Abstract
Background:
The relationship between coronavirus disease 2019 (COVID-19) infection and multiple sclerosis (MS) relapse and disease progression remains unclear. Previous studies are limited by small sample sizes and most lack a propensity-matched control cohort.
Objective:
To evaluate the effect of COVID-19 infection on MS disease course with a large propensity-matched cohort.
Design:
This multi-centre cohort study analysed relapse and disability outcomes post-COVID-19 infection after balancing covariates using a propensity score matching method. The study period was from the 11th of September 2019 to the 16th of February 2023. The mean follow-up period was 1.7 years.
Methods:
Data were retrieved from the MSBase Registry. Propensity scores were obtained based on age, sex, disease duration, baseline Expanded Disability Status Scale (EDSS), MS course, relapses pre-baseline, disease-modifying therapy (DMT) class and country. Primary outcomes were time to first relapse, annualised relapse rate (ARR) and time to confirm EDSS progression. Secondary outcomes were time to EDSS of 3, 4 or 6. Sensitivity analyses with baseline DMT classes were performed.
Results:
The study included 2253 cases and 6441 controls. After matching, there were 2161 cases and an equal number of matched controls. Cases had a significantly higher ARR (ARR = 0.10 [95% CI 0.09–0.11]) compared to controls (ARR = 0.07 [95% CI 0.06–0.08]). Cases had a significantly greater hazard of time to first relapse compared to controls (hazard ratio (HR) = 1.54 [95% CI 1.29–1.84]). There was no association between COVID-19 infection and 24-week EDSS progression (HR = 1.18 [95% CI 0.92–1.52]), or time to EDSS of 3, 4 or 6. For patients on interferons and glatiramer acetate (BRACE), COVID-19 infection was associated with a greater hazard of time to first relapse (HR = 1.83 [95% CI 1.25–2.68]) and greater hazard of time to EDSS of 3 (HR = 2.04 [95% CI 1.06–3.90]) compared to patients on BRACE therapy without COVID-19 infection.
Conclusion:
COVID-19 infection was associated with a significantly increased MS relapse rate and a shorter time to first relapse. There was no effect on confirmed EDSS progression over the short term. These results support ongoing COVID-19 risk minimisation strategies to protect patients with MS.
Keywords: COVID-19, disease progression, multiple sclerosis, relapse
Background
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It was declared a public health emergency of international concern by the World Health Organization (WHO) in January 2020 and has caused nearly 7 million deaths worldwide. 1
Previous studies have shown an association between viral infections, multiple sclerosis (MS) relapses and MRI lesions.2–4 While the exact mechanism is unclear, infections are thought to cause a systemic inflammatory response, leading to an increased expression of pro-inflammatory cytokines and chemokines. The alterations in the inflammatory mediators are crucial in the pathogenesis of COVID-19 symptoms and possibly could increase susceptibility to MS relapse.5,6 Given previous reports of viral infections as a trigger for demyelination, it is important to explore whether COVID-19 infection can lead to an increase in the rate of MS relapse or disability progression. 4
The effect of COVID-19 on MS disease activity is unclear. Previous studies have shown inconsistent results likely due to their retrospective nature, small sample sizes and lack of propensity-matched controls.7–15 This study addresses these limitations by evaluating the effect of COVID-19 infection on MS disease course using a matched cohort study design.
Methods
Standard protocol approvals, registrations and patient consent
The MSBase Registry is an international observational cohort study of patients with MS (pwMS) established in 2000. 16 The MSBase COVID-19 Substudy is an ongoing international and multi-site observational cohort substudy of MSBase comprising pwMS who have been infected (confirmed and suspected) with COVID-19. It was established in July 2020. Data for the analysis were extracted on the 16th of February 2023.
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (Supplemental Material).
Study inclusion criteria, data collection and definitions
Inclusion criteria for this study were as follows: (1) Definite diagnosis of MS; (2) Expanded Disability Status Scale (EDSS) baseline recorded within 6 months of the declaration of COVID-19 as a pandemic by the WHO (11 March 2020) for the control cohort 17 ; (3) complete information for sex, age, duration of disease, the date of starting and/or stopping disease-modifying therapies (DMTs), EDSS assessments and dates of relapses for the duration of the study; (4) at least two follow-up visits with a minimum 6-month gap and complete EDSS assessments to allow for the calculation of confirmed disability progression (CDP); and (5) 18 years of age or over. Exclusion criteria for this study were diagnosis of neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein antibody disorder (MOGAD) or radiologically isolated syndrome (RIS). All MSBase members were invited to participate in this study, with 25 centres accepting the invitation. We included pwMS diagnosed with COVID-19 on polymerase chain reaction (PCR) or rapid antigen (RAT) tests. Cases were included from the time of COVID-19 infection to the time of their last recorded registry visit if they met inclusion criteria. Patients from the participating MSBase COVID-19 Substudy centres meeting the above criteria but without documented COVID-19 infection were used as a control cohort.
These medications – natalizumab, ocrelizumab, rituximab, alemtuzumab, cladribine, fingolimod, siponimod and ofatumumab – were classified as high-efficacy DMTs. All others were classified as low-moderate efficacy DMTs.
DMT classes described for each patient were recorded at baseline.
Patient data were recorded during routine clinical visits at participating centres via the locally installed iMed or MSBase data entry systems (MDS).
Baseline EDSS for the control cohort was defined as the closest visit within 6 months of the declaration of COVID-19 as a pandemic by the WHO (11 March 2020). Cases were included from the date of their infection and subsequently followed up.
Study endpoints
The primary study outcomes were annualised relapse rate (ARR, calculated by dividing the total number of relapses by the total number of person-years at risk), time to first relapse and 24-week CDP.
CDP was defined as an increase of ⩾1.5 EDSS steps from a baseline score of 0, 1 step from baseline scores 1.0–5.5 or 0.5 step from a baseline score ⩾5.5, sustained at two or more consecutive visits separated by ⩾180 days. 18
Relapse was defined as a clinical episode lasting at least 24 h, in the absence of fever, infection or acute concurrent medical illness. There also must have been a preceding 30-day period without clinical relapse.
Secondary outcomes were time to EDSS of 3, 4 or 6. We also repeated the primary and secondary outcomes stratified by baseline DMT classes. DMTs were categorised as glatiramer acetate/interferon beta-1 (BRACE), teriflunomide, dimethyl fumarate, sphingosine-1-phosphate receptor antagonists (fingolimod, siponimod, ponesimod, ozanimod), anti-CD20s (ocrelizumab, ofatumumab, rituximab) and other monoclonal antibodies (natalizumab, alemtuzumab).
Statistical analysis
The demographic information and the baseline characteristics were reported as numbers and percentages for discrete variables and as mean (standard deviation (SD)) or median (interquartile range (IQR)) for continuous variables, as appropriate.
We applied 1:1 propensity score matching to match COVID-19 cases and controls to mitigate baseline differences between the groups and selection bias. 19 The matching method used was k-nearest neighbour matching with a caliper of 0.05. The propensity score was calculated using logistic regression with COVID-19 infection as the dependent outcome variable and the following baseline predictors as explanatory covariates: age, sex, disease duration, baseline EDSS, MS course, relapses pre-baseline, DMT class and country. The covariate balance was evaluated using the absolute standardised difference (ASD) measure, where ASD > 0.1 indicates an imbalance. 20
A negative binomial model was used to compare ARRs, with the relapse count as a dependent variable, and COVID-19 as an independent variable, offset by the follow-up time. We used marginal Cox regression models with robust standard errors and Kaplan–Meier cumulative hazard curves to assess and visualise differences between cases and controls for the time-to-event outcomes. Participants were censored at the time of the event or the last available follow-up visit in those without an event.
