Abstract
Background
In the pursuit of global health security, continuous monitoring of vaccine effectiveness across various viral strains emerges as a crucial imperative. The emergence of SARS-CoV-2 major variants of concern (VOCs), including Alpha, Beta, Delta, and Omicron, has added complexity to the COVID-19 vaccination landscape.
Objectives
To assess illness severity, evaluate vaccine efficacy across varying doses and types, and determine effectiveness against major VOCs within the population.
Methods
This retrospective cohort study, conducted in Abu Dhabi, United Arab Emirates, focuses on a cohort of 44,073 SARS-CoV-2 positive cases from February 2021 to May 2022, dominated by the Delta and Omicron variants. The study employed a nested case-control design, analyzing hospital admissions for confirmed SARS-CoV-2 infection.
Results
Vaccine effectiveness was higher among heterologus-boosted individuals at 87% (95% CI:79%-93%) compared to homologus-boosted individuals at 59% (95% CI: 48%-68%) and fully vaccinated, non-boosted adults at 53% (95% CI: 46%-59%). These findings highlight the importance of heterologous boosting, particularly against rapidly evolving viral variants, offering valuable insights for refining pandemic response strategies.
Conclusion
The study underscores the critical need for ongoing assessment and adaptation of vaccination strategies to the evolving viral landscape.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10124-6.
Keywords: SARS CoV-2, Pandemic, Vaccine, Heterologous, Homologous, Booster, Variant of concern, Severity, virus
Introduction
In the pursuit of global health security, continuous monitoring of vaccine effectiveness across various viral strains is crucial. This approach safeguards communities against the evolving threat of infectious diseases, serves as a key avenue for strategy improvement, and enhances the mitigation measures. Vaccines play a pivotal role in both individual and population-level immune responses, serving as essential tools in reducing fatalities and potentially lowering infection rates, even against highly infectious viruses such as SARS-CoV-2. As of August 2024, 13.53 billion doses have been administered globally, including 10.73 billion initial protocol doses and 2.8 billion booster doses [1]. The vaccination program against SARS-CoV-2 in the United Arab Emirates (UAE) was initiated in December 2020. Five types of two-dose vaccine regimens were approved for emergency use, including BBIBP-CorV (brand name: Sinopharm), BNT162b2 (brand name: Pfizer/BioNTech), rAd26-S + rAd5-S (brand name: Sputnik V Gam-COVID-Vac), ChAdOx1 (brand name: Oxford-AstraZeneca) and mRNA-1273 (brand name: Moderna).
Since the end of 2020, a series of novel variants of concern (VOCs) have emerged globally and been reported in the UAE, including B.1.1.7 (Alpha), B.1.351 (Beta), B.1.617.2 (Delta) and B.1.1.529 (Omicron) [2]. Alpha variant (B.1.1.7 and its descendent lineages) demonstrated over 50% increased transmissibility [3], while the Beta variant (B.1.351 and its descendent lineages) and Gamma variant (P.1 and its descendent lineages) showed the capacity to evade neutralizing antibodies [4]. The Delta variant (B.1.617.2 and its descendent lineages) led to a significant rise in infections in India in early 2021 and became the dominant strain globally until late 2021 [5, 6]. While the Omicron variant (B.1.1.529 and its descendent lineages) spread at an unprecedented rate, studies have indicated its ability to evade a majority of existing SARS-CoV-2 neutralizing antibodies [7–9].
Reports on the efficacy of different vaccines vary based on factors such as population demographics, vaccine types, dosage, time since vaccination, and more. In late 2021, many cohort studies involving the BBIBP-CorV vaccine were conducted, predominantly within our study population. In Hungary, the BBIBP-CorV vaccine demonstrated 66.1% effectiveness in preventing infection and 87.8% effectiveness in preventing death [10]. Another study conducted in Morocco on the long-term effectiveness of the inactivated BBIBP-CorV vaccine revealed a decline from 88% in the first month to 64% beyond the sixth month, with variations observed predominantly in the older population (> 60 years of age) [11].
In Abu Dhabi, UAE, a cohort study focusing on individuals with confirmed SARS CoV-2 infection who received only two doses of the BBIBP-CorV vaccine demonstrated 80% effectiveness in preventing hospitalization and 97% effectiveness in preventing death [12]. With access to high-quality surveillance data, this study aims to investigate the severity of illness and effectiveness of different COVID-19 vaccination doses and types, particularly after booster administration. Additionally, this study aims to evaluate the effectiveness of COVID-19 vaccines against major VOCs within a cohort of SARS-CoV-2 patients in the UAE. Understanding vaccine effectiveness is essential for informing future pandemic strategies and guiding the development of diverse vaccine modalities to combat a spectrum of viruses, thereby enhancing global preparedness and adaptability.
