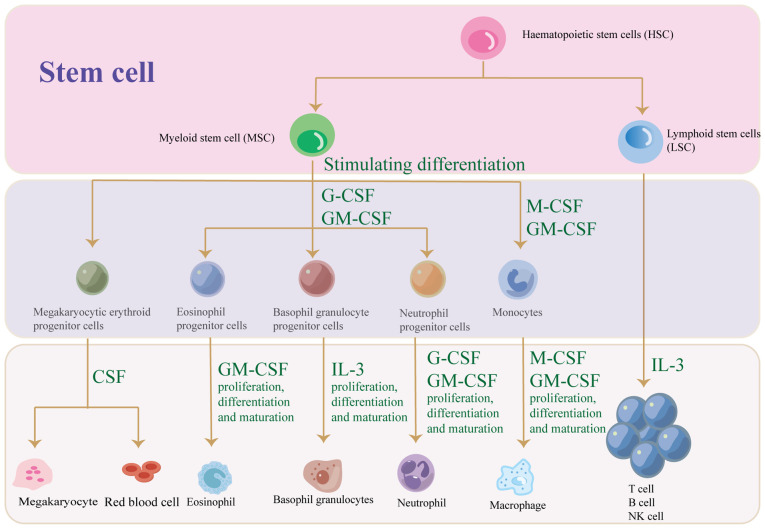
CSFs were first identified in an in vitro study on hematopoietic cells. In 1966, macrophage and granulocyte colony formation was discovered in hematopoietic cell studies, leading to the naming of CSFs in 1967 (9,11,12). Research has shown that CSFs are classified into four types: M-CSF (CSF1), GM-CSF (CSF2), G-CSF (CSF3) and multi-CSF (IL-3; Table I). CSF promotes the differentiation of hematopoietic stem cells into different blood cells, such as eosinophils, basic granulocytes and neutrophils (Fig. 2). Studies have concluded that CSF influences BM in various tumors (Table II). Although CSF has been extensively studied, it remains an area of active research. The physiological functions and effects of each CSF on cancer and BM are described in detail following.
Table I.
Classification and effects of CSF.
| Name | Receptor | Generated cell | Effect | Clinical application | (Refs.) |
|---|---|---|---|---|---|
| M-CSF | M-CSFR | Macrophage, megakaryocytes | Stimulates macrophage colony formation affects granulocyte function and lowers blood cholesterol. | Used to enhance macrophage function to improve immunity. | (25–27) |
| G-CSF | G-CSFR | Fibroblasts, bone marrow stromal cells, eosinophils, basophils, neutrophils, macrophages, fibroblasts, eosinophils, basophils, megakaryocytes, hemocytes, neutrophils | Stimulates granulocyte colony formation and stimulates granulocyte function. | For the treatment of leukopenia following chemotherapy to reduce the incidence of infection. | (8,13,14) |
| GM-CSF | GM-CSFR | Macrophages, fibroblasts, eosinophils, basophils, megakaryocytes, hemocytes, neutrophils | Stimulates granulocyte and macrophage colony formation and affects granulocyte function. | Used for myelo-suppression caused by chemotherapy or radiotherapy and for hematopoietic recovery after bone marrow transplantation. | (28,47–50) |
| IL-3 | IL-3R | Mast cells, basophils, eosinophils, hemocytes, megakaryocytes, neutrophils, macrophages, T cells, B cells, NK cells | Stimulates the formation of progenitor cell colonies of granulocytes, monocytes, erythrocytes and macrophages; enhances phagocytosis of macrophages; stimulates the proliferation of hematopoietic stem cells; promotes the proliferation and differentiation of mast cells, basophils and eosinophils. | Used to increase neutrophil fine and platelet in tumor patients after chemotherapy. | (64–67) |
M-CSF, macrophage-colony-stimulating factor; G-CSF, granulocyte-colony-stimulating factor; GM-CSF, granulocyte-macrophage-colony-stimulating factor.
Figure 2.
CSF promotes the differentiation of hematopoietic stem cells into different blood cells, such as eosinophils, basophil granulocytes, monocytes and neutrophils. CSF, colony-stimulating factor; G-CSF, granulocyte-CSF; M-CSF, macrophage-CSF; GM-CSF, granulocyte-macrophage CSF.
Table II.
Mechanisms of CSF in bone metastasis of different cancers.
| Type of CSF | Type of disease | Type of bone metastasis | Incidence of bone metastasis (%) | Mechanism of action of CSF affecting bone metastasis | Subjects | (Refs.) |
|---|---|---|---|---|---|---|
| M-CSF | Breast cancer bone metastasis | Osteolytic metastasis | 70 | M-CSF affects osteoclast function to promote bone metastasis. | Cell, Mice | (34,35) |
| Prostate cancer bone metastasis | Osteogenic metastasis | >70 | M-CSF alter the bone microenvironment and promote bone metastasis. | Cell, Mice | (40) | |
| Lung cancer bone metastasis | Osteolytic metastasis | 40 | M-CSF increases osteoclast activity and bone metastasis. | Cell, Mice | (43,44) | |
| Renal cell carcinoma bone metastasis | Osteolytic metastasis | 30 | M-CSF increases osteoclast activity and promotes bone metastasis. | Cell | (46) | |
| G-CSF | Breast cancer bone metastasis | Osteolytic metastasis | 70 | G-CSF alters the bone microenvironment to promote bone metastasis. | Mice | (24) |
| GM-CSF | Breast cancer bone metastasis | Osteolytic metastasis | 70 | GM-CSF, alter the bone microenvironment and promote bone metastasis. | Cell, Mice | (59) |
| Nasopharyngeal cancer bone metastasis | Osteolytic metastasis | 64-67 | GM-CSF secreted by tumor cells promotes osteoclast differentiation and bone metastasis. | Cell | (61) | |
| IL-3 | Breast cancer bone metastasis | Osteolytic metastasis | 70 | IL-3 affects osteoclast function. | Cell | (71) |
| Prostate cancer bone metastasis | Osteogenic metastasis | >70 | IL-3 secreted by prostate cancer is associated with prostate cancer BM. | Clinical pathology | (72) |
CSF, colony-stimulating factor; M-CSF, macrophage-colony-stimulating factor; G-CSF, granulocyte-colony-stimulating factor; GM-CSF, granulocyte-macrophage-colony-stimulating factor; IL-3, interleukin-3; EMT, epithelial-mesenchymal transition.
