Abstract
Importance
Head and neck squamous cell carcinomas (HNSCC) are responsible for a significant amount of morbidity and mortality in Canada. Surgical margins are one of the most important factors used to guide treatment; however, currently there is a lack of consensus on the ideal surgical margin definition, sampling, and assessment method.
Objective
To understand the current perspectives and practice patterns of Canadian head and neck surgeons with respect to surgical margin: (1) definition, (2) sampling, (3) pathological assessment.
Design
A 24-question cross-sectional survey was sent via email through the Canadian Society of Otolaryngology—Head & Neck Surgery (CSOHNS), and responses were gathered from December 19, 2023, to March 12, 2024. Responses were aggregated and reported using descriptive statistics.
Setting/Participants
The survey was conducted in Canada among self-reported staff head and neck oncology surgeons with membership in the CSOHNS.
Results
A total of 36 staff head and neck oncology surgeons responded from across Canada. The most common (58.3%) definition of a negative surgical margin for oral cavity HNSCC was “>5 mm formalin fixed paraffin embedded distance.” To obtain surgical margins, surgeons were split with 44.1% using only a tumor bed approach and 32.4% using only a specimen-driven approach. A dedicated head and neck pathologist is always available more commonly for final pathological assessment (63.6%) versus intraoperative frozen section assessment (15.5%). Finally, most surgeons reported having a synoptic standardized reporting system for annotating margin status (78.8%).
Conclusions/Relevance
The results of this survey provide a current-state analysis of head and neck surgeons across Canada and set the stage for future efforts to be directed toward standardizing the collection method and reporting criteria for surgical margins in HNSCC.
Keywords: survey, surgical margins, head and neck oncology, pathology
Graphical abstract.
Background
In Canada, there are over 7500 new cases and over 2000 new deaths attributable to head and neck squamous cell carcinomas (HNSCC) annually. 1 The primary treatment for most HNSCC is surgical resection with or without neck dissection followed by selective use of adjuvant therapy with radiation and/or systemic chemotherapy. During primary resection, obtaining negative surgical margins is correlated with better locoregional control, disease-free survival, and overall survival. Therefore, the status of surgical margins during primary resection is one of the main factors used to guide adjuvant therapy.2,3
Historically, negative surgical margins have been defined as a 1 cm gross margin or a formalin-fixed paraffin-embedded (FFPE) distance >5 mm. Conversely, positive margins have been defined as either at the inked surface or FFPE distance <1 mm. Between these cutoffs, the surgical margins are often referred to as “close” with varying opinions on how this should impact the need for adjuvant therapy. Given the somewhat arbitrary nature of these cutoffs, there has been a recent call for reevaluation and investigation in the literature. 4 Several retrospective studies have suggested similar oncologic outcomes when using cutoffs for negative surgical margins under the pervasive 5 mm standard.5-8 Similarly, there is a lack of consensus on the designation of positive margins in the case of carcinoma in situ (CIS) and dysplasia.9,10 These conflicting reports have led to the most recent 2024 National Comprehensive Cancer Network (NCCN) guidelines removing their previous definitions for clear (>5 mm) and close (2-5 mm) surgical margins and instead stating that currently there is no universal definition for what constitutes a clear/close surgical margin in HNSCC. 11
Another area of ongoing discussion is how to manage and sample pathological specimens for margin assessment. To achieve this goal, two main approaches are described in the literature, which include a specimen-driven approach and a tumor bed approach. The specimen-driven approach involves removing the entire tumor and then obtaining margins directly from the specimen at different orientations. The tumor bed approach involves removing margins directly from the tissue bed after resection. Several studies including a randomized controlled trial performed by Amit et al. have shown a higher rate of negative margins and locoregional control with a specimen-driven approach; however, both methods continue to be used in practice.12,13
Once processed, surgical margins can either be assessed after formalin fixation or in real time using intraoperative frozen sections. Head and neck surgeons are reported to be among the highest users of intraoperative frozen sections and use the initial report on margin clearance to guide further resection or closure. 14 Several weeks after surgery, a more thorough final pathological assessment is reported. If a close or positive margin is identified, surgeons and their patients are faced with discussing the need for re-resection, adjuvant therapy, or surveillance. Currently, the practice patterns of Canadian head and neck surgeons with respect to pathological assessment and decision making are unknown.