Methodology
Study design
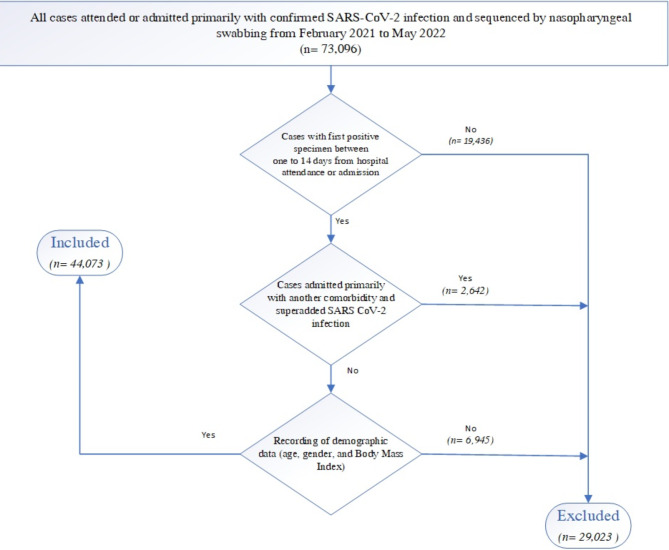
This retrospective cohort study, with a nested case-control component, was conducted in hospitals managing and admitting SARS-CoV-2 positive and severe cases in the Emirate of Abu Dhabi, UAE. All cases attended or admitted primarily for confirmed SARS-CoV-2 infection were included, and their viral sequencing for SARS-CoV-2 was performed using nasopharyngeal swabbing, from February 2021 to May 2022, during which the Delta and Omicron variants were dominant [2].
The inclusion and exclusion criteria for the study participants are detailed in Fig. (1). To be included in the study, cases had to be confirmed by laboratory diagnosis one to 14 days from hospital attendance or admission. This approach minimized biases from routine testing for non-COVID-19 cases and excluded cases with positive tests more than 14 days after admission, which may indicate hospital-acquired SARS-CoV-2 infections. Cases were excluded if they were admitted primarily with another comorbidity and had a superimposed SARS-CoV-2 infection. Cases missing essential demographic information such as age, gender, or BMI, were excluded. To maintain the accuracy of our findings, cases with a death reporting delay exceeding 28 days were also excluded to avoid the introduction of confounding factors related to prolonged reporting intervals.
Fig. 1.
Flowchart illustrating the inclusion and exclusion criteria for the study participants
Data collection
Data were collected locally using a hospital laboratory information system in conjunction with information from Malaffi, a unified electronic medical record accessible to healthcare providers for every citizen and resident in Abu Dhabi, facilitated through the Department of Health’s initiatives [13]. For each case, comprehensive data retrieval was conducted from multiple sources, including the hospital laboratory information system, electronic health records, and Infectious Disease Notification system. The extracted information included demographic details such as gender, age, ethnicity, weight, height, and comorbidities. Additionally, data on admission type, instances of hospitalization, ICU admission, length of stay, provision of ventilatory support, and death status, if applicable, were recorded. Vaccination data were also extracted from Malaffi, including details on vaccine type, dosage, and date of vaccination.
This thorough data extraction process ensures a robust foundation for analysis, providing a comprehensive understanding of the factors influencing COVID-19 outcomes in the studied population. Considering the possibility of participants having multiple positive RT-PCR tests, each individual was included only once, primarily considering the episode with viral sequencing. Vaccination status was classified into three categories according to the vaccination schedule, irrespective of the type of the vaccine: no vaccination (including incomplete vaccination), full vaccination, and full vaccination with a booster. Furthermore, booster doses were classified according to the vaccine type, distinguishing between homologous (if identical to initial doses) and heterologous (if different from initial vaccine doses).
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Science (SPSS) version 25 and R (version 3.4.1). Descriptive analyses were conducted using frequency analysis for categorical variables, while the mean and standard error of mean were used for continuous variables. Pearson Chi-square test and Fisher’s Exact test (two-tailed) were used to analyze categorical variables through cross-tabulation. Logistic regression models were applied to test multivariate relationships between symptom severity and vaccination status. The models were adjusted for potential confounders, including age group, sex, nationality, body mass index (BMI), and comorbidities. The categorization of comorbidities distinguished between the absence of any conditions, the presence of a single condition, or the presence of at least two conditions while accounting for previous SARS-CoV-2 infection. To quantify vaccine effectiveness (VE) against symptomatic SARS-CoV-2 infection, the following equation was applied: VE = (1 – adjusted odds ratio) × 100% [14]. All statistical tests were set at the 0.05 significance level.