We then ran a sensitivity analysis for the relapsing–remitting multiple sclerosis (RRMS) subgroup to assess the potential impact of confounding errors that may result from the inclusion of different MS subtypes.
All statistical tests were two-sided with a statistical significance defined as p < 0.05. All analyses were performed in R, V.4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
A total of 2253 cases and 6441 controls were included (baseline characteristics in Table A1). At baseline, COVID-19 cases were younger, had shorter disease duration, had lower baseline EDSS, had lower pre-baseline relapse activity and were more likely to be on high-efficacy DMTs. After propensity score matching, 2161 cases and an equal number of controls were identified. Baseline characteristics of the matched study population are presented in Table 1. After matching, all baseline covariates were balanced (ASD < 0.1). In all, 239 patients infected with COVID-19 were excluded because they had no baseline EDSS recorded (Figure 1). The distribution of COVID-19 severity in the cohort was: 2.64% had severe COVID-19 infection (ICU admission), 10.00% had moderate severity COVID-19 infection (inpatient admission), 80.66% had mild COVID-19 infection (outpatient setting) and 6.71% had no severity reported.
Table 1.
Baseline characteristics of the matched study population by COVID cases versus controls.
| Baseline factor | Category/metric | Cases (n = 2161) | Controls (n = 2161) | Standardised difference |
|---|---|---|---|---|
| Age | Mean (SD) | 40.63 (11.41) | 40.45 (11.68) | 0.02 |
| Sex | Female | 1506 (69.7) | 1515 (70.1) | −0.01 |
| Male | 655 (30.3) | 646 (29.9) | ||
| Disease duration (years) | Mean (SD) | 9.71 (8.18) | 9.94 (7.23) | −0.03 |
| Baseline EDSS | Median (IQR) | 1.5 (1–3) | 1.5 (1–3) | 0.02 |
| MS course | CIS | 177 (8.2) | 154 (7.1) | 0.03 |
| PP | 76 (3.5) | 139 (6.4) | ||
| PR | 24 (1.1) | 21 (1.0) | ||
| RR | 1751 (81.0) | 1733 (80.2) | ||
| SP | 133 (6.2) | 96 (4.4) | ||
| Not reported | 0 (0.0) | 18 (0.8) | ||
| Relapses 1-year pre-baseline | Mean (SD) | 0.07 (0.30) | 0.07 (0.29) | −0.02 |
| Relapses 2-year pre-baseline | Mean (SD) | 0.10 (0.43) | 0.11 (0.39) | −0.03 |
| DMT class | High efficacy | 1058 (49.0) | 1109 (51.3) | 0.03 |
| Low-moderate efficacy | 746 (34.5) | 686 (31.7) | ||
| No baseline DMT | 357 (16.5) | 366 (16.9) | ||
| Country | Argentina | 20 (0.9) | 67 (3.1) | −0.03 |
| Australia | 254 (11.8) | 271 (12.5) | ||
| Belgium | 52 (2.4) | 109 (5.0) | ||
| Brazil | 21 (1.0) | 19 (0.9) | ||
| Spain | 231 (10.7) | 177 (8.2) | ||
| Croatia | 179 (8.3) | 7 (0.3) | ||
| Italy | 285 (13.2) | 93 (4.3) | ||
| Kuwait | 137 (6.3) | 363 (16.8) | ||
| Mexico | 3 (0.1) | 2 (0.1) | ||
| Romania | 3 (0.1) | 2 (0.1) | ||
| Saudi Arabia | 9 (0.4) | 27 (1.3) | ||
| Turkey | 967 (44.8) | 1024 (47.4) |
Values are expressed as n (%), median (IQR) or mean (SD). Standardised difference is the difference in means or proportions divided by the standard error. An imbalance was defined as an ASD > 0.10.
CIS, clinically isolated syndrome; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; IQR, interquartile range; MS, multiple sclerosis; PP, primary progressive; PR, progressive relapsing; RR, relapsing remitting; SD, standard deviation; SP, secondary progressive.
Figure 1.
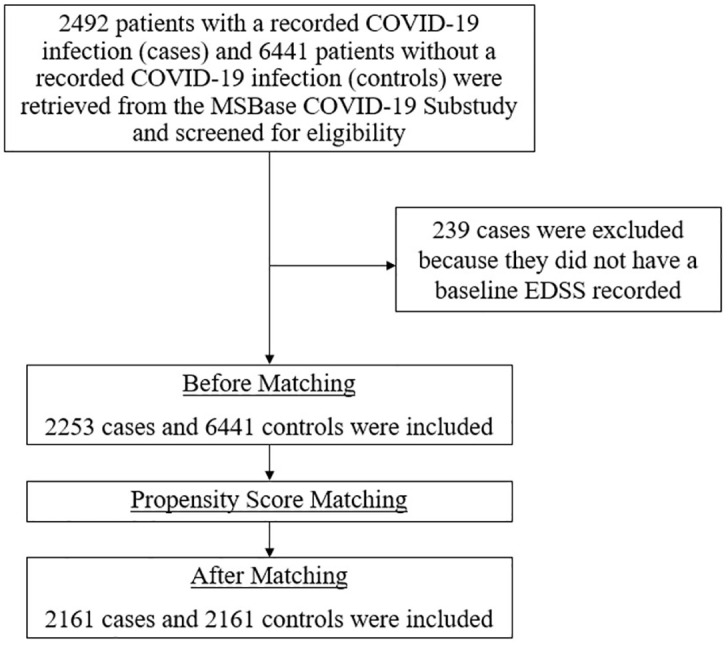
Flow diagram of patients selected from the MSBase COVID-19 registry and 1:1 propensity score matching of cases and controls.
COVID-19, coronavirus disease 2019; EDSS, Expanded Disability Status Scale.
Primary analyses
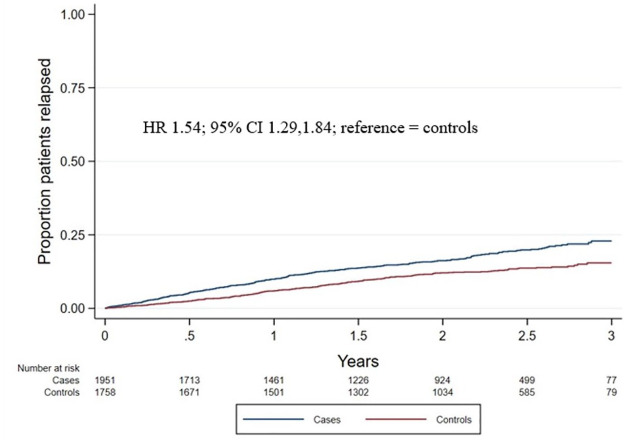
The total number of post-baseline relapses was 380 in COVID-19 cases and 255 in controls. Follow-up person-years were 3689 and 3718 for COVID-19 cases and controls, respectively. COVID-19 infection in pwMS was associated with a significantly higher ARR (ARR = 0.10 [95% CI 0.09–0.11]) compared to matched controls without COVID-19 infection (ARR = 0.07 [95% CI 0.06–0.08]). Consistent results were found for time to first relapse, with cases having a significantly greater hazard of time to first relapse compared to controls (hazard ratio (HR) = 1.54 [1.29, 1.84]; Figure 2).
Figure 2.
Cumulative hazard of time to first relapse. Kaplan–Meier curves were applied to show the cumulative hazard of time to first relapse in COVID-19 cases (blue line) and controls (red line).