Given the lack of consensus on surgical margin definition and assessment, Bulbul et al. conducted a survey in 2021 investigating margin practices of American Head and Neck Society (AHNS) members. 15 In our study, we aimed to build upon this work by conducting a cross-sectional survey of head and neck surgeons with membership in the Canadian Society of Otolaryngology—Head & Neck Surgery (CSOHNS). The main objectives were to (1) determine how Canadian surgeons define margins; (2) investigate how Canadian surgeons sample margins; and (3) investigate the pathological assessment of margins and the impact on clinical practice.
Methods
A cross-sectional survey was designed by the authors after several iterations with expert opinion from head and neck oncology surgeons from across Canada. It was hosted on the third-party website SurveyMonkey and consisted of 24 questions split into 4 sections: (1) demographics, (2) defining surgical margins, (3) sampling surgical margins, and (4) pathological assessment of surgical margins. The sections included questions with binary (yes/no) answers, absolute numbers (e.g. years in practice), and select all that apply questions. Institutional ethics approval for the survey was obtained from the Research Ethics Board of Sunnybrook Health Sciences Centre.
The survey was distributed via email to self-reported Canadian head and neck oncology surgeons with membership in the CSOHNS using the society’s email list. Responses were collected from December 19, 2023, to March 12, 2024, and 1 reminder email was sent to encourage completion. A study information sheet was included with distribution, which emphasized the target population for the survey—surgeons with a practice focused on head and neck oncology. Consent for participation was outlined in the study information sheet and was implicit on response. The total survey time was approximately 5 to 10 minutes.
The survey data was extracted from SurveyMonkey and exported into Microsoft Excel (Microsoft© v16.82 2021) for analysis. Descriptive statistics were used to report quantitative data (binary questions). In the case of qualitative data (open ended questions/comments), responses were analyzed and reported narratively if a relevant theme emerged (2 responses with a similar comment). Participant responses were excluded if they did not complete data beyond the demographics section or if they were not currently-practicing staff surgeons.
Results
A total of 41 head and neck surgical staff and trainees with membership in the CSOHNS responded to the survey. Of the 41 participants, 36 were currently-practicing staff surgeons and 33 of them completed all the questions. The other 5 participants were 3 residents, 1 fellow, and 1 retired staff surgeon. Demographic data (Table 1) and survey responses are reported for the currently-practicing staff surgeons.
Table 1.
Demographics.
| Sex, n (%) | |
| Female | 7 (19.4) |
| Male | 29 (80.6) |
| Mean age (years) | 46.5 |
| Mean years of practice (years) | 14.4 |
| Location of practice, n (%) | |
| Alberta | 5 (13.9) |
| British Columbia | 4 (11.1) |
| Manitoba | 2 (5.6) |
| New Brunswick | 3 (8.3) |
| Newfoundland & Labrador | 3 (8.3) |
| Northwest Territories | 0 (0.0) |
| Nova Scotia | 1 (2.8) |
| Nunavut | 0 (0.0) |
| Ontario | 8 (22.2) |
| Prince Edward Island | 0 (0.0) |
| Quebec | 9 (25.0) |
| Saskatchewan | 1 (2.8) |
| Yukon | 0 (0.0) |
Defining Surgical Margins
When asking surgeons what they considered a negative surgical margin in oral cavity cancer, the most common answer was “>5 mm FFPE distance” (58.3%) followed by “>3 cm fixed paraffin embedded distance” (16.7%) (Figure 1). Next, they were asked whether their definition for a negative margin would change depending on tumor subsite with 52.8% answering “yes” and 47.2% answering “no.” Then, they were asked whether they considered CIS a positive margin with 52.8% answering “yes” and 19.4% answering “no.” Another 27.8% of surgeons answered “it depends,” with many free-text answers mentioning that it hinged on location/subsite and on whether it was contiguous with the gross disease or separate with the implication that it could be field change. Finally, surgeons were asked whether they considered different levels of dysplasia a positive margin. When stratifying by severity, 33.3% answered “yes—only severe,” 5.6% answered “yes—only moderate or severe,” 8.3% answered “yes—mild, moderate, or severe,” and finally 52.8% answered “no” (Table 2).