Results
This study included a total sample size of 44,073 cases, with 75% being adults. Approximately 26.1% of the overall study population had an underlying chronic illness, with hypertension (16.7%) and diabetes mellitus (15.4%) being the most prevalent among adults. Among pediatric cases, bronchial asthma was the most frequently encountered condition (15.4%). Within the total infected cases, 27.3% were attributed to VOCs, with the Delta (B.1.617.2) variant being the most common among both adults (23.2%) and pediatric patients (18.1%). Regarding vaccination data, 34.9% of adults were unvaccinated or incompletely vaccinated, 42.9% were fully vaccinated, and 22.3% had received a booster dose. Among the boosted adults, 4308 were primed and boosted (homologous) with BBIBP-CorV, and 2709 were primed with BBIBP-CorV and boosted with BNT162b2 (heterologous). In contrast, most of the pediatric population were unvaccinated or incompletely vaccinated (82.1%), 17.2% were fully vaccinated, and 0.8% were fully vaccinated with a booster dose. The majority of the study population were non-hospitalized (96.5%). Among adults, 2.8% were hospitalized without ICU admission, 0.8% were hospitalized with ICU admission, and 0.4% resulted in in-hospital mortality due to COVID-19. Among pediatric cases, 1.4% were hospitalized without ICU admission, 0.6% required ICU admission during hospitalization, and only 2 cases resulted in in-hospital mortality due to COVID-19. The median hospital length of stay was 4.13 days for adults and 1.98 days for pediatric patients. The median ICU length of stay was 7 days for adults and 2 days for pediatric patients (Table 1).
Table 1.
Characteristics of the Study Population
| Variable | Total n = 44,073 (%) |
Adult n = 33,003 (74.9%) |
Pediatrics n = 11,070 (25.1%) |
|---|---|---|---|
| Gender | |||
| Male | 23,631 (53.6) | 17,743 (53.8) | 5888 (53.2) |
| Female | 20,442 (46.4) | 15,260 (46.2) | 5182 (46.8) |
| Age (Median, SD) | -- | ||
| < 2 Years | 1658 (3.8) | -- | 1658 (3.8) |
| 3–5 Years | 1605 (3.6) | -- | 1605 (3.6) |
| 6–11 Years | 4296 (9.7) | -- | 4296 (9.7) |
| 12–17 Years | 3511 (8) | -- | 3511 (8) |
| 18–39 Years | 19,470 (44.2) | 19,470 (44.2) | -- |
| 40–59 Years | 10,564 (24) | 10,564 (24) | -- |
| >= 60 Years | 2969 (6.7) | 2969 (6.7) | -- |
| Ethnicity | |||
| Middle Eastern | 27,333 (62) | 18,499 (56.1) | 8834 (79.8) |
| Asian | 12,915 (29.3) | 11,392 (34.5) | 1523 (13.8) |
| African | 1767 (4) | 1590 (3.6) | 177 (0.4) |
| European | 1268 (2.9) | 1005 (3) | 263 (2.4) |
| Others | 790 (1.8) | 517 (2.8) | 273 (3.6) |
| Chronic diseases | 11,484 (26.1) | 9424 (28.6) | 2060 (18.6) |
| Hypertension | 5651 (12.8) | 5520 (16.7) | 131 (1.2) |
| Diabetes Mellitus | 5169 (11.7) | 5070 (15.4) | 99 (0.9) |
| Bronchial Asthma | 4776 (10.8) | 3066 (9.3) | 1710 (15.4) |
| Chronic Kidney Disease | 2259 (5.1) | 2064 (6.3) | 195 (1.8) |
| Neoplasm | 1643 (3.7) | 1503 (4.6) | 140 (1.3) |
| Heart Diseases | 1515 (3.4) | 1503 (4.6) | 12 (0.1) |
| Immunodeficiency | 478 (1.1) | 298 (0.9) | 180 (1.6) |
| Respiratory Disease** | 402 (0.9) | 379 (1.1) | 23 (0.2) |
| Organ Transplantation | 190 (0.4) | 165 (0.5) | 25 (0.2) |
| Others | 216 (0.5) | 188 (0.6) | 28 (0.3) |
| Comorbidities | |||
| 1 | 6158 (14) | 4409 (13.4) | 1749 (15.8) |
| 2 | 2464 (5.6) | 2255 (6.8) | 209 (1.9) |
| 3 | 1368 (3.1) | 1312 (4) | 56 (0.5) |
| 4 | 761 (1.7) | 734 (2.2) | 27 (0.2) |
| 5 | 434 (1) | 420 (1.3) | 14 (0.1) |