Sensitivity analysis of the RRMS subgroup demonstrated no significantly different results. COVID-19 infection in pwMS with the RRMS subtype was associated with a significantly higher ARR (ARR = 0.1009 [0.0901, 0.1126]) compared to matched controls without COVID-19 infection (ARR = 0.0735 [0.0642, 0.0838]). Consistent results were found for time to first relapse, with cases having a significantly greater hazard of time to first relapse compared to controls (HR = 1.40 [1.16, 1.69]).
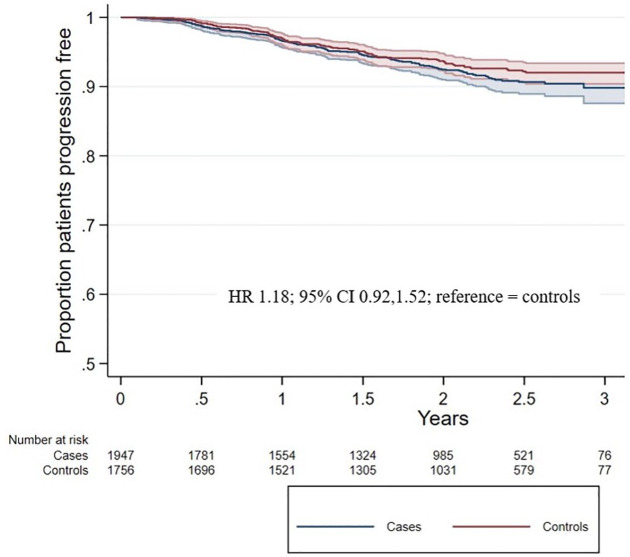
Of the COVID-19 cases, 133 (6.2%) experienced a CDP event, while 111 (5.1%) controls experienced a CDP event. There was no association between COVID-19 infection and 24-week EDSS progression (HR = 1.18 [0.92, 1.52]; Figure 3). The sensitivity analysis for the RRMS subtype also found no association between COVID-19 infection and 24-week EDSS progression (HR = 1.19 [0.86, 1.65]).
Figure 3.
Cumulative hazard of time to CDP. Kaplan–Meier curves were applied to show the cumulative hazard of time to first relapse in COVID-19 cases (blue line) and controls (red line).
CDP, confirmed disability progression.
Secondary analyses
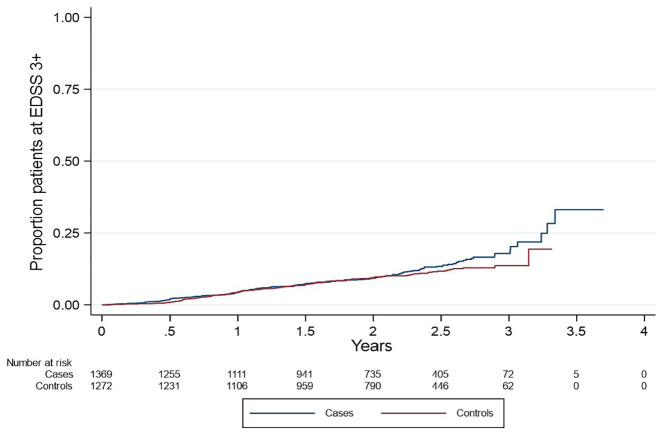
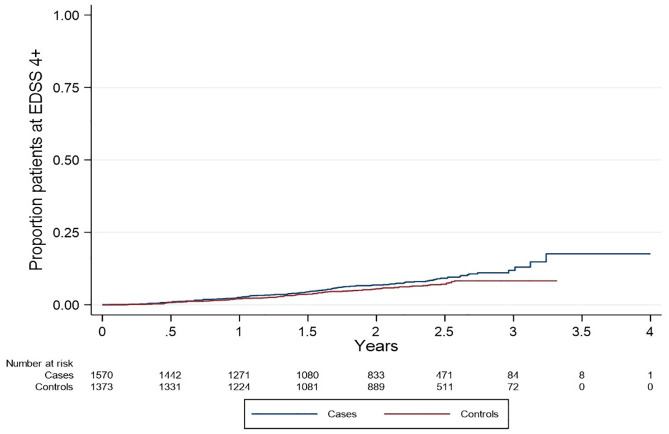
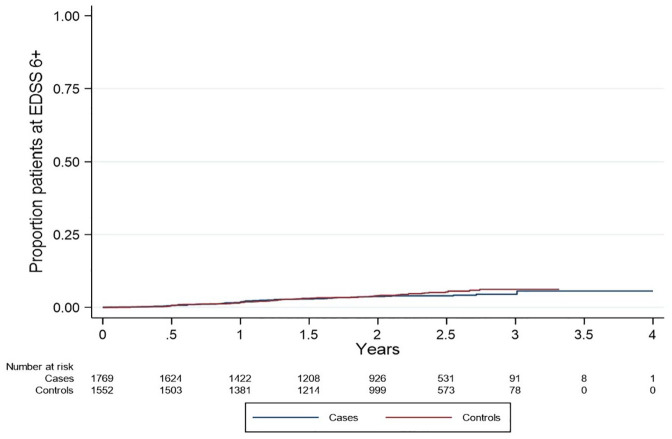
There was no association between COVID-19 infection and time to EDSS of 3 (HR = 1.17 [0.93, 1.49]), 4 (HR = 1.31 [0.99, 1.74]) or 6 (HR = 0.84 [0.59, 1.19]; Figures A1–A3, respectively).
COVID-19 cases who were on interferons or glatiramer acetate (BRACE) had a greater hazard of time to first relapse (HR = 1.54 (95% CI 1.29–1.84)) and time to EDSS of 3 (HR = 2.04 (95% CI 1.06–3.90)) compared to similarly treated controls. There was no difference in the hazard of time to first relapse, CDP or time to EDSS or 3, 4 or 6 for other intra-DMT comparisons (Table 2).
Table 2.
Hazard ratios of COVID-19 cases versus controls for time to first relapse, confirmed disability progression and time to EDSS of 3, 4 and 6, stratified by DMT classes.
| Outcomes | Overall (n = 4322) | BRACE (n = 733) | Teriflunomide (n = 267) | DMF (n = 354) | S1PR modulators (n = 887) | Anti-CD20s (n = 882) | Other mAbs (n = 389) | Cladribine (n = 73) |
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) p value* | Hazard ratio (95% CI) p value* | Hazard ratio (95% CI) p value* | Hazard ratio (95% CI) p value* | Hazard ratio (95% CI) p value* | Hazard ratio (95% CI) p value* | Hazard ratio (95% CI) p value* | Hazard ratio (95% CI) p value* | |
| Time to first relapse | 1.54 (1.29, 1.84) <0.001 | 1.83 (1.25, 2.68) 0.002 | 1.60 (0.87, 2.95) 0.134 | 1.71 (0.92, 3.19) 0.092 | 1.25 (0.86, 1.84) 0.246 | 1.50 (0.89, 2.54) 0.132 | 1.74 (0.86, 3.52) 0.126 | 2.16 (0.43, 10.70) 0.347 |
| 24-week CDP | 1.18 (0.92, 1.52) 0.198 | 1.02 (0.36, 2.93) 0.963 | 0.98 (0.34, 2.80) 0.975 | 1.25 (0.44, 3.54) 0.679 | 1.13 (0.58, 2.20) 0.710 | 0.86 (0.57, 1.30) 0.466 | 1.99 (0.78, 5.04) 0.149 | 0.72 (0.10, 5.14) 0.747 |
| Time to EDSS ⩾ 3 | 1.17 (0.93, 1.49) 0.181 | 2.04 (1.06, 3.90) 0.032 | 0.53 (0.21, 1.33) 0.177 | 0.81 (0.37, 1.77) 0.596 | 1.13 (0.65, 1.94) 0.671 | 0.79 (0.47, 1.35) 0.390 | 1.73 (0.85, 3.50) 0.129 | 0.78 (0.15, 3.94) 0.761 |
| Time to EDSS ⩾ 4 | 1.31 (0.99, 1.74) 0.062 | 1.85 (0.78, 4.43) 0.164 | 0.90 (0.32, 2.48) 0.834 | 0.75 (0.27, 2.10) 0.581 | 1.93 (0.96, 3.88) 0.065 | 0.90 (0.50, 1.60) 0.712 | 0.83 (0.35, 1.97) 0.677 | 0.37 (0.03, 4.27) 0.426 |
| Time to EDSS ⩾ 6 | 0.84 (0.59, 1.19) 0.321 | 1.66 (0.40, 6.95) 0.488 | 0.34 (0.04, 3.25) 0.347 | 0.58 (0.04, 9.20) 0.696 | 1.67 (0.53, 5.26) 0.381 | 0.63 (0.38, 1.04) 0.072 | 0.72 (0.24, 2.22) 0.572 | 1.28 (0.12, 14.18) 0.841 |
Values are expressed as hazard ratio (95% CI) p value. Controls (pwMS without COVID-19) were taken to be the reference group in the above analyses. Bold values indicate statistical significance at p < 0.05.