Figure 1.
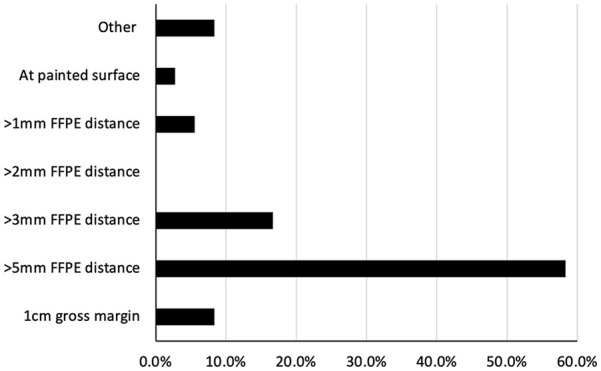
The definition of a negative surgical margin for oral cavity cancer among surveyed Canadian head and neck surgeons.
Abbreviation: FFPE, formalin-fixed paraffin-embedded.
Table 2.
Questions Related to Defining Surgical Margins.
| Question | Response, n (%) | |
|---|---|---|
| Does your definition of margin clearance differ based on subsite? | ||
| Yes | 19 (52.8) | |
| No | 17 (47.2) | |
| Do you consider carcinoma in situ a positive margin? | ||
| Yes | 19 (52.8) | |
| No | 7 (19.4) | |
| It depends | 10 (27.8) | |
| Do you consider dysplasia a positive margin? | ||
| Yes—only mild, moderate, or severe | 3 (8.3) | |
| Yes—only moderate or severe | 2 (5.6) | |
| Yes—only severe | 12 (33.3) | |
| No | 19 (52.8) | |
Sampling Surgical Margins
To gain insight into the methods used in sampling and displaying surgical margins to pathologists, surgeons were asked 10 directed questions, and the results are summarized in Figure 2 and Table 3.
Figure 2.
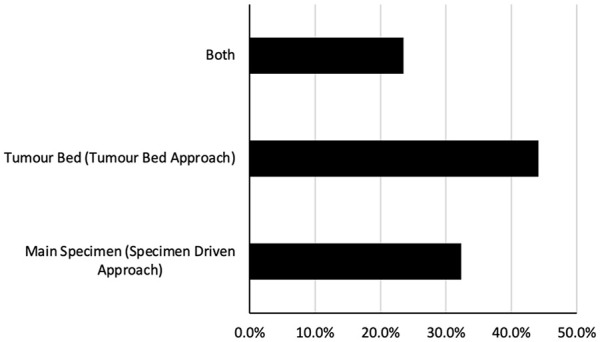
The primary methods used for obtaining surgical margins among surveyed Canadian head and neck surgeons.
Table 3.
Questions Related to Obtaining Surgical Margins.
| Question | Response, n (%) |
|---|---|
| How do you sample margins? Please select all that may apply. a | |
| Cold steel | 82.5% |
| Laser | 2.5% |
| Needle tip bovie | 10.0% |
| Spatula tip bovie | 5.0% |
| Do you use intraoperative frozen sections? | |
| Yes | 30 (88.2) |
| No | 4 (11.8) |
| If your initial frozen section margins are positive for tumor, and further resection results in negative frozen section margins, do you consider that patient’s margin positive or negative? | |
| Positive | 4 (11.8) |
| Negative | 23 (67.7) |
| Other (please specify) | 7 (20.6) |
| How do you orient specimens 3-dimensionally at the time of excision? Please select all that may apply. a | |
| Surgical marking with clips or sutures | 45.6% |
| Pinned | 7.0% |
| Specimen map | 14.0% |
| Ink | 15.8% |
| Other | 17.5% |
| How often do you personally mark or denote the closest representative margin at the time of major specimen handoff? | |
| Routinely | 14 (41.2) |
| Occasionally | 13 (38.2) |
| Never | 1 (2.9) |
| Yes, if area of concern or intraoperative re-resection | 6 (17.7) |
| How would you determine and ensure adequate margin clearance on the deep surface? Please select all that may apply. a | |
| Palpation | 61.1% |
| Intraoperative navigation | 7.4% |
| Bisection method | 18.5% |
| Other | 13.0% |
| Would you utilize intraoperative image guidance (e.g. ultrasound, optical coherece tomography, molecular based imaging) if it were cost-effective and could accurately allow the visualization of the tumor edge? | |
| Yes | 30 (88.2) |
| No | 4 (11.8) |
| When would you consider additional access procedures to ensure 3-dimensional clearance as it relates to oral tongue cancer surgery? Please select all that may apply. a | |
| If transoral approach is attempted and not feasible | 27.8% |
| If there is associated extensive floor of mouth disease | 16.5% |
| If there is bone involvement | 26.8% |
| If there is extension beyond the midline raphe | 6.2% |
| If there is extension beyond circumvallate raphe | 17.5% |
| Other | 5.2% |
| What additional access procedures would you consider for more advanced oral tongue cancers? Please select all that may apply. a | |
| Endoscope | 12.8% |
| TORS | 30.8% |
| Lingual release | 64.1% |
| Mandibular access/mandibulotomy | 92.3% |
| Other | 7.7% |
Abbreviation: TORS, transoral robotic surgery.