| 6 | 212 (0.5) | 207 (0.6) | 5 (0) |
| 7 | 74 (0.2) | 74 (0.2) | -- |
| 8 | 12 (0) | 12 (0) | -- |
| 9 | 1 (0) | 1 (0) | -- |
| VOC infection | 12,041 (27.3) | 9584 (29) | 2457 (22.2) |
| Alpha (B.1.1.7) | 581 (1.3) | 472 (1.4) | 109 (1) |
| Beta (B.1.351) | 705 (1.6) | 596 (1.8) | 109 (1) |
| Gamma (P.1) | 2 (0) | 2 (0) | -- |
| Delta (B.1.617.2) | 9653 (21.9) | 7647 (23.2) | 2006 (18.1) |
| Omicron (B.1.1.529) | 1100 (2.5) | 867 (2.6) | 233 (2.1) |
| Vaccination details | |||
| Non-Vaccinated & incomplete vaccination | 20,599 (46.7) | 11,513 (34.9) | 9086 (82.1) |
| Full vaccination | 16,041 (36.4) | 14,142 (42.9) | 1899 (17.2) |
| Sino pharm | 13,791 (86) | 12,564 (88.8) | 1227 (64.6) |
| Pfizer | 2177 (13.6) | 1505 (10.6) | 672 (35.4) |
| Others# | 73 (0.4) | 73 (0.6) | ---- |
| Vaccine type (Booster) | 7433 (16.9) | 7348 (22.3) | 85 (0.8) |
| Homologues | |||
| Sino pharm | 4360 | 4308 | 52 |
| Pfizer | 314 | 313 | 1 |
| Heterologous (Primed by Sino pharm and booster by Pfizer) | 2741 | 2709 | 32 |
| Clinical data | |||
| Clinical Outpatient | 34,592 (78.5) | 25,983 (78.7) | 8609 (77.8) |
| Emergency | 7601 (17.2) | 5491 (16.6) | 2110 (19.1) |
| Inpatient acute care | 1441 (3.3) | 1231 (3.7) | 210 (1.9) |
| Urgent care | 406 (0.9) | 284 (0.9) | 122 (1.1) |
| Observation (medical short stay) | 14 (0) | 11 (0) | 3 (0) |
| Observation (Pediatric short stay) | 15 (0) | -- | 15 (0) |
| Day surgery (surgical short stay) | 4 (0) | 3 (0) | 1 (0) |
| Phenotype | |||
| Non – hospitalized | 42,521 (96.5) | 31,664 (95.9) | 10,857 (98.1) |
| Hospitalized – No ICU admission | 1088 (2.5) | 938 (2.8) | 150 (1.4) |
| Hospitalized & ICU admission | 319 (0.7) | 258 (0.8) | 61 (0.6) |
| In hospital COVID-19 mortality | 145 (0.3) | 143 (0.4) | 2 (0) |
| (without Critical Care admission) | 41 | 41 | 0 |
| (with Critical care admission) | 104 | 102 | 2 |
| Utilization | |||
| Hospitalized | 1519 (3.4) | 1306 (4) | 213 (1.9) |
| Hospital Length of stay (Min, Max, Mean ± SD, Median) | (1,98,6.74 ± 8.12,3.87) | (1,98,7.28 ± 10.9,4.13) | (1,29,3.45 ± 4.41,1.98) |
| Critical care admission | 423 (1) | 360 (1.1) | 63 (0.6) |
| Critical care Length of stay (Min, Max, Mean ± SD, Median) | (1,85,9.7 ± 10.61,6) | (1,85,10.42 ± 10.96,7) | (1,37,5.57 ± 7.09,2) |
| Received ventilatory support | 294 (0.7) | 269 (0.8) | 25(0.2) |
*Others include European, American, South American, and Oceanic Regions.
** Other than Bronchial Asthma
# Others include Spuntik, AsteraZeneca, Sinovac, Moderna, Johnson & Johnson
No vaccination: no vaccination, > 14 days for individuals who received only one dose and < 14 days for individuals who received two doses.
Full vaccination: ≥14 days for individuals who received two doses.
Booster dose: >6 months for individuals after they received two doses.
The association between different levels of vaccination among BBIBP-CorV vaccinated adult cases and population characteristics revealed significant associations (Table S.1.). There was a significantly lower risk of severe outcomes including hospitalization, ICU admission, and death (p < 0.001) between the fully vaccinated and unvaccinated groups. Additionally, significant differences were noted in age (p < 0.001), gender (p < 0.001), nationality (p < 0.001), BMI (p < 0.001), variant infection (p < 0.001), and specific comorbidities, including bronchial asthma (p < 0.001), chronic kidney disease (p < 0.001), diabetes (p < 0.001), cardiovascular disease (p = 0.003) and hypertension (p < 0.001).