BRACE, glatiramer acetate/interferon beta-1; CDP, confirmed disability progression; DMF, dimethyl fumarate; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; mAbs, monoclonal antibodies; MS, multiple sclerosis; S1PR, sphingosine-1-phosphate receptor; SD, standard deviation
Discussion
In this large multi-national observational cohort study, we demonstrate that pwMS infected with COVID-19 have a significantly increased relapse rate and shorter time to first relapse than matched controls without COVID-19 infection. However, there is no difference in confirmed EDSS progression between the groups. This suggests that COVID-19 infection can acutely exacerbate neurological symptoms. Our results suggest that COVID-19 potentially does not increase disability progression over 1.7 years of follow-up. However, these analyses need to be repeated with longer follow-up times in the future.
The association of COVID-19 infection and higher ARR is consistent with previous reports of viral infections as a trigger for demyelination and clinical relapse in pwMS.2–4 Reports of other respiratory tract infections leading to an increased risk of relapse in pwMS are also consistent with our findings.2,3 Many pathophysiological processes have been postulated to explain this process. Preliminary studies looking at the effect of COVID-19 on the immune system have suggested COVID-19 infection is characterised by misdirected host immune responses that can exacerbate pre-existing autoimmune disease. 21 Furthermore, the increased pro-inflammatory state caused by COVID-19 infection may lead to the recruitment and activation of pro-inflammatory leucocytes ‘bystanders’ that can cross the blood–brain barrier, increasing the risk of clinical MS relapse. 6 The literature on the relationship between COVID-19 and MS relapse has been controversial. For example, studies by Etemadifar et al. 13 and Bsteh et al. 22 found no association between COVID-19 and relapse, or even a lower exacerbation incidence in pwMS infected with COVID-19. However, these studies were limited by small sample sizes.
Including a time variable in our analysis allows us to evaluate the effect of COVID-19 on the time to first relapse. Other studies have used a binary relapse variable to assess the risk of relapse by comparing MS exacerbation in a cohort for a fixed time before the pandemic and a fixed time before and after COVID-19 infection.12,13
Our study found no association between COVID-19 and EDSS progression. This is consistent with several smaller studies. However, these results contradict findings from Peeters et al., 7 who suggest that COVID-19 is linked to clinical worsening in MS patients. This study had a relatively small sample size and no control group. A systematic review and meta-analysis by Aghajanian et al. 23 indicated no impact of COVID-19 infection on MS relapse rates. However, the studies in this review had small sample sizes, limited follow-up duration and did not evaluate incidence through time-to-event analyses. Similarly, a propensity score-matched case-control study by Vercellino et al. 24 found no differences in relapse or MRI activity post-COVID-19 infection, though it also did not analyse relapse incidence through time-to-event methods. 24
Our study also looked at the effect of DMTs on outcome measures. Patients with COVID-19 on BRACE therapies demonstrated a reduced time to first relapse and time to EDSS of 3. Low-efficacy medications are not as effective as other DMTs in suppressing MS activity. As a result, a viral infection like COVID-19 could exacerbate partially controlled MS-related inflammatory activity and result in a relapse. However, these findings must be interpreted with caution, as other unmeasured confounding factors could influence DMT choice and relapse activity in this cohort. For example, insurance status, variation in prescribing at a site level, smoking and obesity were not measured. The BRACE analysis findings should be understood in the context of reports that COVID-19 infection may be less severe in pwMS on BRACE therapy. 25 Our study suggests that the benefit of less severe COVID-19 infection in these patients must be balanced against the increased possibility of MS relapse triggered by COVID-19.
A substantial body of literature suggests that anti-CD20 therapies can worsen the severity of COVID-19.26–31 However, other studies suggest that ocrelizumab is not a risk factor for severe COVID-19 infection, indicating the need for further research. 32 We did not find an association between COVID-19 infection and MS disease course for pwMS on anti-CD20 therapies. This may suggest that anti-CD20 therapies effectively suppress MS disease activity, limiting infection-related relapse activity. Again, this calls for a balance between minimising the severity of COVID-19 infection and mitigating MS disease progression and relapse.
These results support COVID-19 risk minimisation strategies to protect pwMS from neurological exacerbation and relapse. It is also important that pwMS are aware of the risk of infection with COVID-19 on MS relapse and neurological exacerbation to make informed decisions about their lifestyle and health. These decisions should also consider the specific DMT therapy the patient is on and include other disease-related factors such as age, sex, disease duration, MS course and history of past relapse. While the WHO has declared that COVID-19 is no longer a global health emergency, it remains important for pwMS and healthcare providers to be vigilant about the continued presence of this disease in the community. 33
Strengths and limitations
The strengths of this study include the analysis of data from 25 sites incorporating populations from 12 countries. Including multiple sites increases the generalisability of these findings and the large sample size reduces selection bias and improves the accuracy of results. The propensity-matched control cohort mitigated confounding factors such as age, sex, history of relapse, disease duration, baseline EDSS, MS course, DMT class (high efficacy, low-moderate efficacy, no baseline DMT) and country.34–39 Including a time variable allowed for the calculation of the hazard ratio, which is a unique outcome variable of this paper.
There are some limitations to our study. A lack of PCR sequencing means that results cannot be stratified to different strains of COVID-19. Inconsistent vaccine data across sites meant we could not assess the effect of COVID-19 vaccination on relapse and disability. Although we were unable to incorporate vaccination data in our analysis, it is important to address potential interactions between vaccination and our outcomes of interest. This retrospective, observational cohort study found a mild increase in ARR after vaccination. 40 However, more recent studies have not found increased relapse activity after COVID-19 vaccine administration. 41 A systematic review and meta-analysis by Stefanou et al. 42 included 19 observational studies consisting of 14,755 pwMS and concluded that COVID-19 vaccination did not appear to increase the risk of relapse. A limitation of this review is that it only consists of observation studies and so results are only single-arm estimates due to the lack of robust control cohorts.