Select all that apply questions were reported as percentages of the total number of responses received.
When asking surgeons which approach they used to obtain surgical margins, 44.1% reported using only a tumor bed approach, 32.4% reported only using a specimen-driven approach, and 23.5% reported using both approaches (Figure 2). Regardless of the approaches, the most common method reported to sample the margin was cold steel (82.5%), followed by needle tip bovie (10.0%), spatula tip bovie (5.0%), and laser (2.5%). Most surgeons (88.2%) reported using intraoperative frozen sections. If initial frozen section margins are positive, but further resection is negative, most surgeons (67.7%) would consider the margin negative. With that said, 20.6% of surgeons said their opinion would depend on discussion with their pathologist and final pathology report, while 11.8% reported they would consider the margin positive (Table 3).
Once margins or specimens are removed, it is essential to orient the specimen for pathological assessment. The most common method that surgeons reported using for orientation was clips or sutures (45.6%). There was also a wide distribution among using ink (15.8%), specimen map (14.0%), and pinning (7.0%). 17.5% of surgeons reported using other methods, with the most prevalent theme being performing orientation directly with the pathologist. When performing major specimen handoff to the pathologist, the majority of surgeons reported either routinely (41.2%) or occasionally (38.2%) marking the closest representative margin (Table 3).
Ensuring deep margin clearance is often the most challenging part of an ablative procedure. The most common method that surgeons reported using was palpation (61.1%). The bisection method was also relatively common at 18.5%. Another 7.4% reported using intraoperative navigation and 13.0% reported using other methods, including visual inspection, frozen sections, and bisection directly with pathology. Most surgeons (88.2%) would utilize intraoperative image guidance if it were cost-effective and could accurately allow visualization of the tumor edge (Table 3).
Given the difficulty involved in accessing advanced oral tongue cancers, surgeons were first asked when they would consider additional access procedures to ensure 3-dimensional (3D) clearance. The most common answers were when a transoral approach is attempted but not feasible (27.8%) or when there is bone involvement (26.8%). Several surgeons also mentioned that extension beyond circumvallate raphe (17.5%), midline raphe (6.2%), and extensive floor of mouth disease (16.5%) would cause them to consider an alternative approach. Surgeons were then asked what specific access procedures they would perform in these cases. Mandibular access/mandibulotomy was the most common answer at 92.3%; however, many surgeons would also consider lingual release (64.1%), transoral robotic surgery (30.8%), and endoscopy (12.8%) (Table 3).
Pathological Assessment of Margins and Impact on Clinical Practice
To gain a better understanding of the role of the pathologist in assessing margins and their impact on surgeon clinical practice, a series of 5 questions were posed to the surveyed head and neck surgeons with the results summarized in Table 4.
Table 4.