In comparison to the fully vaccinated group, the homologous boosted groups showed significantly less severe outcomes (p < 0.001). However, significant differences were also observed in age (p < 0.001), nationality (p < 0.001), variant infection (p < 0.001), and specific comorbidities such as diabetes (p = 0.009), cardiovascular disease (p = 0.047), hypertension (p < 0.001), neoplasm (p = 0.011), and respiratory disease (p = 0.042). Additionally, among fully vaccinated and heterologous boosted groups with BNT162b2, there was a significantly lower risk of developing severe outcomes (p < 0.001). Significant differences were noted in gender (p < 0.001), nationality (p < 0.001), variant infection (p < 0.001), and specific comorbidities, including chronic kidney disease (p < 0.001), diabetes (p = 0.002), and cardiovascular disease (p < 0.001). These findings highlight the complex relationships between vaccination status and various demographic and health characteristics, providing valuable insights into the effects of immunization strategies.
A bivariate logistic regression analysis was conducted to estimate the crude and adjusted odds of outcomes among VOC and non-VOC-infected adults and the pediatrics group, where non-VOC-infected cases were considered the reference group (Table 2). The adjusted odd ratios were significantly higher among VOC-infected adults regarding hospitalization (p < 0.001), longer hospital stays (p < 0.001), ICU admission (p < 0.001), extended ICU stays (p < 0.001), need for ventilatory support (p < 0.001) and in hospital COVID-19 mortality(p < 0.001). For VOC-infected pediatric patients, no significant differences were noted between the groups (Table 3).
Table 2.
Crude and adjusted odds ratios for outcomes of VOC infection in adult and pediatric populations
| VOC Infected | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adults | Pediatrics | ||||||||
| Outcome |
Crude OR
(95% CI) |
p-value |
Adjusted OR
(95% CI) |
p-value |
Crude OR
(95% CI) |
p-value |
Adjusted OR
(95% CI) |
p-value | |
| Hospitalization | 2.08 (1.86–2.32) | < 0.001 | 1.54 (1.36–1.74) | < 0.001 | 1.16 (0.85–1.59) | 0.341 | 1.00 (0.69–1.44) | 0.999 | |
| Above Median hospital LOS | 4.15 (3.54–4.87) | < 0.001 | 3.53 (2.72–4.12) | < 0.001 | 0.82 (0.53–1.27) | 0.389 | 0.75 (0.45–1.25) | 0.283 | |
| Critical care admission | 3.59 (3.19–4.89) | < 0.001 | 2.69 (2.13–3.39) | < 0.001 | 0.82 (0.43–1.54) | 0.547 | 0.73 (0.37–1.45) | 0.381 | |
| Above Median CCU LOS | 7.21 (5.30–9.80) | < 0.001 | 4.74 (3.23–6.97) | < 0.001 | 0.94 (0.48–1.84) | 0.872 | 0.10 (0.33–1.49) | 0.363 | |
| Need for ventilatory support | 4.93 (3.82–6.36) | < 0.001 | 3.012 (2.30–3.94) | < 0.001 | -- | -- | -- | -- | |
| In hospital COVID-19 Mortality | 6.13 (4.22–8.82) | < 0.001 | 3.73 (2.53–5.51) | < 0.001 | -- | -- | -- | -- | |
LOS = length of stay
Bivariate analysis (Non- VOC & VOC infected) was used for the regression models, non-VOC was considered the reference group in all analyses, presented as crude OR and adjusted OR for sex, nationality, BMI, comorbidities, and vaccination status.
-- Sample size ≤ 20
Table 3.