Granulocyte-colony-stimulating factor
The G-CSF is a 30 kDa glycosylated peptide primarily produced by monocytes and macrophages (13). G-CSF acts mainly on granulocytes. Through binding to its receptor (G-CSFR), G-CSF activates signaling pathways such as Janus tyrosine kinase (JAK)/signal transducers and activators of transcription (STAT), Mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), ultimately leading to the differentiation of hematopoietic stem cells towards the granulocyte lineage and the promotion of neutrophil production (8). G-CSF promotes the proliferation, differentiation, maturation and release of neutrophils while also enhancing the chemotaxis, phagocytosis and bactericidal capacity of mature granulocytes (13). G-CSF downregulates the inflammatory response by inhibiting the production of pro-inflammatory cytokines in activated monocytes and macrophages and by regulating peripheral lymphokine levels (14). Studies have shown that elevated G-CSF often indicates a poor cancer prognosis. G-CSF also stimulates proliferation and metastasis in various cancer cells (15). For example, G-CSF promotes the progression of skin cancer and head and neck squamous cell carcinoma through autocrine and paracrine mechanisms (16,17). Similarly, G-CSF stimulates tumor progression by suppressing innate and adaptive immunity while also promoting angiogenesis and tumor growth (18). While G-CSF can inhibit human meningioma development, it promotes cancer cell proliferation through angiogenesis in other types of cancer (19). G-CSF is significantly correlated with the aggressiveness of cancer cells. For example, G-CSF increases the invasiveness of lung cancer cells through autocrine secretion (20). An in vivo study showed that G-CSF-induced pre-metastatic microenvironment was more favorable for cancer cell migration and increased the expression of MMP-9, S100A8 and S100A9 pro-metastatic molecules through Ly6G+Ly6C expression (21). Elevated G-CSF receptor expression in patients with cancer is associated with an increased risk of advanced metastasis (15). A clinical study compared serum G-CSF levels between patients with non-small cell lung cancer and normal individuals and revealed that G-CSF was significantly elevated in patients with lung cancer, which significantly decreased after the surgery. Therefore, it was inferred that G-CSF is important in diagnosing non-small cell lung cancer (22). Clinical studies have demonstrated that tumor-derived G-CSF is associated with poor patient prognosis. In addition, the patients with retroperitoneal tumor have significantly elevated G-CSF and develop liver and kidney metastases 3 months after primary tumor resection, BM after 8 months and succumbed after 17 months (23). Notably, in a mouse model of breast cancer BM, G-CSF is found to create a metastasis-promoting tumor microenvironment and G-CSF inhibition attenuated bone marrow vascular remodeling and BM incidence (24).
Macrophage-colony-stimulating factor (M-CSF)
M-CSF is a hematopoietic growth factor that plays a key role in regulating mature myeloid cell populations (25). It comprises various cells, including endothelial, fibroblasts, OCs, smooth muscle and macrophages. M-CSF primarily acts on the monocyte-macrophage cell line, promoting monocyte and macrophage production and regulating functions such as antigen presentation, phagocytosis, cytokine secretion and tissue repair. M-CSF stimulates the differentiation of myeloid progenitor cells into monocytes, macrophages, dendritic cells and osteoblasts. M-CSF promotes macrophage growth and function by binding to its receptor (CSF-1R) and activating PI3K and MAPK signaling pathways (26). It has been shown in animal studies that daily administration of recombinant human G-CSF enhances the recovery of stem cells, progenitor cells and blood neutrophils in mice (27). GM-CSF and M-CSF promote the survival and activation of macrophages, neutrophils and eosinophils, as well as dendritic cell maturation. However, certain M-CSF variants specifically promote the survival, proliferation and differentiation of macrophage lineages (28). M-CSF and GM-CSF differ in their roles in regulating macrophage differentiation phenotypes (25). M-CSF stimulates the M1 macrophage phenotype, while GM-CSF promotes M2 phenotype activation (29). In addition, M-CSF modulates macrophage phenotype and regulates their function in the tumor microenvironment (30). M-CSF facilitates tumor invasion, metastasis and immune evasion by modulating tumor-associated macrophages (TAMs) in the tumor microenvironment. Studies have shown that M-CSF levels are elevated in cancer, inflammation and autoimmune diseases. Animal cancer models reveal that M-CSF antibody administration or inhibition of CSF-1R improves inflammation and cancer metastasis (31–33).
M-CSF has been closely associated with breast cancer BM. In a mouse model of breast cancer, M-CSF gene knockdown reduced the occurrence of distant metastasis. However, M-CSF gene knockdown did not affect cancer cell proliferation or development. By contrast, mice with M-CSF gene overexpression exhibit a significant increase in late-stage cancer and lung metastases (34). A clinical study revealed significantly elevated serum M-CSF levels in patients with head and neck tumors, as well as in patients with advanced prostate and breast cancer with BM (35). A clinical trial in 1996 found that serum M-CSF levels are significantly higher in patients with metastatic breast cancer compared with those with primary breast cancer (36). In an in vivo experiment, it was reported that the expression of osteoblasts and OCs in the bone microenvironment can be altered by reducing the expression of M-CSF, thereby inhibiting the occurrence of breast cancer BM (37,38). In addition, M-CSFR phosphorylation reduces bone tumor growth and inhibits osteolysis. Breast cancer BM has also been associated with mesenchymal stem cells (MSCs). In addition, the MCSs of patients with advanced breast cancer had worse self-renewal and proliferation ability than normal individuals. The reduced function of MSCs may be attributed to the increased expression of pro-osteoclastogenic genes, such as CCL-2, MMP-9 and M-CSF (39). M-CSF has been found to influence BM in prostate cancer. A study indicated that in a mouse model of prostate cancer BM, osteoblasts in the bone microenvironment could be altered by the inhibition of RANKL and M-CSF, reducing BM occurrence (40). These results were consistent with another animal experiment which indicated that OCs inhibition in a mouse model of prostate BM decreases M-CSF expression which decreases the occurrence of BM and bone destruction (41). A clinical study compared serum M-CSF levels between patients with prostate cancer with BM, patients with prostate cancer without metastases and healthy individuals. There were no significant differences in M-CSF between healthy subjects and patients with prostate cancer without metastases. However, serum M-CSF levels were significantly higher in patients with prostate cancer with BM compared with those without metastases (42). The study concluded that the occurrence of prostate cancer BM is related to the M-CSF/M-CSFR signaling pathway (42). Similar findings were observed in lung cancer BM. In vitro and in vivo studies have both demonstrated that osteoclastogenesis can be promoted by upregulating M-CSF and RANKL in human lung adenocarcinoma A549 cells, leading to an increased BM development (43,44). M-CSF inhibition reduces the M-CSF/RANKL-induced AMT/mTOR signaling pathway, decreasing OC differentiation. M-CSF inhibition also reduces lung adenocarcinoma-mediated interactions between OCs and osteoblasts, thereby decreasing the occurrence of this ‘vicious circle’ and osteolytic metastases (43). This feedback loop plays a crucial role in lung cancer BM. Another cellular study demonstrated that increased OC activity in non-small cell lung cancer cells via the cyclic effects of parathyroid hormone-related peptide (PTHrP)/IL-8 interference with osteoblasts and OCs increased the incidence of BM in NSCLC (45). In a cellular experiment, co-culture of kidney cancer cells with osteoblasts demonstrated that osteoblasts promote tumor cell proliferation, suggesting that osteoclasts create a more favorable environment for tumor survival. In addition, BM occurrence could be effectively reduced by inhibiting the growth of OCs (46). M-CSF is an important factor in inducing OC differentiation; therefore, it is hypothesized that it is a potential target for treating or preventing BM in renal cell carcinoma.