Questions Related to Pathological Assessment of Margins and Impact on Clinical Practice.
| Question | Response, n (%) |
|---|---|
| Is a synoptic or standardized reporting system that is capable of clearly-annotating margin status used at your institution? | |
| Yes | 26 (78.8) |
| No | 7 (21.2) |
| Is there a dedicated head and neck pathologist available for intraoperative frozen section analysis for major ablative head/neck surgical cases at your institution? | |
| Always | 5 (15.5) |
| Most of the time | 10 (30.3) |
| Occasionally, if specifically requested | 7 (21.2) |
| Infrequently | 8 (24.2) |
| Never | 3 (9.1) |
| Is there a dedicated head and neck pathologist available to report the final pathology for major ablative head/neck surgical cases at your institution? | |
| Always | 21 (63.6) |
| Most of the time | 2 (6.1) |
| Occasionally, if specifically requested | 4 (12.1) |
| Infrequently | 3 (9.1) |
| Never | 3 (9.1) |
| What percentage of the time do you think that final pathology reverses or is discordant with intraoperative frozen sections? | |
| Less than 5% | 15 (45.5) |
| 5% to 10% | 15 (45.5) |
| Greater than 10% | 3 (9.1) |
| How do you manage final pathology with positive margins? Please select all that apply. a | |
| Attempt re-excision for clear margin if easily accessible | 36.1% |
| Attempt re-excision all of the time with intraoperative frozen, even if it requires general anesthesia | 13.1% |
| Refer for consideration of radiation and/or chemotherapy as appropriate | 45.9% |
| Other | 4.9% |
Select all that apply questions were reported as percentages of the total number of responses received.
First, surgeons were asked whether a synoptic or standardized reporting system for clearly-annotating margin status was used at their respective institutions. The majority reported that they did have such a system (78.8%). Surgeons were then asked whether a dedicated head and neck pathologist was available for the analysis of intraoperative frozen sections and final pathology for major ablative head and neck oncology cases. For intraoperative frozen sections, 15.5% of surveyed surgeons reported that a dedicated head and neck pathologist was always available, 30.3% stated they were available most of the time, 21.1% reported occasionally, 24.2% reported infrequently, and 9.1% reported never. In contrast, for final pathology, a larger proportion of surveyed surgeons reported having a dedicated head and neck pathologist either always (63.6%) or most of the time (6.1%). Then, 12.2% reported occasionally, and an equal amount (9.1%) reported either infrequently or never having one available (Table 4).
Next, surgeons were asked what percentage of the time they believed final pathology was discordant with intraoperative frozen sections. An equal proportion (45.5%) believed this occurred less than 5% of the time or between 5% and 10% of the time and 9.1% believed it occurred >10% of the time. Finally, surgeons were asked how they would manage their respective patients in cases of positive margins on final pathology. The most common answer was referral for the consideration of radiation and/or chemotherapy as appropriate (45.9%) (Table 4).
Discussion
Obtaining clear surgical margins intraoperatively is the primary goal of any HNSCC oncologic procedure and has significant implications on patient morbidity and prognosis.2,3 Despite its critical importance, head and neck cancers have some of the highest rates of positive margins among solid cancers, ranging from 10% to 30%. 16 The high rate of positive surgical margins can be in part attributed to the variability in margin definition, margin sampling, and pathological assessment, which we explored in this survey.
Among our group of Canadian head and neck surgeons, over half continue to use the standard 1 cm gross margin or >5 mm FFPE distance as their optimal cutoff for negative surgical margins. The next most common definition was >3 mm FFPE distance, which likely reflects the significant number of retrospective studies advocating for its potential as the new standard cutoff.5,7,8 A recent systematic review by Young et al evaluated the relative risk ratios of stratified surgical margins on local recurrence and found that using a cutoff of >4 mm has the same relative risk as using >5 mm. In their analysis, however, they cautioned against using the data to guide changes in clinical practice as most studies on the topic are retrospective with a lack of standardization in terms of tumor subsite, margin sampling, and adjuvant therapy. 17 It appears that without concrete prospective data and unified consensus, most Canadian head and neck surgeons will continue to use the standard 1 cm gross margin or >5 mm FFPE distance as their cutoff for negative surgical margins.