Nested case-control analyses by vaccination status of adult cases with Non-VOC or VOC SARS CoV-2 infection, either non-hospitalized or hospitalized individuals
| Control | Case | P-Value | Crude OR (95% CI) | P-Value | Adjusted OR (95% CI) | Adjusted P-Value | Vaccine Effectiveness | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Full Vaccination | Non-VOC | Non-Hospitalized | 3979 (95.2%) | 5260 (95.9%) | 0.102 | 1 | 0.002 | 1 | < 0.001 |
56% (45%, 65%) |
| Hospitalized | 201 (4.8%) | 226 (4.1%) | 0.85 (0.70, 1.03) | 0.44 (0.35, 0.55) | ||||||
| VOC | Non-Hospitalized | 6842 (94.2%) | 6707 (95.3%) | < 0.001 | 1 | < 0.001 | 1 | < 0.001 |
50% (41%, 58%) |
|
| Hospitalized | 424 (5.8%) | 328 (4.7%) | 0.78 (0.69, 0.92) | 0.50 (0.42, 0.59) | ||||||
| Booster Dose with BBIBP-CorV | Non-VOC | Non-Hospitalized | 5260 (95.9%) | 603 (98.0%) | 0.008 | 1 | 0.01 | 1 | 0.003 |
60% (26%, 78%) |
| Hospitalized | 226 (4.1%) | 12 (2.0%) | 0.46 (0.25, 0.83) | 0.40 (0.22, 0.74) | ||||||
| VOC | Non-Hospitalized | 6706 (95.3%) | 3609 (97.7%) | < 0.001 | 1 | < 0.001 | 1 | < 0.001 |
62% (51%, 71%) |
|
| Hospitalized | 328 (4.7%) | 86 (2.3%) | 0.48 (0.38, 0.62) | 0.38 (0.29, 0.49) | ||||||
| Booster Dose with BNT162b2 | Non-VOC | Non-Hospitalized | 5260 (95.9%) | 103 (98.1%) | 0.256 | 1 | 0.268 | 1 | 0.258 | NA |
| Hospitalized | 226 (4.1%) | 2 (1.9%) | 0.45 (0.11, 1.84) | 0.43 (0.10, 1.83) | ||||||
| VOC | Non-Hospitalized | 6706 (95.3%) | 2588 (99.4%) | < 0.001 | 1 | < 0.001 | 1 | < 0.001 |
89% (80%, 94%) |
|
| Hospitalized | 328 (4.7%) | 16 (0.6%) | 0.12 (0.07, 0.21) | 0.11 (0.06, 0.20) |
Full vaccination: ≥14 days for individuals who received two doses; control accounts to those that have not been vaccinated; cases accounts to those that have been fully vaccinated.
Booster dose: >6 months for individuals after they received two doses; control accounts to those that have been fully vaccinated; cases accounts to those that have been booster vaccinated.
Bivariate analysis (non-Hospitalized vs. Hospitalized) was used for the regression models, presented as crude OR and adjusted OR for age, sex, nationality, BMI, comorbidities, timeframe and variant type.
Bivariate analysis was performed to determine the adjusted odds ratios of hospitalization (including ICU admission and mortality) among fully vaccinated, homologous boosted, and heterologous boosted adults, using non-hospitalized cases as the reference group (Table S.2). The results indicated that the odds of hospitalization were significantly higher among the fully vaccinated compared to the boosted groups, and higher among homologous boosted than heterologus boosted cases. Vaccine Effectiveness was also higher among heterologus boosted cases 87% (79%-93%) compared to homologus boosted 59% (48%-68%) and fully vaccinated without booster 53% (46%-59%).
A stepwise nested case-control analysis was performed within the adult population, focusing on vaccine protection outcomes relative to the number and type of booster doses (Table 3). Among all analyses, the non-hospitalized cases were considered the reference group. In the initial analysis, where controls comprised individuals who had not received any vaccination, and cases consisted of those who were fully vaccinated, no significant difference in hospitalization rates was observed in the non-VOC infected group between cases and controls. However, there was a significant difference in hospitalization in the VOC-infected group between cases and controls.
Being fully vaccinated was significantly protective against hospitalization among both crude and adjusted odds ratios. Notably, the vaccine effectiveness decreased among non-boosted adults when affected with VOC infections. In the subsequent analysis, controls were fully vaccinated individuals, while cases were those who had received booster vaccinations. Significant hospitalization rate differences were observed in VOC and non-VOC cases compared to controls. With homologus-boosted adults, vaccine effectiveness was slightly higher among VOC-infected individuals than non-VOC-infected individuals. No significant difference (p = 0.258) in hospitalization in the non-VOC-infected group was noted between cases and controls with the heterologous-boosted adults. On the other hand, there was a significant difference (p < 0.001) in hospitalization in the VOC-infected group between cases and controls.
Discussion
Within the context of our study, focusing on the high-risk and rapidly evolving nature of COVID-19, a crucial insight was noted: the choice of booster vaccination plays a pivotal role in effectively mitigating the impact of these ever-changing viral strains. Our findings strongly support the efficacy of a heterologous booster approach in addressing viruses with high mutation rates. As such viruses continue to pose significant global health challenges, our research highlights the importance of strategic vaccination methods, offering valuable insights that contribute to ongoing pandemic preparedness and response efforts.