Granulocyte-macrophage-colony-stimulating factor (GM-CSF)
GM-CSF is a 22 kDa glycosylated secretory protein that promotes the proliferation and maturation of neutrophils, eosinophils and macrophages from bone marrow progenitor cells (28). In addition, GM-CSF acts synergistically with other cytokines as a growth factor for erythroid and megakaryocyte progenitors. In addition, it modulates progenitor cells and interacts with erythropoietin to stimulate the in vitro formation of eosinophil and megakaryocyte colonies (9). GM-CSF promotes bi-directional differentiation of hematopoietic stem cells to granulocytes and macrophages by binding to GM-CSFR and activating signaling pathways such as JAK2/STAT5, MAPK and PI3K/AKT (28). GM-CSF has been associated with inflammatory responses, activating neutrophil activity in the human body (47). It has been observed that GM-CSF and G-CSF can induce endothelial cell proliferation and migration, thereby promoting angiogenesis (48). These properties indicate that GM-CSF may have potential applications in adjuvant tumor therapy (49). Angiogenesis not only promotes cancer cell growth but significantly increases distance metastasis and advances tumor stages more quickly (50). In addition, serum GM-CSF receptor levels are elevated in patients with advanced cancer and distant metastases (15). Studies have shown that GM-CSF promotes the development of tumors such as lung, breast, pancreatic, prostate, skin, colon, rectal, head and neck squamous cell carcinomas (16,17,51–55). In vivo and in vitro research shows that GM-CSF overexpression elevates tumor cell migration and invasion (56). In 1999, a study reported that GM-CSF could promote the invasiveness of lung cancer cells (20). In addition, a mouse tumor model revealed that GM-CSF gene expression is correlated with tumor metastasis in mice (57). GM-CSF plays a dual role in tumor development, both promoting and inhibiting tumor growth, depending on the context. GM-CSF has a complex role in the tumor microenvironment, either suppressing tumors by enhancing anti-tumor immune responses or promoting tumor growth and metastasis by promoting the activity of tumor-associated macrophages (58).
A significant association between GM-CSF and BM has been observed. A study revealed that CTNND1 gene knockdown accelerated the differentiation of immature bone marrow cells and promoted BM development. This may be due to CTNND1 knockdown enhancing PI3K/AKT/HIF-1α/CXCR4 pathway expression, promoting EMT in tumor cells. When the CTNND1 knockdown tumor cells reach the bone, they secrete more GM-CSF and IL-8, enhancing immature bone marrow cells (especially neutrophils) and promoting BM development (59). In a mouse model of breast cancer BM, GM-CSF promotes the metastatic ability of cancer cells and BM (60). For nasopharyngeal cancer, bone is the most common metastatic site. Among patients with advanced nasopharyngeal cancer, 64–67% carry BM and the most common BM type in nasopharyngeal cancer is osteolytic (61). GM-CSF secreted by cancer cells can promote BM by promoting the secretion of IGF-1 from OCs, which in turn promotes the proliferation of nasopharyngeal carcinoma cells via the IGF-1/IGF-1R signaling pathway (61). In addition, metastatic breast cancer was also associated with arthritis progression, indicating a ‘vicious cycle’ (62). The M-CSF and GM-CSF are important pro-inflammatory factors associated with rheumatoid arthritis (63). Therefore, it is suggested that M-CSF and GM-CSF may contribute to the development of breast cancer BM by influencing the course of arthritis.
IL-3
IL-3, also called multi-CSF, is a hematopoietic factor produced by activated T-cells and NK-cells. In addition, it promotes the growth and differentiation of bone-marrow-derived T-cells in the immune response. Similarly, it increases the formation of fibroblasts, granulocytes, macrophages, megakaryocytes, eosinophils and mast cell colonies (9). IL-3 is recognized as an essential early hematopoietic growth factor that regulates hematopoiesis. IL-3 mainly acts on early hematopoietic progenitor cells to promote their proliferation and differentiation. In the later stages, IL-3 acts in conjunction with hematopoietic growth factors such as erythropoietin, GM-CSF and thrombopoietin to promote the proliferation and differentiation of myeloid hematopoietic stem cells. IL-3 stimulates the formation of progenitor cell colonies of granulocytes, monocytes, erythrocytes and macrophages; enhances macrophage phagocytosis; promotes hematopoietic stem cell proliferation; and facilitates the proliferation and differentiation of mast cells, basophils and eosinophils (64,65). IL-3 and GM-CSF were first reported in 1988 to co-stimulate hematopoiesis in primates (66). Evidence suggests that IL-3 is involved in the onset and progression of various hematological diseases, including acute myeloid leukemia, chronic myeloid leukemia and myelodysplastic syndromes (64). IL-3 has been identified as a potential marker for the severity and mortality of COVID-19-associated pneumonia during SARS-CoV-2 infection. Thus, IL-3 serves as a predictive marker for the severity of SARS-CoV-2 infection and a potential therapeutic target for COVID-19-associated pneumonia (67). In addition, since IL-3 can influence the development of basophils and mast cells, it is often associated with allergies, asthma, inflammation and other diseases. Extensive data suggest that IL-3 is closely associated with various types of cancer. For instance, IL-3 levels were significantly higher in the serum of patients with colorectal cancer than in healthy subjects (68). Therefore, it is inferred that IL-3 influences cancer development in several ways. IL-3 alters the tumor microenvironment by affecting basophils, thereby promoting cancer development (69). Further, IL-3 promotes cancer cell proliferation by inducing angiogenesis (70). In cellular assays, it is found that osteoclast differentiation could be inhibited by adding benzyl isothiocyanate with zoledronic acid to a breast cancer-conditioned medium. Osteoclast inhibition is accompanied by a significant increase in IL-3 (71). Immunohistochemical analysis of patients with prostate cancer reveal that IL-3 is implicated in the development of prostate cancer BM (72). However, the relationship between IL-3 and BM requires further experimental validation.
3. Mechanisms of CSF effects in bone metastases
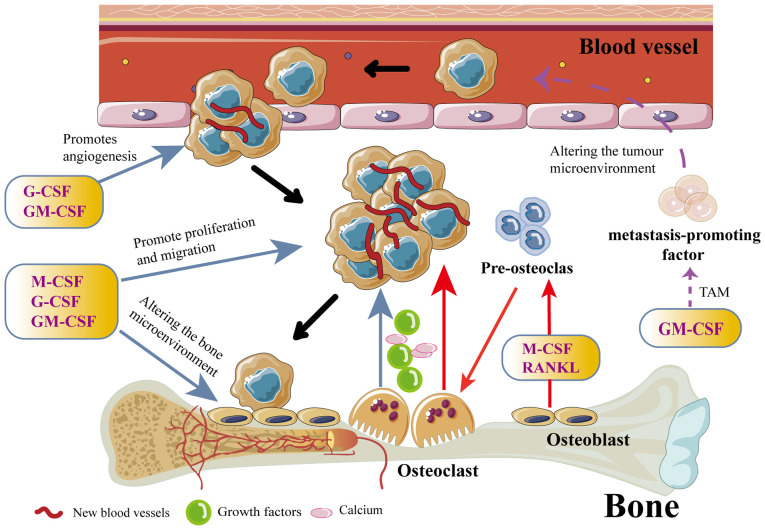
CSFs play complex and diverse roles in developing and progressing BM in various types of cancer. They affect not only the hematopoietic system but also the skeletal microenvironment and the behavior of tumor cells through various mechanisms (Fig. 3). First, CSF promotes the bone metastatic potential of tumor cells. CSF increases the metastatic potential of cancer cells by promoting neoangiogenesis and enhancing their invasiveness (73). Studies have shown that G-CSF and GM-CSF can enhance the BM in tumor cells through multiple pathways. For example, G-CSF promotes tumor cell growth and metastasis by increasing angiogenesis and improving nutrient and oxygen availability to tumor cells (74). In addition, G-CSF can improve the tolerance of tumor cells to chemotherapeutic drugs, making it easier for them to survive and proliferate in the bone (75,76). GM-CSF enhances the pro-metastatic properties of the tumor microenvironment by promoting the generation and activation of TAMs, which are capable of secreting a variety of pro-angiogenic and pro-metastatic factors (for example, VEGF, MMPs and IL-10), further promoting BM of tumor cells (73).