When considering the definition of positive surgical margins, the surveyed group was split on whether CIS and different levels of dysplasia meet definition for a positive margin. Currently, the available literature regarding the impact of different degrees of dysplasia on prognosis is limited.9,10 Thus, the most updated NCCN guidelines suggest that a positive margin is only defined by CIS or invasive carcinoma at the margin of resection. 11
The specimen-driven approach and tumor bed approach are the two most commonly-used methods for sampling margins and are often sent for intraoperative frozen section analysis by pathology. Most of our surveyed Canadian head and neck surgeons reported using intraoperative frozen sections. Interestingly, despite literature supporting the specimen-driven approach for improved locoregional control, a larger proportion of our surveyed group reported using only the tumor bed approach and approximately one quarter reported using both methods.12,13 Furthermore, when initial frozen sections are reported as a positive margin, but re-resection is negative, most of our surveyed group would categorize the patient’s margins as negative, despite literature eluding to a significantly-worse oncologic prognosis in this situation.18,19 These results may serve as opportunities for further investigation and education among our group of Canadian head and neck surgeons.
Arguably, the most important aspect of margin assessment is ensuring that there is a reliable and consistent pathway in place for intraoperative and final pathology. Current methods have inherent limitations, which include tissue shrinkage during fixation, variability in specimen handling, processing, and sampling, and poor-to-moderate interobserver reliability between pathologists.20-23 To assist pathologists at an individual level, the Canadian surgeons surveyed in this study reported orienting specimens 3-dimensionally using various methods and marking the closest representative margin. At an institutional level, employment of dedicated head and neck pathologists, implementation of synoptic reporting pathways, standardization of laboratory processing, and external quality assurance through regular audits are vital. Another area that is being actively investigated is the use of 3D modeling to enhance communication between surgeons and pathologists. Saturno et al and Sharif et al used similar approaches, whereby surgically-resected specimens were scanned to create 3D virtual maps that pathologists could annotate and use to communicate with surgeons intraoperatively and postoperatively. Given that this technology is still in the investigative phase it was not directly explored in this survey, but it could serve as a useful adjunct in the future.24,25
In our study, most of the surveyed Canadian surgeons reported having standardized synoptic reporting systems in place. Not surprisingly, surgeons reported having access to a dedicated head and neck pathologist more often for final pathology, rather than intraoperative pathology. With that said, the majority reported at least occasionally having one available in both instances and the reported percentage of discordance between intraoperative frozen sections and final pathology was split between 5% and 10% and under 10%, which is consistent with the standard in the literature.26,27
Beyond enhancing current pathology standards, several intraoperative imaging guidance systems are being investigated as new methods for margin assessment. The overwhelming majority of the surveyed Canadian head and neck surgeons would use these technologies if cost-effective and could accurately visualize the tumor edge. While there are many experimental options including molecular-based imaging with fluorescence, the most promising may be intraoperative ultrasound in large part due to its widespread availability, affordability, and familiarity in head and neck surgery.28-30 One of its primary applications could be for ensuring deep margin clearance, which is one of the most challenging aspects of head and neck cancer resections. Currently, our surveyed group of surgeons are primarily using palpation and the bisection method, but a recent study using intraoperative ultrasound in oral tongue cancer resections demonstrated the feasibility of visualizing the deep resection margin with a trend toward a decrease in the number of insufficient histopathological deep margins. 31 Overall, there is clearly an interest in using intraoperative imaging guidance for accurate margin assessment, and thus, further research in this area is warranted with potential for implementation into clinical practice.
When comparing our survey results to the AHNS survey from 2021, we found several similarities and a few subtle differences between our Canadian and the overarching North American perspective. The notable similarities include the following: (1) “>5 mm FFPE” being the most common definition of a negative surgical margin, (2) the widespread use of intraoperative frozen sections, (3) similar levels of discordance when categorizing CIS and dysplasia as a positive versus negative margin, and (4) openness to the use of new intraoperative image guidance. The subtle differences include the following: (1) a larger proportion of Canadian surgeons reporting different definitions of margin clearance dependent on tumor subsite, and (2) slightly more Canadian surgeons favoring a tumor driven approach, while slightly more AHNS surgeons favoring a specimen-driven approach to margin sampling. Overall, it appears that our Canadian perspective mostly parallels the results of the overarching North American perspective. 15
The limitations of our study are inherent to the cross-sectional survey design. Firstly, there may have been sampling bias given that the majority of respondents were from Ontario and Quebec with their unique interprovincial perspectives potentially being overrepresented in the data. Secondly, while efforts were made to maximize it, the response rate is still relatively low. Finally, there is the potential for response bias, as answers may not have truly represented the clinical perspectives or practice patterns of the surveyed surgeons.