Additional potential confounders that may affect outcomes post-COVID-19, such as comorbidities, frailty and body composition, were not controlled for in our study.38,43–46 Confounders described to be associated with an increased risk of hospitalisation in pwMS and COVID-19 such as diabetes, smoking history and hypertension were not accounted for in our analysis. 47
There is a lack of Multiple Sclerosis Functional Composite score which could indicate the possibility of progression independent of relapse activity. We could not corroborate relapse with MRI findings. It is possible that some members of the MSBase control cohort, who were not reported to have COVID-19, may have had an unknown or unreported past infection. To minimise the risk of selecting patients with unknown COVID-19 infection, controls were retrieved from sites participating in the MSBase COVID-19 Substudy. Additionally, there may be a reporting bias of COVID-19 in patients presenting with relapse. We conducted a sensitivity analysis for the RRMS subgroup to assess the potential impact of confounding errors that may result from the inclusion of different MS subtypes. This demonstrated no significant difference in results compared to the total cohort. We have also not included the severity of COVID-19, so we cannot comment on its relationship with the MS disease course. The severity and duration of relapse were not represented in our study. Some countries had different quarantine measures based on age. This may have impacted exposure to COVID-19 infection, as well as access to MS services or pharmacies to receive usual (prescribed) MS DMTs. This study only analysed the effect of baseline DMTs. It did not consider the duration or proportion of time a patient was on specific DMT therapies. The interpretation of the outcome measure of CDP may also be limited in this paper given the relatively short follow-up period of 1.7 years. The cessation of DMTs and irregular compliance were not accounted for in our analysis. If treatment was interrupted due to infection, reported increased relapse rates may be confounded by MS disease activity rather than be caused by COVID-19 directly.
Conclusion
In our study, COVID-19 infection was associated with a significantly increased MS relapse rate and a shorter time to first relapse. There was no difference in confirmed EDSS progression over a short follow-up period of 1.7 years. BRACE therapy was associated with a greater hazard of time to first relapse and time to EDSS of 3 in cases compared to controls.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864241278496 for The impact of COVID-19 infection on multiple sclerosis disease course across 12 countries: a propensity-score-matched cohort study by David Levitz, Yi Chao Foong, Paul Sanfilippo, Tim Spelman, Louise Rath, Angie Roldan, Anoushka Lal, Mastura Monif, Vilija Jokubaitis, Serkan Ozakbas, Raed Alroughani, Cavit Boz, Murat Terzi, Tomas Kalincik, Yolanda Blanco, Matteo Foschi, Andrea Surcinelli, Katherine Buzzard, Olga Skibina, Guy Laureys, Liesbeth Van Hijfte, Cristina Ramo-Tello, Aysun Soysal, Jose Luis Sanchez-Menoyo, Mario Habek, Elisabetta Cartechini, Juan Ignacio Rojas, Rana Karabudak, Barbara Willekens, Talal Al-Harbi, Yara Fragoso, Tamara Castillo-Triviño, Danny Decoo, Maria Cecilia Aragon de Vecino, Eli Skromne, Carmen-Adella Sirbu, Chao Zhu, Daniel Merlo, Melissa Gresle, Helmut Butzkueven and Anneke Van Der Walt in Therapeutic Advances in Neurological Disorders
Acknowledgments
We thank the MSBase Registry for access to its data.
Appendix
Table A1.
Baseline characteristics of unmatched study population by COVID cases versus controls.
| Baseline factor | Category/metric | Cases (n = 2253*) | Controls (n = 6441) | Standardised difference |
|---|---|---|---|---|
| Age | Mean (SD) | 40.26 (11.45) | 43.03 (12.59) | −0.230 |
| Sex | Female | 1576 (70.0) | 4474 (69.5) | 0.011 |
| Male | 677 (30.0) | 1967 (30.5) | ||
| Disease duration (years) | Mean (SD) | 9.45 (8.14) | 11.34 (8.99) | −0.221 |
| Baseline EDSS | Median (IQR) | 1.5 (1, 3) | 2 (1, 4) | −0.130 |
| MS course | CIS | 254 (11.3) | 301 (4.7) | −0.257 |
| PP | 85 (3.8) | 292 (4.5) | ||
| PR | 24 (1.1) | 70 (1.1) | ||
| RR | 1757 (78.0) | 5109 (79.3) | ||
| SP | 133 (5.9) | 530 (8.2) | ||
| Not reported | 0 (0.0) | 139 (2.2) | ||
| Relapses 1-year pre-baseline | Mean (SD) | 0.06 (0.29) | 0.21 (0.50) | −0.353 |
| Relapses 2-year pre-baseline | Mean (SD) | 0.10 (0.42) | 0.39 (0.72) | −0.496 |
| DMT class | High efficacy | 1125 (49.9) | 2403 (37.3) | −0.331 |
| Low-moderate efficacy | 771 (34.2) | 2182 (33.9) | ||
| No baseline DMT | 357 (15.9) | 1856 (28.8) | ||
| Country | Argentina | 20 (0.9) | 158 (2.5) | −0.257 |
| Australia | 265 (11.8) | 984 (15.3) | ||
| Belgium | 52 (2.3) | 364 (5.7) | ||
| Brazil | 21 (0.9) | 65 (1.0) | ||
| Spain | 231 (10.3) | 865 (13.4) | ||
| Croatia | 214 (9.5) | 24 (0.4) | ||
| Italy | 296 (13.1) | 392 (6.1) | ||
| Kuwait | 149 (6.6) | 821 (12.8) | ||
| Mexico | 3 (0.1) | 8 (0.1) | ||
| Romania | 3 (0.1) | 2 (0.0) | ||
| Saudi Arabia | 9 (0.4) | 66 (1.0) | ||
| Turkey | 990 (43.9) | 2692 (41.8) |
Values are expressed as n (%), median (IQR) or mean (SD). Standardised difference is the difference in means or proportions divided by the standard error. An imbalance was defined as an ASD > 0.10, indicated in bold.
239 of the original 2492 cases were excluded for not having a baseline EDSS
ASD, absolute standardised difference; CIS, clinically isolated syndrome; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; PP, primary progressive; PR, progressive relapsing; RR, relapsing remitting; SP, secondary progressive; SD, standard deviation.
Figure A1.
Cumulative hazard of time to EDSS equal or greater than 3. Weighted Kaplan–Meier curves were applied to show the cumulative hazard of time to first relapse in COVID-19 cases (blue line) and controls (red line).
EDSS, Expanded Disability Status Scale.
Figure A2.
Cumulative hazard of time to EDSS equal or greater than 4. Weighted Kaplan–Meier curves were applied to show the cumulative hazard of time to first relapse in COVID-19 cases (blue line) and controls (red line).
EDSS, Expanded Disability Status Scale.
Figure A3.
Cumulative hazard of time to EDSS equal or greater than 6. Weighted Kaplan–Meier curves were applied to show the cumulative hazard of time to first relapse in COVID-19 cases (blue line) and controls (red line).
EDSS, Expanded Disability Status Scale.
Footnotes
ORCID iDs: David Levitz  https://orcid.org/0009-0008-3610-1926
https://orcid.org/0009-0008-3610-1926
Vilija Jokubaitis  https://orcid.org/0000-0002-3942-4340
https://orcid.org/0000-0002-3942-4340
Raed Alroughani  https://orcid.org/0000-0001-5436-5804
https://orcid.org/0000-0001-5436-5804
Yolanda Blanco  https://orcid.org/0000-0002-1834-0498
https://orcid.org/0000-0002-1834-0498
Barbara Willekens  https://orcid.org/0000-0002-5212-8837
https://orcid.org/0000-0002-5212-8837
Daniel Merlo  https://orcid.org/0000-0001-8766-3453
https://orcid.org/0000-0001-8766-3453
Helmut Butzkueven  https://orcid.org/0000-0003-3940-8727
https://orcid.org/0000-0003-3940-8727
Anneke Van Der Walt  https://orcid.org/0000-0002-4278-7003
https://orcid.org/0000-0002-4278-7003
Supplemental material: Supplemental material for this article is available online.
Contributor Information
David Levitz, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Yi Chao Foong, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia; Department of Neurology, The Alfred Hospital, Melbourne, VIC, Australia.