Figure 3.
CSF influences the course of cancer development through paracrine secretion and promotes the occurrence of bone metastases. First, CSF promotes angiogenesis, which is the main cause of the transformation of intraepithelial neoplasia into early-stage cancer. Second, CSF can also increase the invasiveness of cancer cells and accelerate their extravasation. Finally, CSF can lead to the development of osteolytic bone metastases by increasing osteoclast activity. Activated osteoclasts can release large amounts of growth factors and Ca from the bone matrix, which increases cancer cell proliferation, creating a vicious circle. CSF, colony-stimulating factor; G-CSF, granulocyte-CSF; M-CSF, macrophage-CSF; GM-CSF, granulocyte-macrophage CSF; TAM, tumor-associated macrophage; RANKL, NF-κB ligand receptor-activating factors.
Second, CSF indirectly affects the occurrence and development of BM by regulating cellular components and factor secretion in the bone marrow microenvironment. Bone is a common site of metastasis for a number of cancers due to the high expression of specific chemokines and growth factors in its microenvironment, such as stromal cell-derived factor 1, which promotes tumor metastasis (77). It is considered that before the primary tumor reaches the bone, it alters the bone microenvironment to promote cancer cell proliferation and colonization. In addition, tumor cells induce metastasis by forming ‘pre-metastatic niches’ in the bone, comprising clusters of bone marrow-derived cells, creating a favorable environment for the subsequent invasion and growth of tumor cells (78). Osteolytic lesions caused by OCs are significantly associated with BM. In osteolytic BM, OCs activating factors act on OCs to induce its formation. CSF and RANKL are important activators of OCs. In addition, osteoclastogenesis is primarily modulated by the interaction of CSF-1R, M-CSF, as well as RANK and RANKL (79). Therefore, M-CSF is essential for OC generation and increased OC activity promotes osteolytic BM. M-CSF is a key factor affecting OC generation and can influence OC precursor's survival and development. Similarly, M-CSF induces cytoskeletal rearrangement of OCs by activating c-Src and phosphocreatine 3-kinase (80). The M-CSF directly affects OCs by binding to M-CSFR, which attracts a signaling complex comprising phosphorylated dDNA-activated protein 12 and the non-receptor tyrosine kinase Syk. In addition, it also activates ERK/growth factor receptor-binding protein 2 and Akt/PI3K signaling to regulate the proliferation, differentiation and growth of OCs and their precursor cells (80). In addition, M-CSF can induce osteoclastogenesis by increasing the OCs precursor RANK expression via RANKL binding (81). In a number of cancer models, tumor cells have been observed to secrete cytokines that stimulate osteoblasts, such as PTHrP, VEGF-A and hepatocyte growth factor. This increases the expression of RANKL and M-CSF, stimulating osteoclastogenesis and altering the bone microenvironment, thereby promoting BM development. M-CSF affects osteoclastogenesis and influences other members of the CSF family on OCs (2–7). In 1993, it was reported that M-CSF, GM-CSF and IL-3 could promote osteoclastogenesis (82). In addition, GM-CSF is stimulated by increased levels of NF-kB, resulting in increased OC activity, which in turn leads to bone destruction and highly metastatic tumor growth (15).
Third, CSF also plays an important role in regulating the immune system, ultimately affecting BM. G-CSF inhibits T-cell activity and reduces the killing effect of the immune system on tumor cells, making it easier for tumor cells to grow and metastasize in the bone (83).
4. Preclinical and clinical application of CSF
BM often has a poor prognosis and high mortality due to delayed diagnosis and limited treatment options. Bone is a common site of metastasis in breast cancer; however, BM in early-stage breast cancer is difficult to detect. As a result, BM in breast cancer is often diagnosed late, leading to delayed treatment. BM has been reported to occur in ~70% of patients with advanced breast cancer. The median overall survival following BM diagnosis was 40 months, indicating a high mortality rate (84). Similarly, BM has been reported in >70% of patients with advanced prostate cancer (1). Epidemiological data from the United States show that lung cancer now has a higher mortality rate than breast and prostate cancers, ranking as the leading cause of cancer-related death (85). Recently, nanoparticles have provided new directions in lung cancer treatment (86,87). Distant metastasis is the primary cause of death in patients with lung cancer. Bone is a common site of metastasis in advanced lung cancer and in advanced patients with lung cancer, 40% develop BM (88). The median survival of patients with lung cancer BM is often <6 months (89). Therefore, developing effective treatments for BM remains a significant challenge for clinicians and researchers.
CSFs are widely used in patients with cancer after chemotherapy to elevate critically low leukocyte levels (9). The clinical role of CSFs has been widely publicized. In 1990, with the availability of recombinant mouse CSF experimental, it was revealed that subcutaneous injections of G-CSF into mice increased blood leukocyte levels. High doses of G-CSF have been associated with erythrocyte suppression in the bone marrow (90). In addition, a 1987 study revealed that injection of recombinant GM-CSF in mice does not significantly increase blood leukocytes; however, it markedly increases the macrophage numbers and activity (91). The most significant increase in the blood of mice injected with recombinant multi-CSF was in eosinophils, followed by neutrophils and monocyte levels (92). Further research is needed to determine whether prolonged use of high-dose CSF has additional side effects. Transgenic mice overexpressing GM-CSF indicates blindness and various inflammatory lesions. In addition, a number of transgenic mice overexpressing GM-CSF died at 2–4 months due to macrophage activation, leading to muscle atrophy (93). In IL-3 overexpressing mice, there were increased progenitor cells in the spleen and peritoneum and decreased bone marrow progenitor cells. In addition, 80% of the mice succumbed within 5 weeks (94). Mice overexpressing G-CSF cause the proliferation of granulocytes and progenitor cells but does not cause severe tissue damage (95). The role of different cytokines in influencing the immunogenicity of tumor cells and the vaccination properties of murine tumor cells have been investigated in an animal experiment. Tumor cells expressing mouse GM-CSF have the most significant stimulatory effect on anti-tumor immunity following irradiation (96). GM-CSF is used in the treatment of melanoma (97).
The first clinical trials of CSF, published in 1988, demonstrated a significant increase in neutrophils when recombinant human G-CSF was administered to patients with cancer prior to chemotherapy (98). In addition, G-CSF-associated side effects are minimal the most common bring bone pain, which might be due to increased bone marrow cell counts (99). A phase II clinical trial at the Cancer Research Institute in Australia found that treatment with recombinant human GM-CSF in 21 patients with advanced cancer resulted in a 10-fold increase in leukocytosis. The trial also observed increases in circulating neutrophils, eosinophils, monocytes and lymphocytes. In addition, side effects, such as bone pain, myalgia, rash and hepatic dysfunction, are observed with high doses of rhGM-CSF (100). The safety and efficacy of GM-CSF in combination with Ipilimumab (Yervoy) for treating metastatic malignant melanoma is in a phase II clinical trial (NCT01363206). In another clinical trial (NCT02156388), G-CSF was administered in patients with advanced metastatic cancer following chemotherapy, which revealed increased neutrophil counts and activity and shorter duration and acute symptoms of neutropenia. This minimized the incidence of serious infections, reflecting improved efficacy and a longer half-life. The therapeutic effect of G-CSF with trastuzumab in metastatic breast cancer has also been studied in a randomized phase II clinical trial (NCT00169104).