Conclusion
Ensuring negative surgical margins in HNSCC greatly impacts the prognosis of patients. In our cross-sectional survey of Canadian head and neck surgeons, there was lack of consensus on the definition of negative and positive margins that coincides with discourse in the literature. Ultimately, this survey serves as a current state analysis to encourage the creation of a standardized universal protocol for margin definition, acquisition, and interpretation that all centers can refer to and align themselves with. Future prospective randomized controlled studies should focus on establishing the gold standard cutoff for clear surgical margins and further validate the best margin sampling technique.
Acknowledgments
None.
Footnotes
Author Contributions: R.C.D.: contributed to the analysis of the results and manuscript preparation. R.C.D., B.Y., S.C., A.C.N., A.E., D.E., and K.H.: contributed to the design and implementation of the research and provided intellectual guidance and insight in the revision of the manuscript.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication: Not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participate: Ethics approval was obtained from the Sunnybrook Research Ethics Board.
ORCID iD: Ryan Daniel  https://orcid.org/0000-0002-6431-9016
https://orcid.org/0000-0002-6431-9016
References
- 1. Brenner DR, Poirier A, Woods RR, et al. Projected estimates of cancer in Canada in 2022. Can Med Assoc J. 2022;194(17):E601-E607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin MC, Leu YS, Chiang CJ, et al. Adequate surgical margins for oral cancer: a Taiwan cancer registry national database analysis. Oral Oncol. 2021;119:105358. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell DA, Kanatas A, Murphy C, Chengot P, Smith AB, Ong TK. Margins and survival in oral cancer. Br J Oral Maxillofac Surg. 2018;56(9):820-829. [DOI] [PubMed] [Google Scholar]
- 4. Zanoni DK, Migliacci JC, Xu B, et al. A proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol Head Neck Surg. 2017;143(6):555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brinkman D, Callanan D, O’Shea R, Jawad H, Feeley L, Sheahan P. Impact of 3 mm margin on risk of recurrence and survival in oral cancer. Oral Oncol. 2020;110:104883. [DOI] [PubMed] [Google Scholar]
- 6. Solomon J, Hinther A, Matthews TW, et al. The impact of close surgical margins on recurrence in oral squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2021;50(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nason RW, Binahmed A, Pathak KA, Abdoh AA, Sándor GKB. What is the adequate margin of surgical resection in oral cancer? Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2009;107(5):625-629. [DOI] [PubMed] [Google Scholar]
- 8. Chiou WY, Lin HY, Hsu FC, et al. Buccal mucosa carcinoma: surgical margin less than 3 mm, not 5 mm, predicts locoregional recurrence. Radiat Oncol. 2010;5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brinkman D, Callanan D, Jawad H, et al. Comparison of Royal College of Pathologists and College of American Pathologists definition for positive margins in oral cavity squamous cell carcinoma. Oral Oncol. 2022;127:105797. [DOI] [PubMed] [Google Scholar]
- 10. Senarath NH, Jayasooriya PR, Siriwardena BSMS, Tilakaratne WM. Epithelial dysplasia at excision margins of oral squamous cell carcinoma: a review on relationship to clinicopathological parameters and prognosis. Asian Pac J Cancer Prev. 2021;22(8):2313-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfister D, Spencer S, Adkins D, et al. NCCN Guidelines Version 3.2024 Head and Neck Cancers. National Comprehensive Cancer Network; 2024. [Google Scholar]
- 12. Amit M, Na’Ara S, Leider-Trejo L, et al. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: a prospective randomized controlled study. Head Neck. 2016;38(Suppl 1):E1803-E1809. [DOI] [PubMed] [Google Scholar]
- 13. Maxwell JH, Thompson LDR, Brandwein-Gensler MS, et al. Early oral tongue squamous cell carcinoma sampling of margins from tumor bed and worse local control. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1104-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McIntosh ER, Harada S, Drwiega J, Brandwein-Gensler MS, Gordetsky J. Frozen section: guiding the hands of surgeons? Ann Diagn Pathol. 2015;19(5):326-329. [DOI] [PubMed] [Google Scholar]