Paul Sanfilippo, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Tim Spelman, Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden.
Louise Rath, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Angie Roldan, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Anoushka Lal, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Mastura Monif, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Vilija Jokubaitis, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Serkan Ozakbas, Dokuz Eylul University, Konak, Turkey.
Raed Alroughani, Division of Neurology, Department of Medicine, Amiri Hospital, Kuwait City, Kuwait.
Cavit Boz, KTU Medical Faculty Farabi Hospital, Trabzon, Turkey.
Murat Terzi, Medical Faculty, 19 Mayis University, Samsun, Turkey.
Tomas Kalincik, Neuroimmunology Centre, Department of Neurology, The Royal Melbourne Hospital, Melbourne, VIC, Australia; CORe, Department of Medicine, University of Melbourne, Melbourne, VIC, Australia.
Yolanda Blanco, Center of Neuroimmunology, Service of Neurology, Hospital Clinic de Barcelona, Barcelona, Spain.
Matteo Foschi, Department of Neuroscience, Neurology Unit, S. Maria delle Croci Hospital of Ravenna, AUSL Romagna, Ravenna, Italy; Department of Biotechnological and Applied Clinical Sciences (DISCAB), University of L’Aquila, L’Aquila, Italy.
Andrea Surcinelli, Department of Neuroscience, Neurology Unit, S. Maria delle Croci Hospital of Ravenna, AUSL Romagna, Ravenna, Italy.
Katherine Buzzard, Department of Neurology, Box Hill Hospital, Melbourne, VIC, Australia; Eastern Health Clinical School, Monash University, Box Hill, VIC, Australia.
Olga Skibina, Department of Neurology, The Alfred Hospital, Melbourne, VIC, Australia; Department of Neurology, Box Hill Hospital, Melbourne, VIC, Australia; Eastern Health Clinical School, Monash University, Box Hill, VIC, Australia.
Guy Laureys, Department of Neurology, University Hospital Ghent, Ghent, Belgium.
Liesbeth Van Hijfte, Department of Neurology, University Hospital Ghent, Ghent, Belgium.
Cristina Ramo-Tello, Hospital Germans Trias i Pujol, Badalona, Spain.
Aysun Soysal, Bakirkoy Education and Research Hospital for Psychiatric and Neurological Diseases, Istanbul, Turkey.
Jose Luis Sanchez-Menoyo, Hospital de Galdakao-Usansolo, Galdakao, Spain; Instituto de Investigacion sanitario Biocruces-Bizkaia, Barakaldo, Spain.
Mario Habek, Department of Neurology, University Hospital Center Zagreb, Zagreb, Croatia; School of Medicine, University of Zagreb, Zagreb, Croatia.
Elisabetta Cartechini, UOC Neurologia, Azienda Sanitaria Unica Regionale Marche – AV3, Macerata, Italy.
Juan Ignacio Rojas, Hospital Universitario de CEMIC, Buenos Aires, Argentina.
Rana Karabudak, Hacettepe University, Ankara, Turkey.
Barbara Willekens, Department of Neurology, Antwerp University Hospital, Edegem, Belgium; Translational Neurosciences Research Group, Faculty of Medicine and Health Sciences, University of Antwerp, Wilrijk, Belgium.
Talal Al-Harbi, King Fahad Specialist Hospital-Dammam, Dammam, Saudi Arabia.
Yara Fragoso, Universidade Metropolitana de Santos, Santos, Brazil.
Tamara Castillo-Triviño, Hospital Universitario Donostia and IIS Biodonostia, San Sebastián, Spain.
Danny Decoo, AZ Alma Ziekenhuis, Damme, Belgium.
Maria Cecilia Aragon de Vecino, Hospital Moinhos de Vento, Porto Alegre, Brazil.
Eli Skromne, Hospital Angeles de las Lomas, Instituto Mexicano de Neurociencias, Huixquilucan, Mexico.
Carmen-Adella Sirbu, Central Military Emergency University Hospital, Bucharest, Romania; Titu Maiorescu University, Bucharest, Romania.
Chao Zhu, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Daniel Merlo, Department of Neurology, Box Hill Hospital, Melbourne, VIC, Australia.
Melissa Gresle, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Helmut Butzkueven, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia; Department of Neurology, The Alfred Hospital, Melbourne, VIC, Australia.
Anneke Van Der Walt, Department of Neuroscience, Central Clinical School, Monash University, 99 Commercial Road, Melbourne, VIC 3004, Australia; Department of Neurology, The Alfred Hospital, Melbourne, VIC, Australia.
Declarations
Ethics approval and consent to participate: Ethics approval for the collection of demographics, COVID-19, clinical and treatment data via the MSBase Registry was obtained from the Alfred Health Human Research Ethics Committee (528/12), and institutional review boards at all participating centres. Written informed consent was obtained from all enrolled registry participants.
Consent for publication: Not applicable.
Author contributions: David Levitz: Investigation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Yi Chao Foong: Investigation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Paul Sanfilippo: Formal analysis; Methodology; Software; Writing – review & editing.
Tim Spelman: Data curation; Formal analysis; Resources; Software; Writing – review & editing.
Louise Rath: Investigation; Writing – review & editing.
Angie Roldan: Investigation; Writing – review & editing.
Anoushka Lal: Investigation; Writing – review & editing.
Mastura Monif: Investigation; Writing – review & editing.
Vilija Jokubaitis: Investigation; Writing – review & editing.
Serkan Ozakbas: Investigation; Writing – review & editing.
Raed Alroughani: Investigation; Writing – review & editing.
Cavit Boz: Investigation; Writing – review & editing.
Murat Terzi: Investigation; Writing – review & editing.
Tomas Kalincik: Investigation; Writing – review & editing.
Yolanda Blanco: Investigation; Writing – review & editing.
Matteo Foschi: Investigation; Writing – review & editing.
Andrea Surcinelli: Investigation; Writing – review & editing.
Katherine Buzzard: Investigation; Writing – review & editing.
Olga Skibina: Investigation; Writing – review & editing.
Guy Laureys: Investigation; Writing – review & editing.
Liesbeth Van Hijfte: Investigation; Writing – review & editing.
Cristina Ramo-Tello: Investigation; Writing – review & editing.
Aysun Soysal: Investigation; Writing – review & editing.
Jose Luis Sanchez-Menoyo: Investigation; Writing – review & editing.
Mario Habek: Investigation; Writing – review & editing.
Elisabetta Cartechini: Investigation; Writing – review & editing.
Juan Ignacio Rojas: Investigation; Writing – review & editing.
Rana Karabudak: Investigation; Writing – review & editing.
Barbara Willekens: Investigation; Writing – review & editing.
Talal Al-Harbi: Investigation; Writing – review & editing.
Yara Fragoso: Investigation.
Tamara Castillo-Triviño: Investigation; Writing – review & editing.
Danny Decoo: Investigation; Writing – review & editing.
Maria Cecilia Aragon de Vecino: Investigation; Writing – review & editing.
Eli Skromne: Investigation; Writing – review & editing.
Carmen-Adella Sirbu: Investigation; Writing – review & editing.
Chao Zhu: Investigation; Supervision; Writing – review & editing.
Daniel Merlo: Investigation; Supervision; Writing – review & editing.
Melissa Gresle: Investigation; Supervision; Writing – review & editing.
Helmut Butzkueven: Conceptualisation; Data curation; Investigation; Methodology; Resources; Supervision; Writing – review & editing.