CSF has been widely used in treating rheumatoid arthritis, coronary atherosclerosis and various inflammatory and autoimmune diseases (28). A number of preclinical trials have demonstrated that CSF is closely associated with mechanisms of BM in various types of cancer. However, more evidence is required to establish its efficacy in treating BM. It is hypothesized that targeted therapies against M-CSF or its signaling pathway may have clinical applications in BM treatment. The effectiveness of combining CSF with existing anti-bone metastatic drugs, such as bisphosphonates or RANKL inhibitors, in inhibiting BM progression warrants further investigation. Therefore, CSF might serve as a therapeutic target for treating BM in different cancers.
5. Summary and outlook
Cancer is the leading cause of death and the incidence of distant metastases significantly reduces survival in patients with cancer. Bone is a common site of metastasis in patients with cancer, often leading to serious complications such as pain and hypercalcemia. In addition, BM significantly reduces quality of life and imposes a substantial economic burden on patients and healthcare systems. In addition, the limited treatment options for BM contribute to significant psychological distress and anxiety in affected patients. A number of studies have indicated that changes in the bone microenvironment are closely related to BM, suggesting that modulating the bone microenvironment may help alleviate metastasis. In BM, cancer cells first undergo EMT, invade the blood and lymphatic vessels and migrate into bone tissue. Prior to entering bone tissue, cancer cells secrete cytokines that modify the bone microenvironment, facilitating their colonization and proliferation. For example, tumor cells can release factors that induce osteoblast formation during BM. This enhances the osteoblast-mediated bone formation while increasing the resorption of mineralized bone by OCs, which severely disrupts normal bone homeostasis (2). CSF has been shown to stimulate the differentiation and proliferation of hematopoietic cells. In addition, the relationship between CSF and BM has been extensively studied. Further, M-CSF is essential for promoting the differentiation of OC precursors. Drugs targeting OCs, such as bisphosphonates and the RANK ligand inhibitor denosumab, have been established to treat BM. It was also observed that administering OC inhibitors such as bisphosphonates, OPG and RANKL antagonists before tumor inoculation reduces the incidence of BM (101,102). However, the clinical application of CSF in treating BM remains underexplored. The treatment of BM remains a significant challenge for oncologists and further clinical trials are required to determine whether CSF could serve as a therapeutic target for BM.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by Hubei Province Key Laboratory of Molecular Imaging (grant no. 2023fzyx025 to HJB), Jingzhou 2023 Medical Health Science and Technology Plan Project (grant no. 2023HC07 to HJB), Hubei Provincial Natural Science Foundation (grant no. 2023AFB969 to HJB). Jingzhou Science and Technology Bureau Project (grant no. 2022HC78 to PXC) and Wujieping Medical Foundation digestive tract cancer research fund (grant no. 320.6750.2024–10-3 to PXC).
Availability of data and materials
Not applicable.
Authors' contributions
XP and JH designed and supervised the study. YH and YW reviewed the references. YH, YW and XP wrote the manuscript. YQ, DC, and TL contributed to tables and figures and XP, JH and WW revised the manuscript. XP and JH acquired funding. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chaoying L, Chao M, Xiangrui Y, Yingjian H, Gang Z, Yunhan R, Yu G. Risk factors of bone metastasis in patients with newly diagnosed prostate cancer. Eur Rev Med Pharmacol Sci. 2022;26:391–398. doi: 10.26355/eurrev_202201_27863. [DOI] [PubMed] [Google Scholar]
- 2.Clézardin P, Coleman R, Puppo M, Ottewell P, Bonnelye E, Paycha F, Confavreux CB, Holen I. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol Rev. 2021;101:797–855. doi: 10.1152/physrev.00012.2019. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 4.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488–494. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- 5.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 6.Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S, Kobayashi Y, Furuya Y, Yasuda H, Fukuda C, Tsuda E. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021;39:19–26. doi: 10.1007/s00774-020-01162-6. [DOI] [PubMed] [Google Scholar]
- 7.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 8.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28:509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf D. The colony-stimulating factors and cancer. Nat Rev Cancer. 2010;10:425–434. doi: 10.1038/nrc2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakefield PE, James WD, Samlaska CP, Meltzer MS. Colony-stimulating factors. J Am Acad Dermatol. 1990;23:903–912. doi: 10.1016/0190-9622(90)70313-7. [DOI] [PubMed] [Google Scholar]
- 11.Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44:287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa Y, Pluznik DH, Sachs L. In vitro control of the development of macrophage and granulocyte colonies. Proc Natl Acad Sci USA. 1966;56:488–495. doi: 10.1073/pnas.56.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartung T. Immunomodulation by colony-stimulating factors. Rev Physiol Biochem Pharmacol. 1999;136:1–164. doi: 10.1007/BFb0032323. [DOI] [PubMed] [Google Scholar]
- 14.Hareng L, Hartung T. Induction and regulation of endogenous granulocyte colony-stimulating factor formation. Biol Chem. 2002;383:1501–1517. doi: 10.1515/BC.2002.172. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Qiao L, Hu P, Deng G, Zhang J, Liang N, Xie J, Zhang J. The effect of granulocyte and granulocyte-macrophage colony stimulating factors on tumor promotion. J BUON. 2017;22:21–28. [PubMed] [Google Scholar]
- 16.Mueller MM, Peter W, Mappes M, Huelsen A, Steinbauer H, Boukamp P, Vaccariello M, Garlick J, Fusenig NE. Tumor progression of skin carcinoma cells in vivo promoted by clonal selection, mutagenesis, and autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Am J Pathol. 2001;159:1567–1579. doi: 10.1016/S0002-9440(10)62541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutschalk CM, Herold-Mende CC, Fusenig NE, Mueller MM. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res. 2006;66:8026–8036. doi: 10.1158/0008-5472.CAN-06-0158. [DOI] [PubMed] [Google Scholar]
- 18.Lee CH, Lin SH, Chang SF, Chang PY, Yang ZP, Lu SC. Extracellular signal-regulated kinase 2 mediates the expression of granulocyte colony-stimulating factor in invasive cancer cells. Oncol Rep. 2013;30:419–424. doi: 10.3892/or.2013.2463. [DOI] [PubMed] [Google Scholar]