This study included a substantial sample size, encompassing both adult and pediatric cohorts. In particular, our analyses of the pediatric population revealed significant disparities in critical care length of stay and the need for ventilatory support among those infected with VOCs. These findings align with a study conducted in the USA during the Omicron wave in New York City, which emphasized the impact of VOC infection on critical care outcomes in pediatric cases [15]. Among adults, VOC infection led to a significant increase in healthcare resource utilization, including hospitalization, ICU admission, extended length of stay, ventilatory support, and a higher risk of in-hospital COVID-19 mortality compared to non-VOC infection. Additionally, significant differences were observed in age, gender, nationality, BMI, infection status, and comorbidities, such as bronchial asthma, chronic kidney disease, diabetes, cardiovascular disease, and hypertension. These findings highlight the impact of vaccination on different demographic and health-related factors, emphasizing the complex interactions between vaccination status and individual characteristics.
Our results revealed the superiority of booster vaccinations over two-dose regimens and the effectiveness of full vaccination compared to no or incomplete vaccination in preventing hospitalization and severe illness. Notably, heterologous boosters, specifically with mRNA vaccine BNT162b2 (Pfizer) booster, exhibited the highest vaccine effectiveness, a trend consistent with other relevant studies [16–19] and local research on different populations’ immune response levels [20]. Heterologous boosting enhanced vaccine-elicited immune responses, including spike-specific CD4 + and CD8 + T cells, and induced higher levels of neutralizing antibodies (NAbs) with broader and longer-lasting neutralizing capacity [20]. A study among healthcare professionals who received the CoronaVac booster showed a significant two-fold increase in Omicron-neutralizing activity post-boost [21]. Similarly, a study conducted in the UK highlighted improved protection against symptomatic COVID-19 following booster doses of BNT162b2 or mRNA-1273 vaccines during the Delta variant’s dominance [22].
Stepwise case-control analyses revealed a decline in vaccine effectiveness against VOC infections among non-boosted, fully vaccinated adults. In contrast, homologous boosters demonstrated a marginal 2% difference in vaccine effectiveness between VOC and non-VOC infections. The highest vaccine effectiveness was observed among VOC-infected adults who received heterologous boosters. The global emergence of the Omicron sublineage has necessitated a strategic shift in vaccination protocols, promoting third and subsequent booster doses, whether administered homologously or heterologously. Our findings align with broader research showing that heterologous booster doses enhance neutralization activity against Omicron and its sublineages [23].
Limitations of our study include the absence of comparisons between different types of VOC infections and a reliance on hospital-registered cases for viral sequence analysis, restricting generalizability to the general population. However, this study features one of the largest sample sizes in the region, offering significant public health insights, encompassing genomic epidemiology, vaccination practices, and the allocation of healthcare resources.
This study demonstrated the effectiveness of heterologous boosting with mRNA-based vaccines as a powerful means of enhancing overall vaccine effectiveness and immunogenicity, providing extended protection against VOCs. The practical application of such “mix and match” strategies presents a promising pathway for streamlining mass vaccination efforts. This flexibility is crucial as the global response to COVID-19 adapts to the emergence of new VOCs, offering a dynamic approach to vaccine administration. As the global community navigates the complexities of COVID-19 vaccination strategies, it is essential to undertake long-term observational studies examining vaccine-induced immunity’s durability. These efforts are vital for refining our understanding of these dynamics and optimizing the long-term efficacy of heterologous vaccine approaches within the broader framework of global vaccination strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Ms. Hanan Al Mutairi, from the Public Health Research section at the Abu Dhabi Public Health Center (ADPHC).
Abbreviations
- BBIBP-CorV
Beijing Bio-Institute of Biological Products COVID-19 vaccine
- BMI
Body Mass Index
- COVID-19
COrona VIrus Disease 2019
- ICD
International Classification of Diseases
- ICU
Intensive Care Unit
- m-RNA
Messenger Ribonucleic Acid
- RT-PCR
Reverse Transcriptase – Polymerase Chain Reaction
- SARS-CoV-2
Severe Acute Respiratory Syndrome CoronaVirus 2
- SPSS
Statistical Package for Social Science (SPSS)
- UAE
United Arab Emirates
- VE
Vaccine Effectiveness
- VOC
Variant of Concern
Author contributions
This study was conceptualized and consensus on methodology was done by RA, HA, NA, FA. Dara collection and mapping was done by FS, JA, AR, Data analysis was done by MM, RA. Manuscript drafting was done by RA, MM. All coauthors provided input and review.
Funding
This article is a part of a public health project conducted by Abu Dhabi Department of Health, UAE, for survaillance of SARS CoV-2 variants, no funding received for conducting this research.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Declarations
Ethics approval and consent to participate
Abu Dhabi Health Research and Technology Ethics Committee, established as by the principles outlined in the Declaration of Helsinki, reviewed and approved the study protocol and the manuscript. The requirement for participants to provide informed consent for this study was waived by the same ethics committee through Institutional Review Board (IRB) number DOH/CVDC/2021/1704.