Anneke Van der Walt: Conceptualisation; Data curation; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Competing interests: David Levitz: Nothing to disclose. Yi Chao Foong: Reports a relationship with Biogen that includes travel reimbursement. Paul Sanfilipino: Nothing to disclose. Tim Spelman: Received compensation for serving on the scientific advisory board for Biogen and speaker honoraria from Novartis. Louise Rath: Nothing to disclose. Angie Roldan: Nothing to disclose. Anoushka Lal: Nothing to disclose. Mastura Monif: MM has served on the advisory board for Merck and Novartis. MM has received speaker honoraria from Merck and Biogen. Her institution receives funding from Merk, the Australian National Health Medical Research Council, Brain Foundation, Charles and Sylvia Viertel Foundation and MS Research Australia. Vilija Jokubaitis: Nothing to disclose. Serkan Ozakbas: Nothing to disclose. Raed Alroughani: Received honoraria as a speaker and for serving on scientific advisory boards from Bayer, Biogen, GSK, Merck, Novartis, Roche and Sanofi-Genzyme. Cavit Boz: Received conference travel support from Biogen, Novartis, Bayer-Schering, Merck and Teva; has participated in clinical trials by Sanofi Aventis, Roche and Novartis. Murat Terzi: Received travel grants from Novartis, Bayer-Schering, Merck and Teva; has participated in clinical trials by Sanofi Aventis, Roche and Novartis. Tomas Kalincik: Served on scientific advisory boards for MS International Federation and World Health Organisation, BMS, Roche, Janssen, Sanofi Genzyme, Novartis, Merck and Biogen, steering committee for Brain Atrophy Initiative by Sanofi Genzyme, received conference travel support and/or speaker honoraria from WebMD Global, Eisai, Novartis, Biogen, Roche, Sanofi-Genzyme, Teva, BioCSL and Merck; and received research or educational event support from Biogen, Novartis, Genzyme, Roche, Celgene and Merck. Yolanda Blanco: Received speaker honoraria/consulting fees from Merck, Biogen, Roche, Brystol, Novartis, Sanofi and Sandoz. Matteo Foschi: Received travel and meeting attendance support from Novartis, Biogen, Roche, Sanofi-Genzyme and Merck. Andrea Surcinelli: Nothing to disclose. Katherine Buzzard: Received speaker honoraria and/or education support from Biogen, Teva, Novartis, Genzyme-Sanofi, Roche, Merck and Alexion; has been a member of advisory boards for Merck and Biogen. Olga Skibina: Received honoraria and consulting fees from Bayer Schering, Novartis, Merck, Biogen and Genzyme. Guy Laureys: Received travel and/or consultancy compensation from Sanofi-Genzyme, Roche, Teva, Merck, Novartis, Celgene and Biogen. Liesbeth Van Hijfte: Received travel compensation from Merck. Cristina Ramo-Tello: Has received research funding, or compensation for consulting services and speaker honoraria, or meetings travel from Biogen, Novartis, Sanofi, Bristol, Roche, Almirall, Janssen and Merck. Aysun Soysal: Nothing to disclose. Jose Luis Sanchez-Menoyo: Accepted travel compensation from Novartis, Merck and Biogen; speaking honoraria from Biogen, Novartis, Sanofi, Merck, Almirall, Bayer and Teva; and has participated in clinical trials by Biogen, Merck and Roche. Mario Habek: Participated as a clinical investigator and/or received consultation and/or speaker fees from Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals and TG Pharmaceuticals. Elisabetta Cartechini: Nothing to disclose. Juan Ignacio Rojas: Nothing to disclose. Rana Karabudak: Nothing to disclose. Barbara Willekens: Received honoraria for acting as a member of Scientific Advisory Boards/Consultancy for Almirall, Biogen, Celgene/BMS, Merck, Janssen, Novartis, Roche, Sandoz and Sanofi-Genzyme; speaker honoraria and travel support from Biogen, Celgene/BMS, Merck, Novartis, Roche and Sanofi-Genzyme; research and/or patient support grants from Biogen, Janssen, Merck, Sanofi-Genzyme and Roche. Honoraria and grants were paid to UZA/UZA Foundation. Further, BW received research funding from FWO-TBM, Belgian Charcot Foundation, Start2Cure Foundation, Queen Elisabeth Medical Foundation for Neurosciences and the National MS Society USA. Talal Al-Harbi: Nothing to disclose. Yara Fragoso: Received honoraria as a consultant on scientific advisory boards by Novartis, Teva, Roche and Sanofi-Aventis and compensation for travel from Novartis, Biogen, Sanofi Aventis, Teva, Roche and Merck. Tamara Castillo-Triviño: Received speaking/consulting fees and/or travel funding from Almirall, Biogen, Bristol Myers Squibb, Janssen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. Danny Decoo: Received compensation for travel, speaker honoraria and consultant fees from Biogen, Novartis, Merck KGaA, Bayer, Sanofi/Genzyme, Roche and Teva, as well as support for research activities from Biogen, Novartis, Merck, Sanofi, Roche and Teva. Maria Cecilia Aragon de Vecino: Nothing to disclose. Eli Skromne: Received speaking honoraria from Biogen, Novartis and Teva. Carmen-Adella Sirbu: Received speaking honoraria from Teva; and travel grants from Bayer-Schering and Teva. Chao Zhu: Nothing to disclose. Daniel Merlo: Reports a relationship with Novartis that includes speaking and lecture fees. Melissa Gresle: Reports a relationship with Biogen that includes funding grants and a relationship with F Hoffmann-La Roche Ltd that includes funding grants. Helmut Butzkueven: Received institutional (Monash University) funding from Biogen, F. Hoffmann-La Roche Ltd, Merck, Alexion, CSL and Novartis; has carried out contracted research for Novartis, Merck, F. Hoffmann-La Roche Ltd and Biogen; has taken part in speakers’ bureaus for Biogen, Genzyme, UCB, Novartis, F. Hoffmann-La Roche Ltd and Merck. He has received travel support from Merck and Novartis. Anneke van der Walt: Served on advisory boards and receives unrestricted research grants from Novartis, Biogen, Merck and Roche; She has received speaker’s honoraria and travel support from Novartis, Roche and Merck; She receives grant support from the National Health and Medical Research Council of Australia and MS Research Australia.
Availability of data and materials: Data: Data available: Yes. Data types: Deidentified participant data, Data dictionary. How to access data: https://www.msbase.org/media/1197/mds-datadictionary.pdf. When available: With publication. Supporting Documents: Document types: None. Additional Information: Who can access the data: Researchers whose proposed use of the data has been approved. Types of analyses: For repeating the analysis. Mechanisms of data availability: Permission from each contributing data controller is required.
References
- 1. World Health Organisation. Rolling updates on coronavirus disease (COVID-19), https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (2020, accessed 27 May 2023).