- 19.Braun B, Lange M, Oeckler R, Mueller MM. Expression of G-CSF and GM-CSF in human meningiomas correlates with increased tumor proliferation and vascularization. J Neurooncol. 2004;68:131–140. doi: 10.1023/B:NEON.0000027751.87894.f0. [DOI] [PubMed] [Google Scholar]
- 20.Pei XH, Nakanishi Y, Takayama K, Bai F, Hara N. Granulocyte, granulocyte-macrophage, and macrophage colony-stimulating factors can stimulate the invasive capacity of human lung cancer cells. Br J Cancer. 1999;79:40–46. doi: 10.1038/sj.bjc.6690009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA. 2010;107:21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mroczko B, Szmitkowski M, Czygier M. Granulocyte colony stimulating factor (G-CSF) in diagnosis and monitoring of non-small-cell lung cancer (NSCLC) Pol Arch Med Wewn. 2000;103:163–168. (In Polish) [PubMed] [Google Scholar]
- 23.Fukuta K, Daizumoto K, Takahashi M, Mori H, Otomi Y, Uehara H, Fukawa T, Yamamoto Y, Yamaguchi K, Kanayama HO. Granulocyte colony-stimulating factor producing retroperitoneal leiomyosarcoma. IJU Case Rep. 2020;4:75–78. doi: 10.1002/iju5.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yip RKH, Rimes JS, Capaldo BD, Vaillant F, Mouchemore KA, Pal B, Chen Y, Surgenor E, Murphy AJ, Anderson RL, et al. Mammary tumour cells remodel the bone marrow vascular microenvironment to support metastasis. Nat Commun. 2021;12:6920. doi: 10.1038/s41467-021-26556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol. 2016;100:481–489. doi: 10.1189/jlb.3RU0316-144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–1820. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- 27.Moore MA, Warren DJ. Synergy of interleukin 1 and granulocyte colony-stimulating factor: In vivo stimulation of stem-cell recovery and hematopoietic regeneration following 5-fluorouracil treatment of mice. Proc Natl Acad Sci USA. 1987;84:7134–7138. doi: 10.1073/pnas.84.20.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 29.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dougherty ST, Eaves CJ, McBride WH, Dougherty GJ. Role of macrophage-colony-stimulating factor in regulating the accumulation and phenotype of tumor-associated macrophages. Cancer Immunol Immunother. 1997;44:165–172. doi: 10.1007/s002620050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han Y, Ma FY, Tesch GH, Manthey CL, Nikolic-Paterson DJ. c-fms blockade reverses glomerular macrophage infiltration and halts development of crescentic anti-GBM glomerulonephritis in the rat. Lab Invest. 2011;91:978–991. doi: 10.1038/labinvest.2011.61. [DOI] [PubMed] [Google Scholar]
- 32.Shaposhnik Z, Wang X, Lusis AJ. Arterial colony stimulating factor-1 influences atherosclerotic lesions by regulating monocyte migration and apoptosis. J Lipid Res. 2010;51:1962–1970. doi: 10.1194/jlr.M005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manthey CL, Johnson DL, Illig CR, Tuman RW, Zhou Z, Baker JF, Chaikin MA, Donatelli RR, Franks CF, Zeng L, et al. JNJ-28312141, a novel orally active colony-stimulating factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor tyrosine kinase inhibitor with potential utility in solid tumors, bone metastases, and acute myeloid leukemia. Mol Cancer Ther. 2009;8:3151–3161. doi: 10.1158/1535-7163.MCT-09-0255. [DOI] [PubMed] [Google Scholar]
- 34.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDermott RS, Deneux L, Mosseri V, Védrenne J, Clough K, Fourquet A, Rodriguez J, Cosset JM, Sastre X, Beuzeboc P, et al. Circulating macrophage colony stimulating factor as a marker of tumour progression. Eur Cytokine Netw. 2002;13:121–127. [PubMed] [Google Scholar]
- 36.Scholl SM, Lidereau R, de la Rochefordiere A, Le-Nir CC, Mosseri V, Noguès C, Pouillart P, Stanley FR. Circulating levels of the macrophage colony stimulating factor CSF-1 in primary and metastatic breast cancer patients. A pilot study. Breast Cancer Res Treat. 1996;39:275–283. doi: 10.1007/BF01806155. [DOI] [PubMed] [Google Scholar]
- 37.Kang J, Choi YJ, Seo BY, Jo U, Park SI, Kim YH, Park KH. A Selective FGFR inhibitor AZD4547 suppresses RANKL/M-CSF/OPG-dependent ostoclastogenesis and breast cancer growth in the metastatic bone microenvironment. Sci Rep. 2019;9:8726. doi: 10.1038/s41598-019-45278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liverani C, Mercatali L, Spadazzi C, La Manna F, De Vita A, Riva N, Calpona S, Ricci M, Bongiovanni A, Gunelli E, et al. CSF-1 blockade impairs breast cancer osteoclastogenic potential in co-culture systems. Bone. 2014;66:214–222. doi: 10.1016/j.bone.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Borzone FR, Giorello MB, Martinez LM, Sanmartin MC, Feldman L, Dimase F, Batagelj E, Yannarelli G, Chasseing NA. Senescent mesenchymal stem/stromal cells in pre-metastatic bone marrow of untreated advanced breast cancer patients. Oncol Res. 2023;31:361–374. doi: 10.32604/or.2023.028104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C, Whang YM, Campbell P, Mulcrone PL, Elefteriou F, Cho SW, Park SI. Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis. Cancer Lett. 2018;414:205–213. doi: 10.1016/j.canlet.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Zhao E, Wang L, Dai J, Kryczek I, Wei S, Vatan L, Altuwaijri S, Sparwasser T, Wang G, Keller ET, Zou W. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology. 2012;1:152–161. doi: 10.4161/onci.1.2.18480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ide H, Hatake K, Terado Y, Tsukino H, Okegawa T, Nutahara K, Higashihara E, Horie S. Serum level of macrophage colony-stimulating factor is increased in prostate cancer patients with bone metastasis. Hum Cell. 2008;21:1–6. doi: 10.1111/j.1749-0774.2007.00042.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsai YM, Chong IW, Hung JY, Chang WA, Kuo PL, Tsai MJ, Hsu YL. Syringetin suppresses osteoclastogenesis mediated by osteoblasts in human lung adenocarcinoma. Oncol Rep. 2015;34:617–626. doi: 10.3892/or.2015.4028. [DOI] [PubMed] [Google Scholar]
- 44.Fujita H, Gomori A, Fujioka Y, Kataoka Y, Tanaka K, Hashimoto A, Suzuki T, Ito K, Haruma T, Yamamoto-Yokoi H, et al. High potency VEGFRs/MET/FMS triple blockade by TAS-115 concomitantly suppresses tumor progression and bone destruction in tumor-induced bone disease model with lung carcinoma cells. PLoS One. 2016;11:e0164830. doi: 10.1371/journal.pone.0164830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hung JY, Chang WA, Tsai YM, Hsu YL, Chiang HH, Chou SH, Huang MS, Kuo PL. Tricetin, a dietary flavonoid, suppresses benzo(a)pyrene-induced human non-small cell lung cancer bone metastasis. Int J Oncol. 2015;46:1985–1993. doi: 10.3892/ijo.2015.2915. [DOI] [PubMed] [Google Scholar]