Consent for publication
We don’t have any content that requires consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rowan Abuyadek and Mira Mousa are first co-author
References
- 1.Edouard Mathieu HR, Lucas Rodés-Guirao C, Appel C, Giattino J, Hasell B, Macdonald S, et al. Coronavirus pandemic (COVID-19). 2024 [cited 2024 Aug 15]. Available from: https://ourworldindata.org/coronavirus
- 2.Abuyadek R, Amirtharaj F, Al Marzooqi S, Mahmoud S, Al Hosani F. Combined epidemiology and genetic sequencing surveillance in the era of COVID-19 pandemic; Abu Dhabi experience, United Arab Emirates. Infect Genet Evol. 2023;109:105411. [DOI] [PubMed] [Google Scholar]
- 3.Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538). [DOI] [PMC free article] [PubMed]
- 4.Hoffmann M, Arora P, Groß R, Seidel A, Hörnich BF, Hahn AS, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184(9):2384–e9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–80. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Ginn HM, Dejnirattisai W, Supasa P, Wang B, Tuekprakhon A, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184(16):4220–e3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603(7902):679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vokó Z, Kiss Z, Surján G, Surján O, Barcza Z, Wittmann I, et al. Effectiveness and waning of Protection with different SARS-CoV-2 primary and Booster vaccines during the Delta Pandemic Wave in 2021 in Hungary (HUN-VE 3 study). Front Immunol. 2022;13:919408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belayachi J, Obtel M, Mhayi A, Razine R, Abouqal R. Long term effectiveness of inactivated vaccine BBIBP-CorV (Vero cells) against COVID-19 associated severe and critical hospitalization in Morocco. PLoS ONE. 2022;17(12):e0278546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Kaabi N, Oulhaj A, Ganesan S, Al Hosani FI, Najim O, Ibrahim H, et al. Effectiveness of BBIBP-CorV vaccine against severe outcomes of COVID-19 in Abu Dhabi, United Arab Emirates. Nat Commun. 2022;13(1):3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu Dhabi Health Data Services- SP LLC. (Malaffi), Page Title. https://malaffi.ae/. (2022) Accessed 1 Jun 2023.
- 14.Shao W, Chen X, Zheng C, Liu H, Wang G, Zhang B, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: a literature review and meta-analysis. Emerg Microbes Infections. 2022;11(1):2383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acker KP, Levine DA, Varghese M, Nash KA, RoyChoudhury A, Abramson EL et al. Indications for hospitalization in children with SARS-CoV-2 infection during the Omicron Wave in New York City. Child (Basel). 2022;9(7). [DOI] [PMC free article] [PubMed]
- 16.Belik M, Jalkanen P, Lundberg R, Reinholm A, Laine L, Väisänen E, et al. Comparative analysis of COVID-19 vaccine responses and third booster dose-induced neutralizing antibodies against Delta and Omicron variants. Nat Commun. 2022;13(1):2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canaday DH, Oyebanji OA, White E, Keresztesy D, Payne M, Wilk D, et al. COVID-19 vaccine booster dose needed to achieve Omicron-specific neutralisation in nursing home residents. eBioMedicine. 2022;80:104066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Beltran WF, St. Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457–e664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lusvarghi S, Pollett SD, Neerukonda SN, Wang W, Wang R, Vassell R, et al. SARS-CoV-2 BA.1 variant is neutralized by vaccine booster–elicited serum but evades most convalescent serum and therapeutic antibodies. Sci Transl Med. 2022;14(645):eabn8543. [DOI] [PMC free article] [PubMed]
- 20.Al-Rifai RH, Alhosani F, Abuyadek R, Atef S, Donnelly JG, Leinberger-Jabari A, et al. Evaluation of post-vaccinationimmunoglobulin G antibodies and T-cell immune response after inoculation withdifferent types and doses of SARS-CoV-2 vaccines: a retrospective cohort study.Front Med. 2022;9:1092646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Chen L, Yin S, Tao Y, Zhu L, Tong X, et al. The third dose of CoronVac vaccination induces broad and potent adaptive immune responses that recognize SARS-CoV-2 delta and omicron variants. Emerg Microbes Infect. 2022;11(1):1524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(4):831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gils MJ, Lavell A, van der Straten K, Appelman B, Bontjer I, Poniman M, et al. Antibody responses against SARS-CoV-2 variants induced by four different SARS-CoV-2 vaccines in health care workers in the Netherlands: a prospective cohort study. PLoS Med. 2022;19(5):e1003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.