- 15. Bulbul MG, Zenga J, Tarabichi O, et al. Margin practices in oral cavity cancer resections: survey of American Head and Neck Society members. Laryngoscope. 2021;131(4):782-787. [DOI] [PubMed] [Google Scholar]
- 16. Orosco RK, Tapia VJ, Califano JA, et al. Positive surgical margins in the 10 most common solid cancers. Sci Rep. 2018;8:5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young K, Bulosan H, Kida CC, Bewley AF, Abouyared M, Birkeland AC. Stratification of surgical margin distances by the millimeter on local recurrence in oral cavity cancer: a systematic review and meta-analysis. Head Neck. 2023;45(5):1305-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agne GR, Kohler HF, Chulam TC, Pinto CAL, Vartanian JG, Kowalski LP. Oncologic outcomes of microscopic tumor cut-through in locally advanced oral squamous cell carcinoma. Arch Head Neck Surg. 2022;51:e20220013. [Google Scholar]
- 19. Guillemaud JP, Patel RS, Goldstein DP, Higgins KM, Enepekides DJ. Prognostic impact of intraoperative microscopic cut-through on frozen section in oral cavity squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2010;39(4):370-377. [PubMed] [Google Scholar]
- 20. Abbey LM, Kaugars GE, Gunsolley JC, et al. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80(2):188-191. [DOI] [PubMed] [Google Scholar]
- 21. Pindborg JJ, Reibel J, Holmstrup P. Subjectivity in evaluating oral epithelial dysplasia, carcinoma in situ and initial carcinoma. J Oral Pathol Med. 1985;14(9):698-708. [DOI] [PubMed] [Google Scholar]
- 22. Pangare TB, Waknis PP, Bawane SS, Patil MN, Wadhera S, Patowary PB. Effect of formalin fixation on surgical margins in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. 2017;75(6):1293-1298. [DOI] [PubMed] [Google Scholar]
- 23. Umstattd LA, Mills JC, Critchlow WA, Renner GJ, Zitsch RP. Shrinkage in oral squamous cell carcinoma: an analysis of tumor and margin measurements in vivo, post-resection, and post-formalin fixation. Am J Otolaryngol Head Neck Med Surg. 2017;38(6):660-662. [DOI] [PubMed] [Google Scholar]
- 24. Saturno MP, Brandwein-Weber M, Greenberg L, et al. Utilizing 3D head and neck specimen scanning for intraoperative margin discussions: proof of concept of our novel approach. Head Neck. 2022;45(1):10-21. doi: 10.1002/hed.27171 [DOI] [PubMed] [Google Scholar]
- 25. Sharif KF, Lewis JS, Jr, Ely KA, et al. The computer-aided design margin: ex vivo 3D specimen mapping to improve communication between surgeons and pathologists. Head Neck. 2022;45(1):22-31. doi: 10.1002/hed.27201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long SM, McLean T, Valero Mayor C, et al. Use of intraoperative frozen section to assess final tumor margin status in patients undergoing surgery for oral cavity squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148(10):911-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kungoane T, Robinson LM, Madiba TK. Frozen sections in head and neck surgery and the impact of intraoperative analysis on final resection margins: an institutional study. S Afr Dent J. 2022;77:18-22. [Google Scholar]
- 28. De Boer E, Warram JM, Tucker MD, et al. In vivo fluorescence immunohistochemistry: localization of fluorescently labeled cetuximab in squamous cell carcinomas. Sci Rep. 2015;5:10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao RW, Teraphongphom NT, van den Berg NS, et al. Determination of tumor margins with surgical specimen mapping using near-infrared fluorescence. Cancer Res. 2018;78(17):5144-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tarabichi O, Kanumuri V, Juliano AF, Faquin WC, Cunnane ME, Varvares MA. Intraoperative ultrasound in oral tongue cancer resection: feasibility study and early outcomes. Otolaryngol Head Neck Surg. 2018;158(4):645-648. [DOI] [PubMed] [Google Scholar]
- 31. Nilsson O, Knutsson J, Landström FJ, Magnuson A, von Beckerath M. Ultrasound-assisted resection of oral tongue cancer. Acta Otolaryngol. 2022;142(9-12):743-748. [DOI] [PubMed] [Google Scholar]