- 46.Spadazzi C, Recine F, Mercatali L, Miserocchi G, Liverani C, De Vita A, Bongiovanni A, Fausti V, Ibrahim T. mTOR inhibitor and bone-targeted drugs break the vicious cycle between clear-cell renal carcinoma and osteoclasts in an in vitro co-culture model. J Bone Oncol. 2019;16:100227. doi: 10.1016/j.jbo.2019.100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki K, Hino M, Hato F, Tatsumi N, Kitagawa S. Cytokine-specific activation of distinct mitogen-activated protein kinase subtype cascades in human neutrophils stimulated by granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor-alpha. Blood. 1999;93:341–349. doi: 10.1182/blood.V93.1.341. [DOI] [PubMed] [Google Scholar]
- 48.Mann A, Breuhahn K, Schirmacher P, Blessing M. Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing: Stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J Invest Dermatol. 2001;117:1382–1390. doi: 10.1046/j.0022-202x.2001.01600.x. [DOI] [PubMed] [Google Scholar]
- 49.Wu FPK, Westphal JR, Hoekman K, Mels AK, Statius Muller MG, de Waal RW, Beelen RHJ, van Leeuwen PAM, Meijer S, Cuesta MA. The effects of surgery, with or without rhGM-CSF, on the angiogenic profile of patients treated for colorectal carcinoma. Cytokine. 2004;25:68–72. doi: 10.1016/j.cyto.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Mueller MM, Fusenig NE. Friends or foes-bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 51.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilalta M, Rafat M, Giaccia AJ, Graves EE. Recruitment of circulating breast cancer cells is stimulated by radiotherapy. Cell Rep. 2014;8:402–409. doi: 10.1016/j.celrep.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obermueller E, Vosseler S, Fusenig NE, Mueller MM. Cooperative autocrine and paracrine functions of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the progression of skin carcinoma cells. Cancer Res. 2004;64:7801–7812. doi: 10.1158/0008-5472.CAN-03-3301. [DOI] [PubMed] [Google Scholar]
- 54.Lahm H, Wyniger J, Hertig S, Yilmaz A, Fischer JR, Givel JC, Odartchenko N. Secretion of bioactive granulocyte-macrophage colony-stimulating factor by human colorectal carcinoma cells. Cancer Res. 1994;54:3700–3702. [PubMed] [Google Scholar]
- 55.Oshika Y, Nakamura M, Abe Y, Fukuchi Y, Yoshimura M, Itoh M, Ohnishi Y, Tokunaga T, Fukushima Y, Hatanaka H, et al. Growth stimulation of non-small cell lung cancer xenografts by granulocyte-macrophage colony-stimulating factor (GM-CSF) Eur J Cancer. 1998;34:1958–1961. doi: 10.1016/S0959-8049(98)00236-6. [DOI] [PubMed] [Google Scholar]
- 56.Gutschalk CM, Yanamandra AK, Linde N, Meides A, Depner S, Mueller MM. GM-CSF enhances tumor invasion by elevated MMP-2, -9, and -26 expression. Cancer Med. 2013;2:117–129. doi: 10.1002/cam4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda K, Hatakeyama K, Tsuchiya Y, Rikiishi H, Kumagai K. A correlation between GM-CSF gene expression and metastases in murine tumors. Int J Cancer. 1991;47:413–420. doi: 10.1002/ijc.2910470318. [DOI] [PubMed] [Google Scholar]
- 58.Kumar A, Taghi Khani A, Sanchez Ortiz A, Swaminathan S. GM-CSF: A double-edged sword in cancer immunotherapy. Front Immunol. 2022;13:901277. doi: 10.3389/fimmu.2022.901277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin Q, Fang X, Liang G, Luo Q, Cen Y, Shi Y, Jia S, Li J, Yang W, Sanders AJ, et al. Silencing CTNND1 mediates triple-negative breast cancer bone metastasis via upregulating CXCR4/CXCL12 axis and neutrophils infiltration in bone. Cancers (Basel) 2021;13:5703. doi: 10.3390/cancers13225703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee SK, Park KK, Kim HJ, Park J, Son SH, Kim KR, Chung WY. Human antigen R-regulated CCL20 contributes to osteolytic breast cancer bone metastasis. Sci Rep. 2017;7:9610. doi: 10.1038/s41598-017-09040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang K, Hu Y, Feng Y, Li K, Zhu Z, Liu S, Lin Y, Yu B. IGF-1R mediates crosstalk between nasopharyngeal carcinoma cells and osteoclasts and promotes tumor bone metastasis. J Exp Clin Cancer Res. 2024;43:46. doi: 10.1186/s13046-024-02970-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Das Roy L, Pathangey LB, Tinder TL, Schettini JL, Gruber HE, Mukherjee P. Breast-cancer-associated metastasis is significantly increased in a model of autoimmune arthritis. Breast Cancer Res. 2009;11:R56. doi: 10.1186/bcr2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuentelsaz-Romero S, Cuervo A, Estrada-Capetillo L, Celis R, Garcia-Campos R, Ramirez J, Sastre S, Samaniego R, Puig-Kröger A, Cañete JD. GM-CSF expression and macrophage polarization in joints of undifferentiated arthritis patients evolving to rheumatoid arthritis or psoriatic arthritis. Front Immunol. 2021;11:613975. doi: 10.3389/fimmu.2020.613975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varricchi G, Poto R, Marone G, Schroeder JT. IL-3 in the development and function of basophils. Semin Immunol. 2021;54:101510. doi: 10.1016/j.smim.2021.101510. [DOI] [PubMed] [Google Scholar]
- 65.Yadav P, Vats R, Bano A, Bhardwaj R. Hematopoietic stem cells culture, expansion and differentiation: An insight into variable and available media. Int J Stem Cells. 2020;13:326–334. doi: 10.15283/ijsc19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donahue RE, Seehra J, Metzger M, Lefebvre D, Rock B, Carbone S, Nathan DG, Garnick M, Sehgal PK, Laston D, et al. Human IL-3 and GM-CSF act synergistically in stimulating hematopoiesis in primates. Science. 1988;241:1820–1823. doi: 10.1126/science.3051378. [DOI] [PubMed] [Google Scholar]
- 67.Bénard A, Jacobsen A, Brunner M, Krautz C, Klösch B, Swierzy I, Naschberger E, Podolska MJ, Kouhestani D, David P, et al. Interleukin-3 is a predictive marker for severity and outcome during SARS-CoV-2 infections. Nat Commun. 2021;12:1112. doi: 10.1038/s41467-021-21310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mroczko B, Szmitkowski M, Wereszczynska-Siemiatkowska U, Okulczyk B. Stem cell factor (SCF) and interleukin 3 (IL-3) in the sera of patients with colorectal cancer. Dig Dis Sci. 2005;50:1019–1024. doi: 10.1007/s10620-005-2697-3. [DOI] [PubMed] [Google Scholar]
- 69.Marone G, Gambardella AR, Mattei F, Mancini J, Schiavoni G, Varricchi G. Basophils in tumor microenvironment and surroundings. Adv Exp Med Biol. 2020;1224:21–34. doi: 10.1007/978-3-030-35723-8_2. [DOI] [PubMed] [Google Scholar]
- 70.Dentelli P, Rosso A, Olgasi C, Camussi G, Brizzi MF. IL-3 is a novel target to interfere with tumor vasculature. Oncogene. 2011;30:4930–4940. doi: 10.1038/onc.2011.204. [DOI] [PubMed] [Google Scholar]