- 2. Edwards S, Zvartau M, Clarke H, et al. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J Neurol Neurosurg Psychiatry 1998; 64(6): 736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kriesel JD, White A, Hayden FG, et al. Multiple sclerosis attacks are associated with picornavirus infections. Mult Scler 2004; 10(2): 145–148. [DOI] [PubMed] [Google Scholar]
- 4. Buljevac D, Flach HZ, Hop WC, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 2002; 125 (Pt 5): 952–960. [DOI] [PubMed] [Google Scholar]
- 5. Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J 2022; 19(1): 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruiz F, Vigne S, Pot C. Resolution of inflammation during multiple sclerosis. Semin Immunopathol 2019; 41(6): 711–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peeters G, Van Remoortel A, Nagels G, et al. Occurrence and severity of Coronavirus Disease 2019 are associated with clinical disability worsening in patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2023; 10(3): e200089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Etemadifar M, Abhari AP, Nouri H, et al. Does COVID-19 increase the long-term relapsing-remitting multiple sclerosis clinical activity? A cohort study. BMC Neurol 2022; 22(1): 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garjani A, Middleton RM, Hunter R, et al. COVID-19 is associated with new symptoms of multiple sclerosis that are prevented by disease modifying therapies. Mult Scler Relat Disord 2021; 52: 102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conway SE, Healy BC, Zurawski J, et al. COVID-19 severity is associated with worsened neurological outcomes in multiple sclerosis and related disorders. Mult Scler Relat Disord 2022; 63: 103946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michelena G, Casas M, Eizaguirre MB, et al. ¿ Can COVID-19 exacerbate multiple sclerosis symptoms? A case series analysis. Mult Scler Relat Disord 2022; 57: 103368. [DOI] [PubMed] [Google Scholar]
- 12. Barzegar M, Vaheb S, Mirmosayyeb O, et al. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult Scler Relat Disord 2021; 52: 102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Etemadifar M, Sedaghat N, Aghababaee A, et al. COVID-19 and the risk of relapse in multiple sclerosis patients: a fight with no bystander effect? Mult Scler Relat Disord 2021; 51: 102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paybast S, Hejazi SA, Molavi P, et al. A one year follow of patients with multiple sclerosis during COVID-19 pandemic: a cross-sectional study in Qom province, Iran. Mult Scler Relat Disord 2022; 60: 103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nowak-Kiczmer M, Kubicka-Bączyk K, Niedziela N, et al. The course of COVID-19 infection in patients with multiple sclerosis – the experience of one center based on the population of Upper Silesia. Mult Scler Relat Disord 2021; 52: 102984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butzkueven H, Chapman J, Cristiano E, et al. MSBase: an international, online registry and platform for collaborative outcomes research in multiple sclerosis. Mult Scler 2006; 12(6): 769–774. [DOI] [PubMed] [Google Scholar]
- 17. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed 2020; 91(1): 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalincik T, Cutter G, Spelman T, et al. Defining reliable disability outcomes in multiple sclerosis. Brain 2015; 138(11): 3287–3298. [DOI] [PubMed] [Google Scholar]
- 19. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46(3): 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28(25): 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021; 595(7866): 283–288. [DOI] [PubMed] [Google Scholar]
- 22. Bsteh G, Assar H, Gradl C, et al. Long-term outcome after COVID-19 infection in multiple sclerosis: a nation-wide multicenter matched-control study. Eur J Neurol. Epub ahead of print June 2022. DOI: 10.1111/ene.15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aghajanian S, Shafiee A, Akhondi A, et al. The effect of COVID-19 on Multiple Sclerosis relapse: a systematic review and meta-analysis. Mult Scler Relat Disord 2024; 81: 105128. [DOI] [PubMed] [Google Scholar]
- 24. Vercellino M, Bosa C, Alteno A, et al. SARS-CoV-2 pandemic as a model to assess the relationship between intercurrent viral infections and disease activity in Multiple Sclerosis: a propensity score matched case-control study. Mult Scler Relat Disord 2023; 74: 104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reder AT, Centonze D, Naylor ML, et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs 2021; 35(3): 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated With SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol 2021; 78(6): 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and Coronavirus Disease 2019 severity in multiple sclerosis. Ann Neurol 2021; 89(4): 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sormani MP, Salvetti M, Labauge P, et al. DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol 2021; 8(8): 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology 2021; 97(19): e1870–e1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spelman T, Forsberg L, McKay K, et al. Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: a study of the Swedish multiple sclerosis registry. Mult Scler 2022; 28(7): 1051–1059. [DOI] [PubMed] [Google Scholar]
- 31. Foong YC, Merlo D, Gresle M, et al. Comparing ocrelizumab to interferon/glatiramer acetate in people with multiple sclerosis over age 60. J Neurol Neurosurg Psychiatry 2024; 95: 767–774. [DOI] [PubMed] [Google Scholar]
- 32. Todorović S, Vojinović S, Savić D, et al. Potential beneficial effect of IFN-β1a and ocrelizumab in people with MS during the COVID-19 pandemic. Acta Neurol Belg 2024; 124(2): 447–455. [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization. Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic, https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (2023, accessed 27 May 2023).
- 34. Magyari M, Koch-Henriksen N. Quantitative effect of sex on disease activity and disability accumulation in multiple sclerosis. J Neurol Neurosurg Psychiatry 2022; 93(7): 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Filippi M, Amato MP, Centonze D, et al. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: an expert opinion. J Neurol 2022; 269(10): 5382–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tremlett H, Yousefi M, Devonshire V, et al. Impact of multiple sclerosis relapses on progression diminishes with time. Neurology 2009; 73(20): 1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Claflin SB, Broadley S, Taylor BV. The effect of disease modifying therapies on disability progression in multiple sclerosis: a systematic overview of meta-analyses. Front Neurol 2018; 9: 1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhargava A, Fukushima EA, Levine M, et al. Predictors for severe COVID-19 infection. Clin Infect Dis 2020; 71(8): 1962–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reilly GD, Mahkawnghta AS, Jelinek PL, et al. International differences in multiple sclerosis health outcomes and associated factors in a cross-sectional survey. Front Neurol 2017; 8: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stastna D, Menkyova I, Drahota J, et al. To be or not to be vaccinated: The risk of MS or NMOSD relapse after COVID-19 vaccination and infection. Mult Scler Relat Disord 2022; 65: 104014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stastna D, Elberling F, Pontieri L, et al. COVID-19 vaccination and relapse activity: a nationwide cohort study of patients with multiple sclerosis in Denmark. Eur J Neurol 2024; 31(3): e16163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stefanou MI, Palaiodimou L, Theodorou A, et al. Safety of COVID-19 vaccines in multiple sclerosis: a systematic review and meta-analysis. Mult Scler 2023; 29(4–5): 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Y, Tan S, Yan Q, et al. Sarcopenia and COVID-19 Outcomes. Clin Interv Aging 2023; 18: 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health 2020; 5(8): e444–e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sudre CH, Murray B, Varsavsky T, et al. Author Correction: Attributes and predictors of long COVID. Nat Med 2021; 27(6): 1116-. [DOI] [PubMed] [Google Scholar]
- 46. Foong YC, Chherawala N, Aitken D, et al. Accelerometer-determined physical activity, muscle mass, and leg strength in community-dwelling older adults. J Cachexia Sarcopenia Muscle 2016; 7(3): 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moreno-Torres I, Meca Lallana V, Costa-Frossard L, et al. Risk and outcomes of COVID-19 in patients with multiple sclerosis. Eur J Neurol 2021; 28(11): 3712–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864241278496 for The impact of COVID-19 infection on multiple sclerosis disease course across 12 countries: a propensity-score-matched cohort study by David Levitz, Yi Chao Foong, Paul Sanfilippo, Tim Spelman, Louise Rath, Angie Roldan, Anoushka Lal, Mastura Monif, Vilija Jokubaitis, Serkan Ozakbas, Raed Alroughani, Cavit Boz, Murat Terzi, Tomas Kalincik, Yolanda Blanco, Matteo Foschi, Andrea Surcinelli, Katherine Buzzard, Olga Skibina, Guy Laureys, Liesbeth Van Hijfte, Cristina Ramo-Tello, Aysun Soysal, Jose Luis Sanchez-Menoyo, Mario Habek, Elisabetta Cartechini, Juan Ignacio Rojas, Rana Karabudak, Barbara Willekens, Talal Al-Harbi, Yara Fragoso, Tamara Castillo-Triviño, Danny Decoo, Maria Cecilia Aragon de Vecino, Eli Skromne, Carmen-Adella Sirbu, Chao Zhu, Daniel Merlo, Melissa Gresle, Helmut Butzkueven and Anneke Van Der Walt in Therapeutic Advances in Neurological Disorders