- 71.Hahm ER, Kim SH, Pore SK, Mathan SV, Singh RP, Singh SV. Mechanism of synergistic inhibitory effect of benzyl isothiocyanate and zoledronic acid combination on breast cancer induction of osteoclast differentiation. Mol Carcinog. 2024;63:301–313. doi: 10.1002/mc.23653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugihara A, Maeda O, Tsuji M, Tsujimura T, Nakata Y, Akedo H, Kotake T, Terada N. Expression of cytokines enhancing the osteoclast activity, and parathyroid hormone-related protein in prostatic cancers before and after endocrine therapy: An immunohistochemical study. Oncol Rep. 1998;5:1389–1394. doi: 10.3892/or.5.6.1389. [DOI] [PubMed] [Google Scholar]
- 73.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aliper AM, Frieden-Korovkina VP, Buzdin A, Roumiantsev SA, Zhavoronkov A. A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med. 2014;3:737–746. doi: 10.1002/cam4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16:10. doi: 10.1186/s12943-016-0577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo S, Li P, Zhang A, Meng L, Huang L, Wu X, Cheng H, Tu H, Gong X. G-CSF improving combined whole brain radiotherapy and immunotherapy prognosis of non-small cell lung cancer brain metastases. Int Immunopharmacol. 2024;130:111705. doi: 10.1016/j.intimp.2024.111705. [DOI] [PubMed] [Google Scholar]
- 77.Psaila B, Lyden D. The metastatic niche: Adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen DX, Bos PD, Massagué J. Metastasis: From dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 80.Győri DS, Mócsai A. Osteoclast signal transduction during bone metastasis formation. Front Cell Dev Biol. 2020;8:507. doi: 10.3389/fcell.2020.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92:860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Povolny BT, Lee MY. The role of recombinant human M-CSF, IL-3, GM-CSF and calcitriol in clonal development of osteoclast precursors in primate bone marrow. Exp Hematol. 1993;21:532–537. [PubMed] [Google Scholar]
- 83.Ray AL, Saunders AS, Nofchissey RA, Reidy MA, Kamal M, Lerner MR, Fung KM, Lang ML, Hanson JA, Guo S, et al. G-CSF is a novel mediator of T-cell suppression and an immunotherapeutic target for women with colon cancer. Clin Cancer Res. 2023;29:2158–2169. doi: 10.1158/1078-0432.CCR-22-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brook N, Brook E, Dharmarajan A, Dass CR, Chan A. Breast cancer bone metastases: Pathogenesis and therapeutic targets. Int J Biochem Cell Biol. 2018;96:63–78. doi: 10.1016/j.biocel.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Bade BC, Dela Cruz CS. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin Chest Med. 2020;41:1–24. doi: 10.1016/j.ccm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y, Cheng W, Xin H, Liu R, Wang Q, Cai W, Peng X, Yang F, Xin H. Nanoparticles advanced from preclinical studies to clinical trials for lung cancer therapy. Cancer Nanotechnol. 2023;14:28. doi: 10.1186/s12645-023-00174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lv T, Meng Y, Liu Y, Han Y, Xin H, Peng X, Huang J. RNA nanotechnology: A new chapter in targeted therapy. Colloids Surf B Biointerfaces. 2023;230:113533. doi: 10.1016/j.colsurfb.2023.113533. [DOI] [PubMed] [Google Scholar]
- 88.Cheng D, Wang J, Wang Y, Xue Y, Yang Q, Yang Q, Zhao H, Huang J, Peng X. Chemokines: Function and therapeutic potential in bone metastasis of lung cancer. Cytokine. 2023;172:156403. doi: 10.1016/j.cyto.2023.156403. [DOI] [PubMed] [Google Scholar]
- 89.Al Husaini H, Wheatley-Price P, Clemons M, Shepherd FA. Prevention and management of bone metastases in lung cancer: A review. J Thorac Oncol. 2009;4:251–259. doi: 10.1097/JTO.0b013e31819518fc. [DOI] [PubMed] [Google Scholar]
- 90.Molineux G, Pojda Z, Dexter TM. A comparison of hematopoiesis in normal and splenectomized mice treated with granulocyte colony-stimulating factor. Blood. 1990;75:563–569. doi: 10.1182/blood.V75.3.563.bloodjournal753563. [DOI] [PubMed] [Google Scholar]
- 91.Metcalf D, Begley CG, Williamson DJ, Nice EC, De Lamarter J, Mermod JJ, Thatcher D, Schmidt A. Hemopoietic responses in mice injected with purified recombinant murine GM-CSF. Exp Hematol. 1987;15:1–9. [PubMed] [Google Scholar]
- 92.Metcalf D, Begley CG, Johnson GR, Nicola NA, Lopez AF, Williamson DJ. Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood. 1986;68:46–57. doi: 10.1182/blood.V68.1.46.bloodjournal68146. [DOI] [PubMed] [Google Scholar]
- 93.Lang RA, Metcalf D, Cuthbertson RA, Lyons I, Stanley E, Kelso A, Kannourakis G, Williamson DJ, Klintworth GK, Gonda TJ, et al. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987;51:675–686. doi: 10.1016/0092-8674(87)90136-X. [DOI] [PubMed] [Google Scholar]
- 94.Chang JM, Metcalf D, Lang RA, Gonda TJ, Johnson GR. Nonneoplastic hematopoietic myeloproliferative syndrome induced by dysregulated multi-CSF (IL-3) expression. Blood. 1989;73:1487–1497. doi: 10.1182/blood.V73.6.1487.bloodjournal7361487. [DOI] [PubMed] [Google Scholar]
- 95.Chang JM, Metcalf D, Gonda TJ, Johnson GR. Long-term exposure to retrovirally expressed granulocyte-colony-stimulating factor induces a nonneoplastic granulocytic and progenitor cell hyperplasia without tissue damage in mice. J Clin Invest. 1989;84:1488–1496. doi: 10.1172/JCI114324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the treatment of cancer. J Interferon Cytokine Res. 2019;39:6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gabrilove JL, Jakubowski A, Fain K, Grous J, Scher H, Sternberg C, Yagoda A, Clarkson B, Bonilla MA, Oettgen HF, et al. Phase I study of granulocyte colony-stimulating factor in patients with transitional cell carcinoma of the urothelium. J Clin Invest. 1988;82:1454–1461. doi: 10.1172/JCI113751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Renwick W, Pettengell R, Green M. Use of filgrastim and pegfilgrastim to support delivery of chemotherapy: Twenty years of clinical experience. BioDrugs. 2009;23:175–186. doi: 10.2165/00063030-200923030-00004. [DOI] [PubMed] [Google Scholar]
- 100.Lieschke GJ, Maher D, Cebon J, O'Connor M, Green M, Sheridan W, Boyd A, Rallings M, Bonnem E, Metcalf D, et al. Effects of bacterially synthesized recombinant human granulocyte-macrophage colony-stimulating factor in patients with advanced malignancy. Ann Intern Med. 1989;110:357–364. doi: 10.7326/0003-4819-110-5-357. [DOI] [PubMed] [Google Scholar]
- 101.Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaissé JM, Clézardin P. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60:2949–2954. [PubMed] [Google Scholar]
- 102.Gao L, Deng H, Zhao H, Hirbe A, Harding J, Ratner L, Weilbaecher K. HTLV-1 Tax transgenic mice develop spontaneous osteolytic bone metastases prevented by osteoclast inhibition. Blood. 2005;106:4294–4302. doi: 10.1182/blood-2005-04-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.